Monascuspiloin from Monascus-Fermented Red Mold Rice Alleviates Alcoholic Liver Injury and Modulates Intestinal Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
2.2. Animal Experiment
2.3. Biochemical Parameter Detection
2.4. Histological Examination
2.5. Analysis of Fecal Short-Chain Fatty Acids (SCFAs) by Gas Chromatograph (GC)
2.6. Amplicon Sequencing and Bioinformatics Analysis
2.7. Metabolomics Analysis
2.8. RT-qPCR
2.9. Western Blot
2.10. Statistical Analysis
3. Results
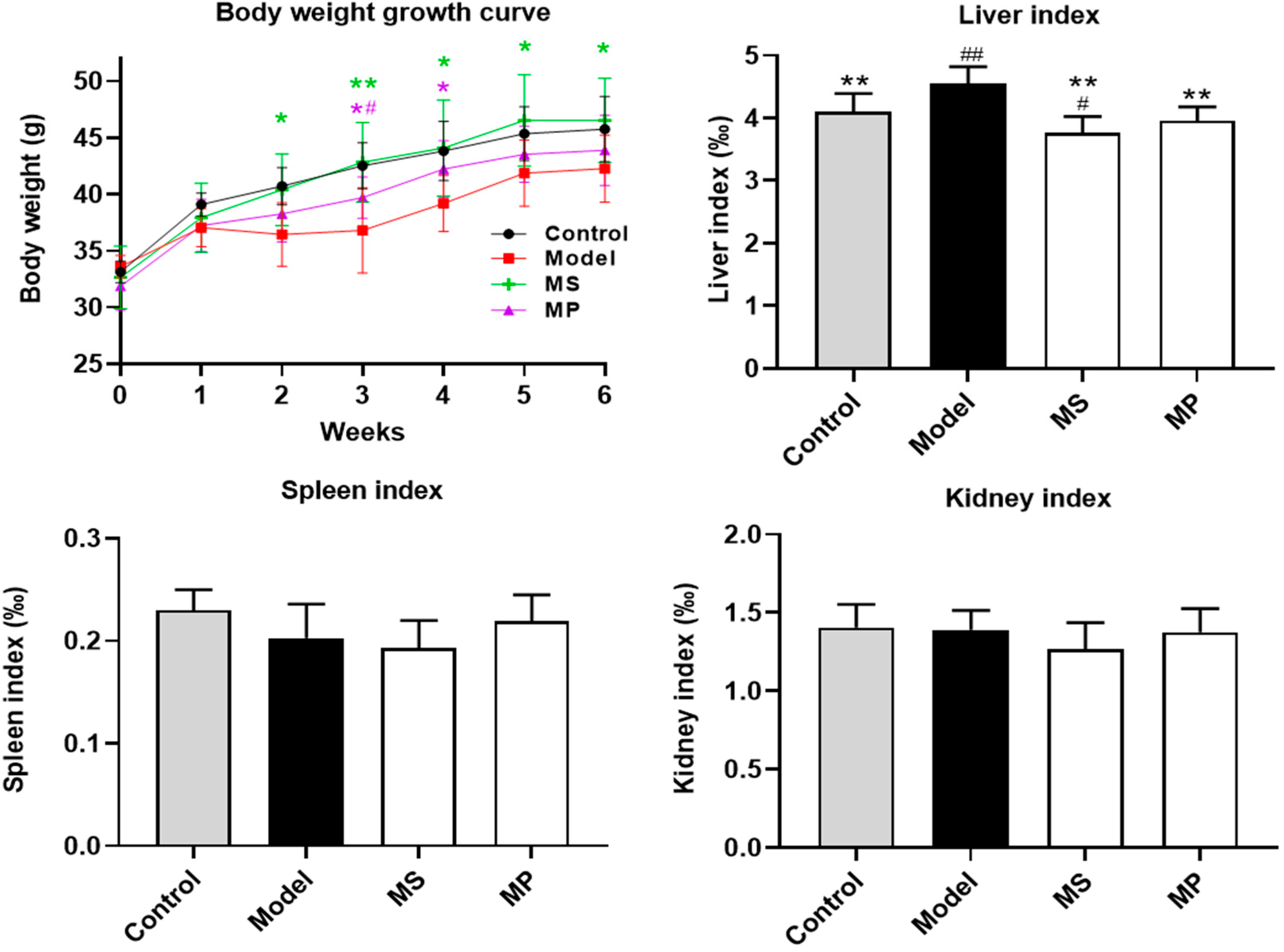
3.1. Effects of MP Intervention on the Body Weight and Organ Indexes
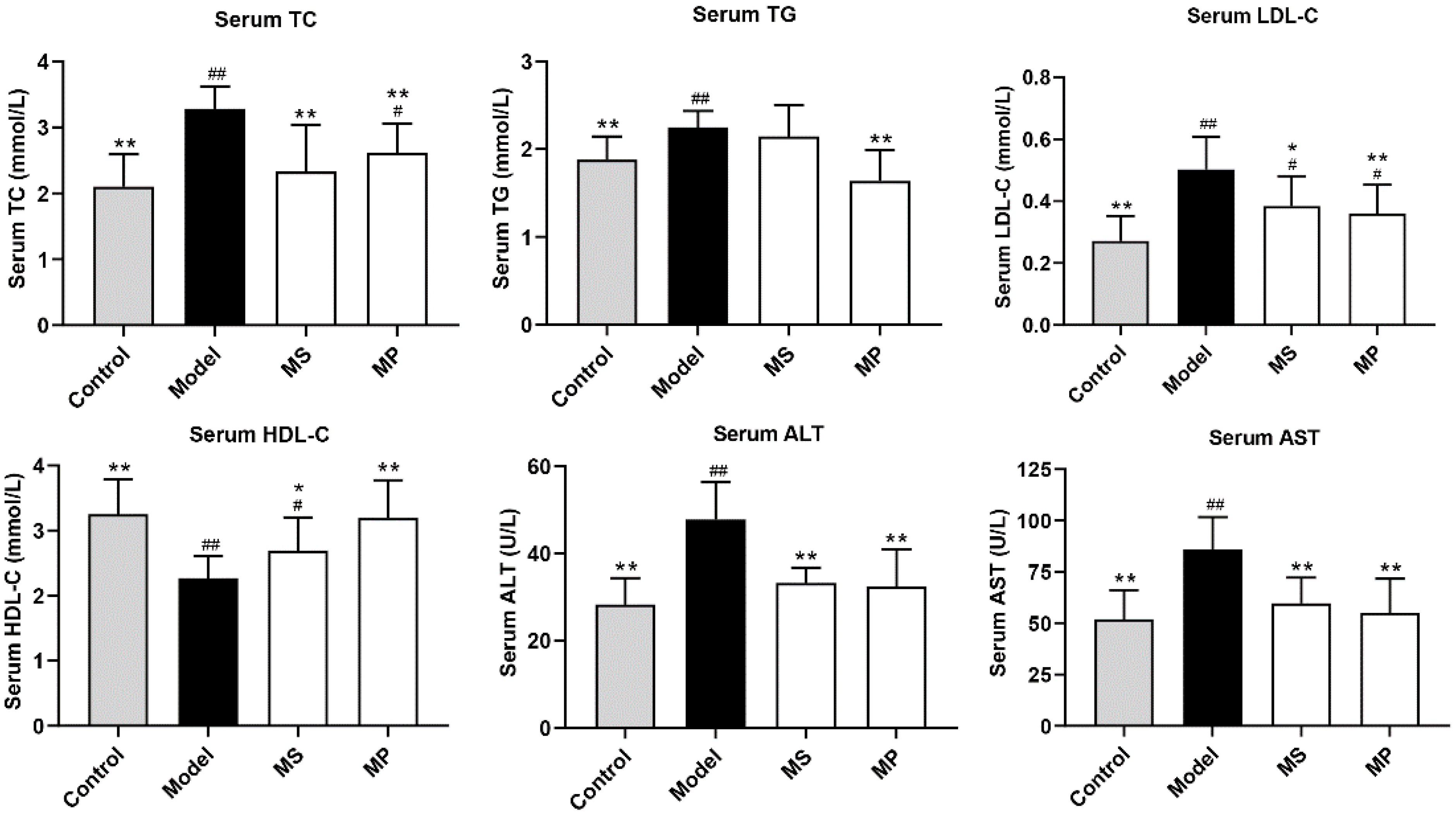
3.2. Effects of MP on Serum Biochemical Parameters
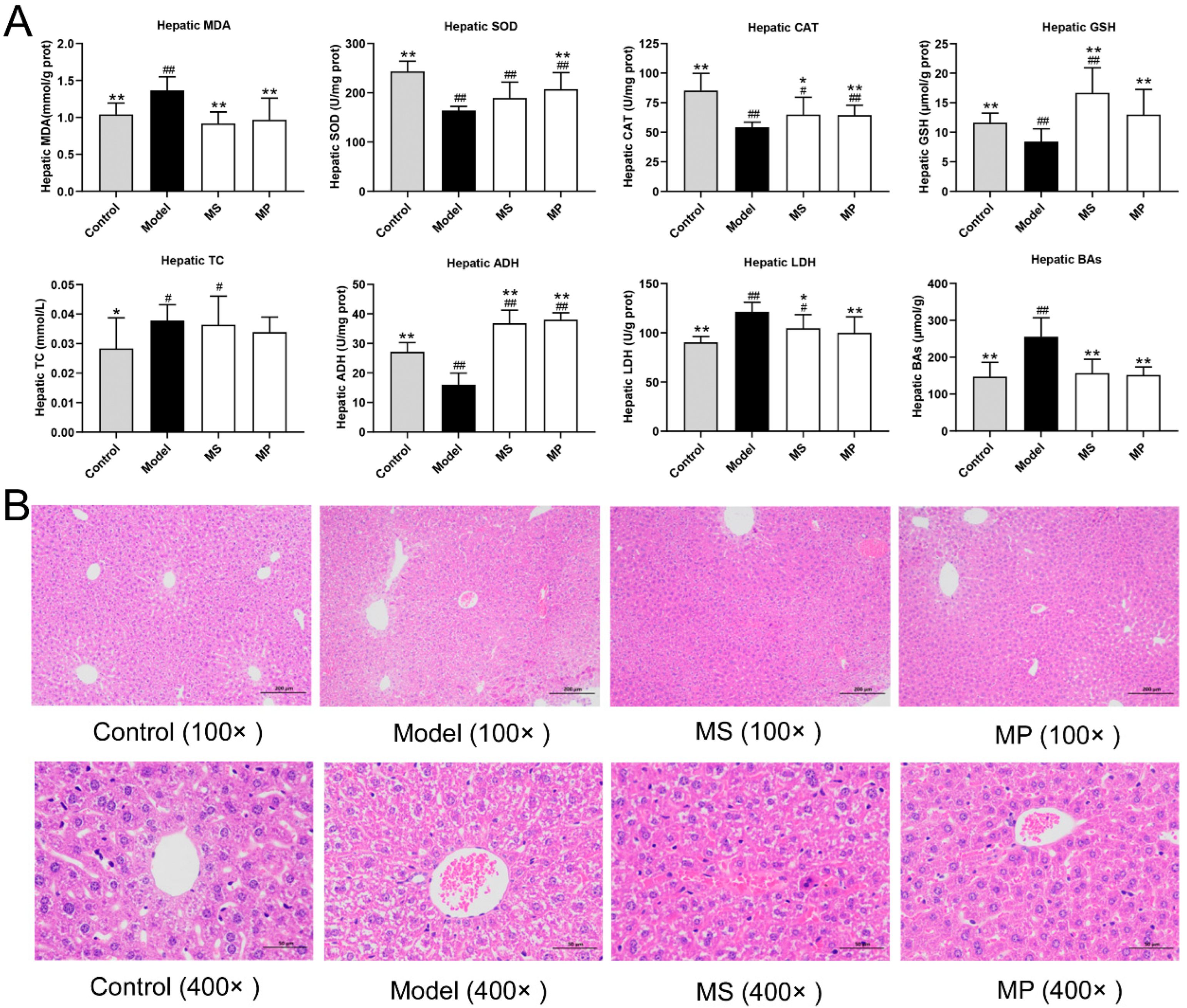
3.3. Effects of MP on Liver Biochemical Parameters and Histopathological Features
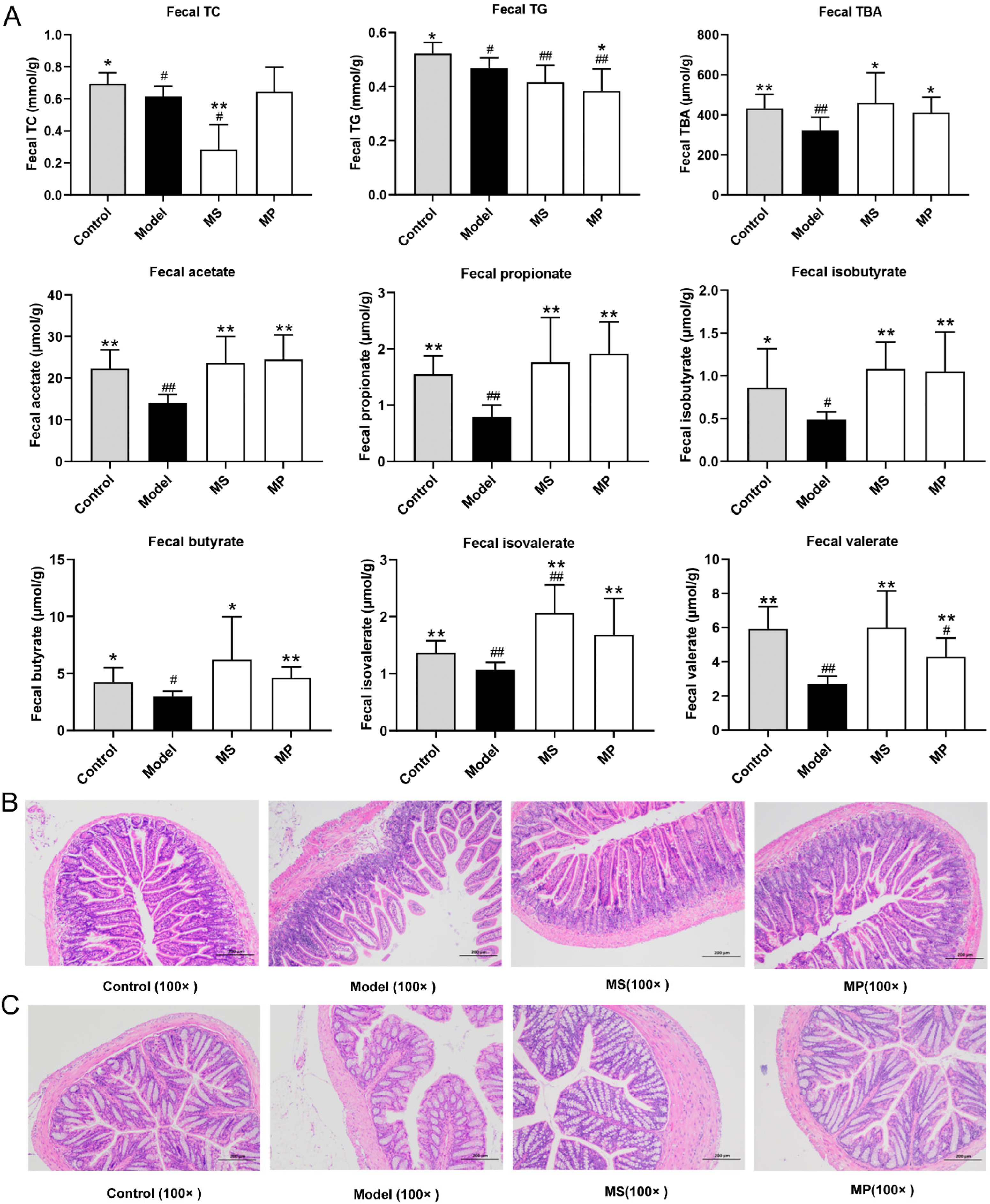
3.4. Effects of MP on Fecal Biochemical Parameters and Intestinal Histopathological Features
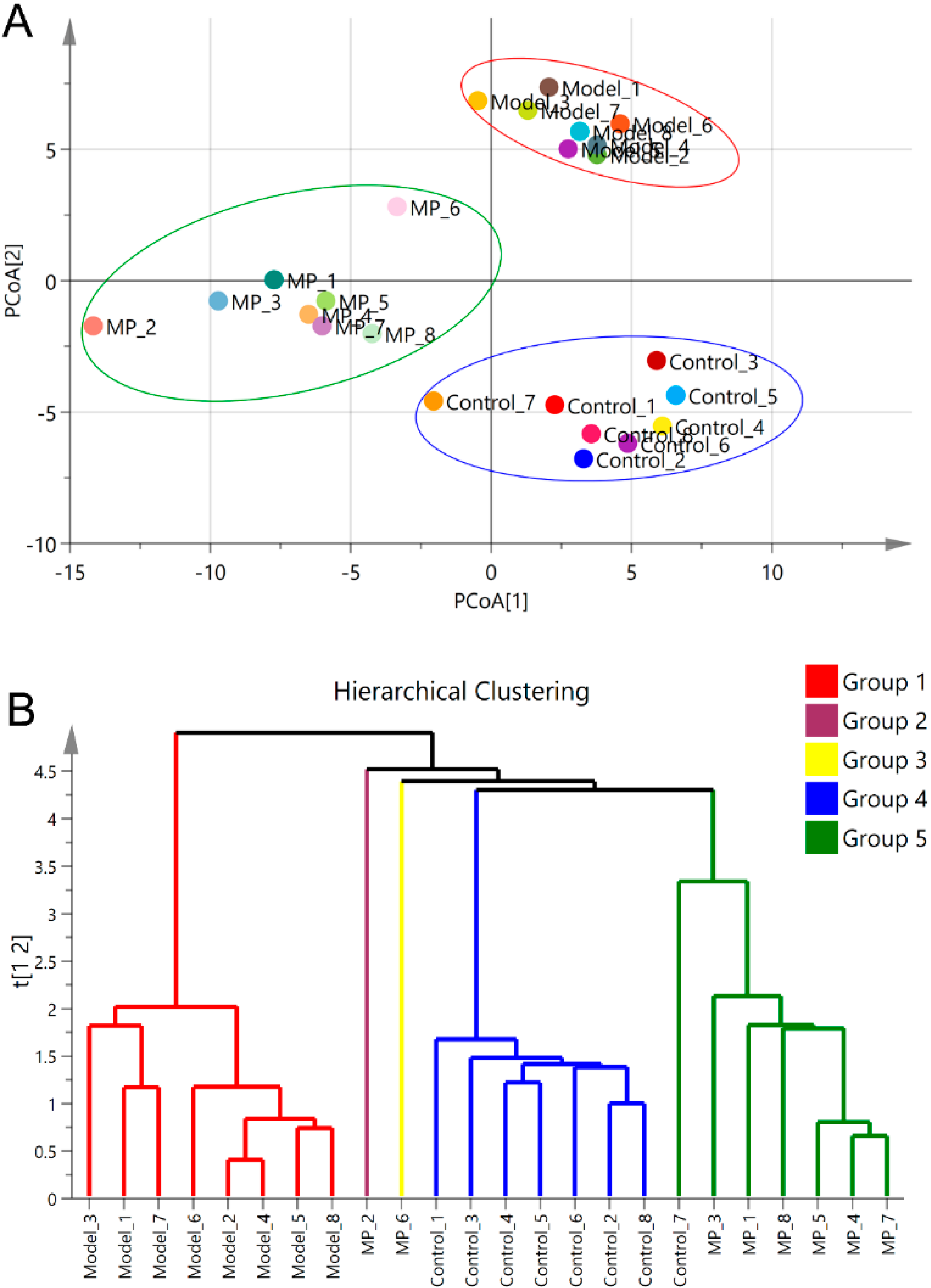
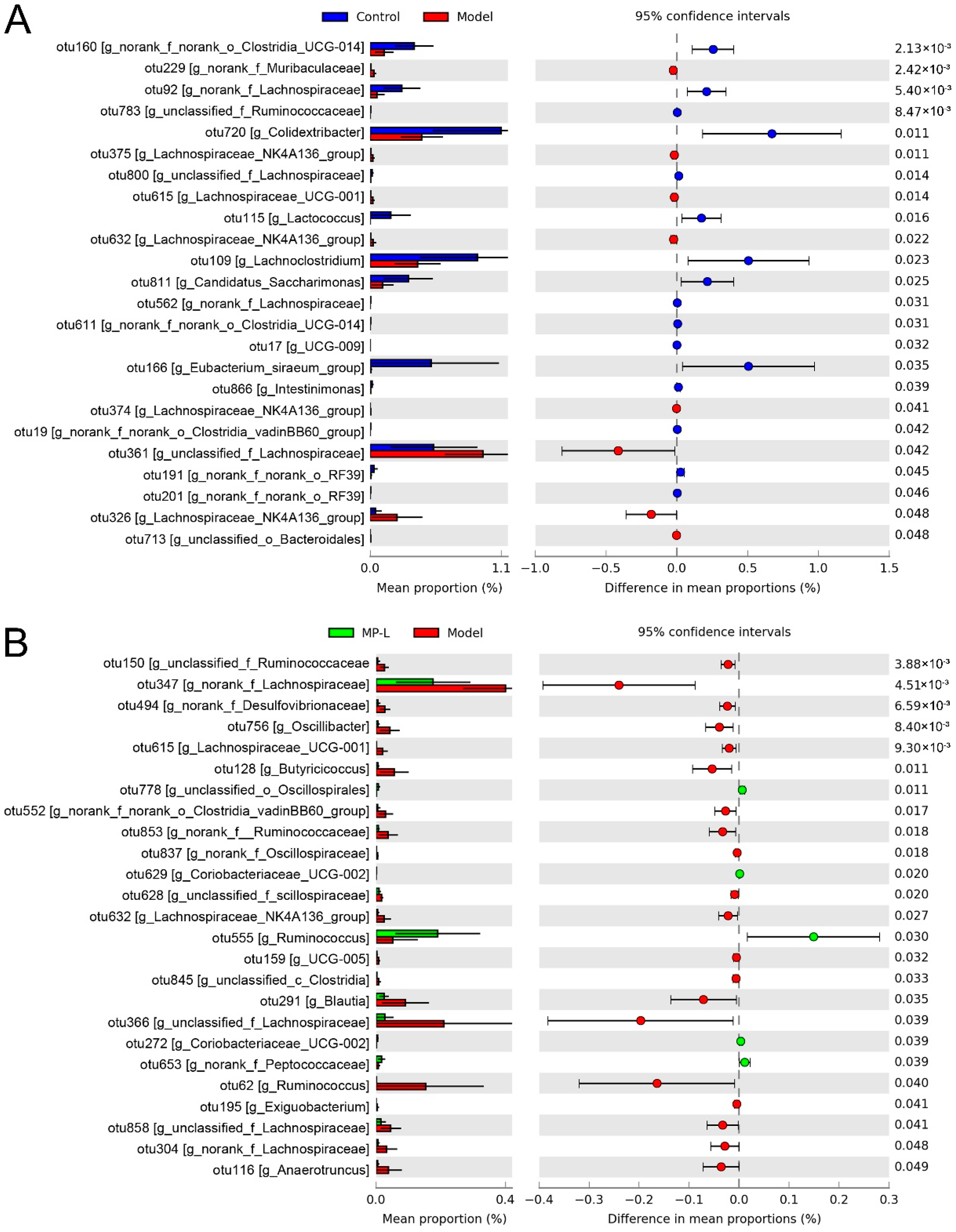
3.5. Effects of MP Intervention on the Composition of Intestinal Microflora
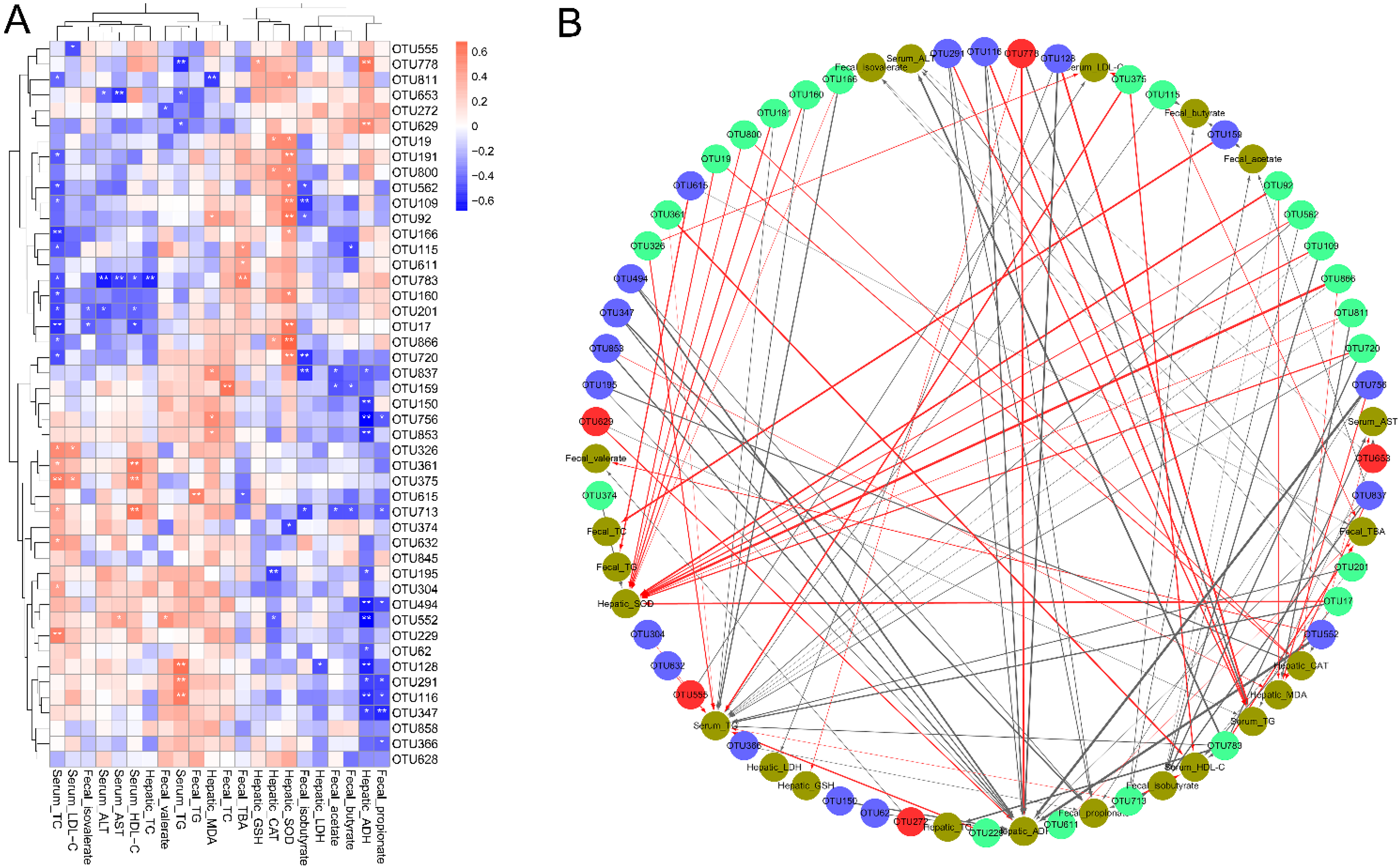
3.6. Correlations of the Key Bacterial OTUs with the Biochemical Parameters
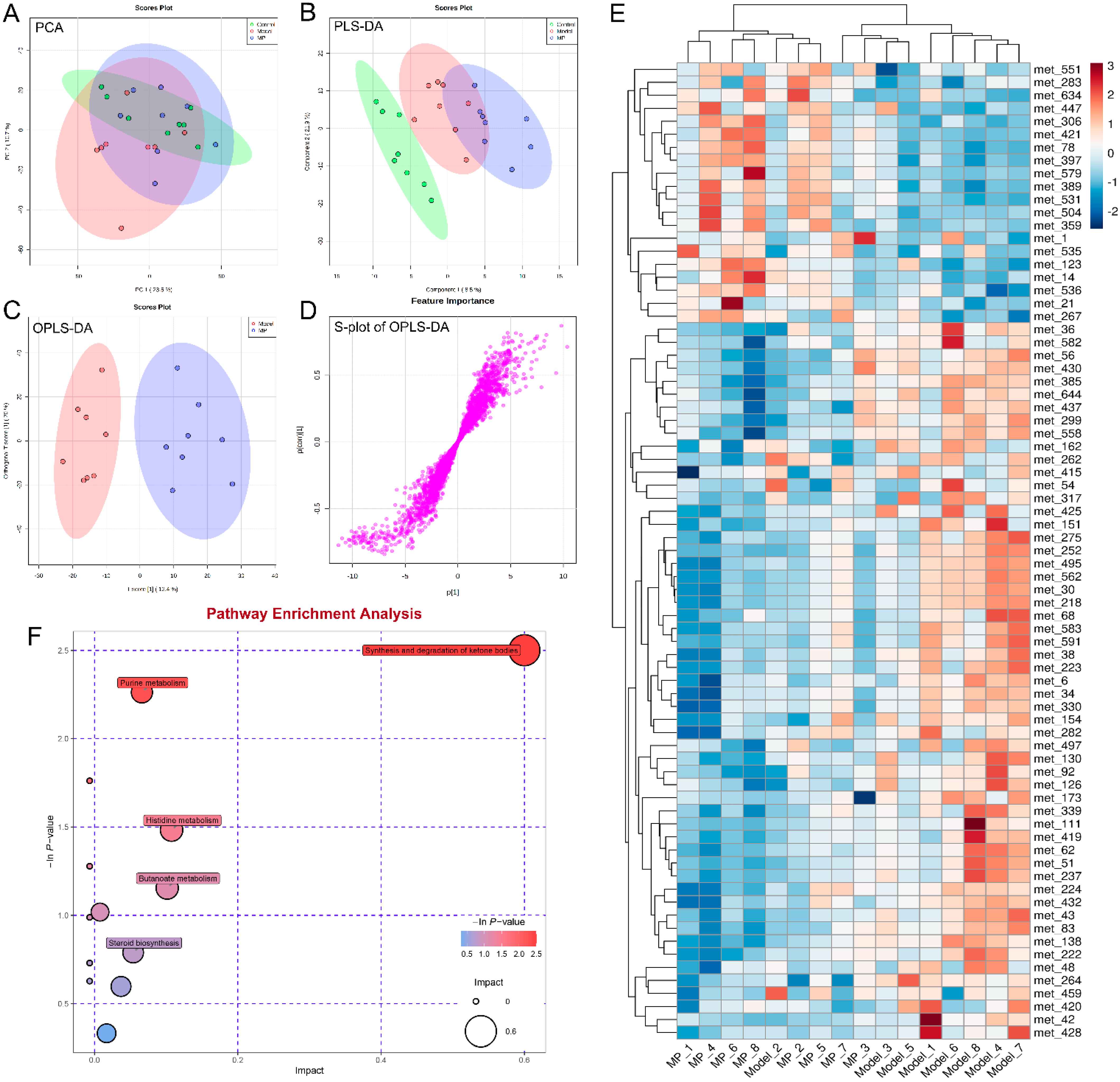
3.7. Effects of MP Intervention on Liver Metabolome in Mice with Excessive Alcohol Intake
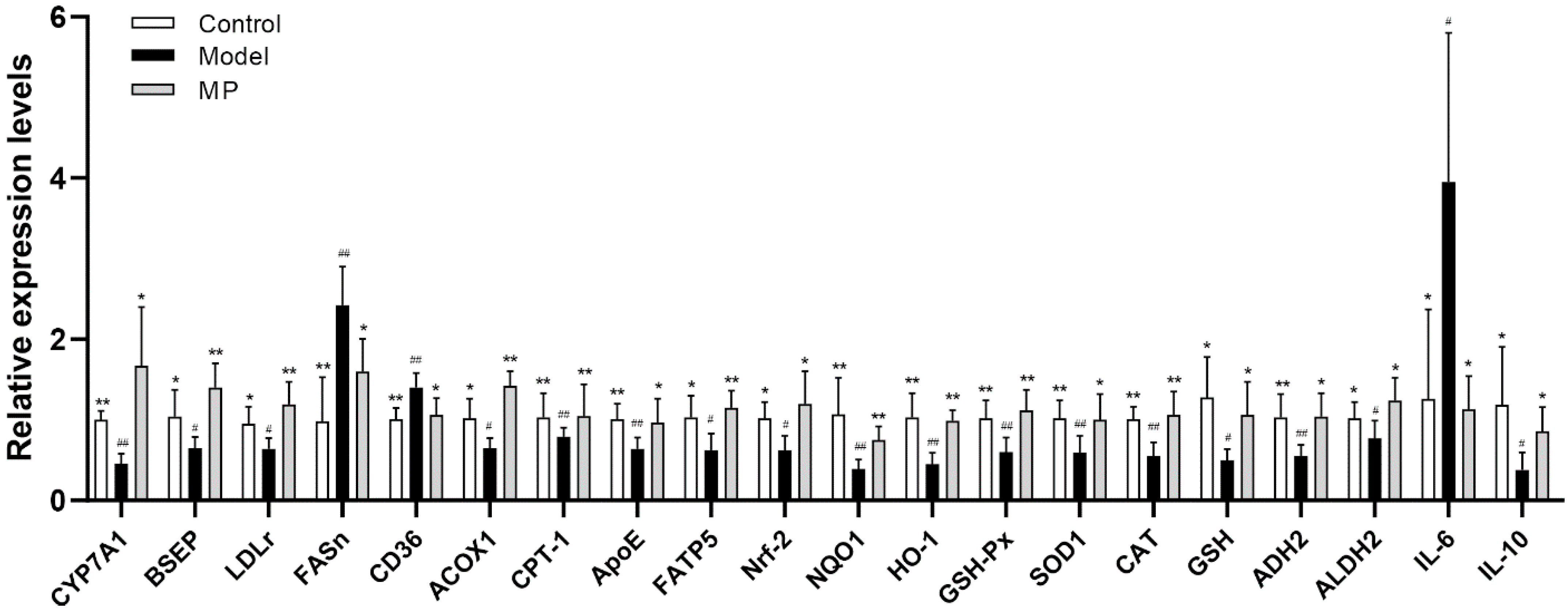
3.8. Effects of MP Intervention on the mRNA Transcription Levels in Liver
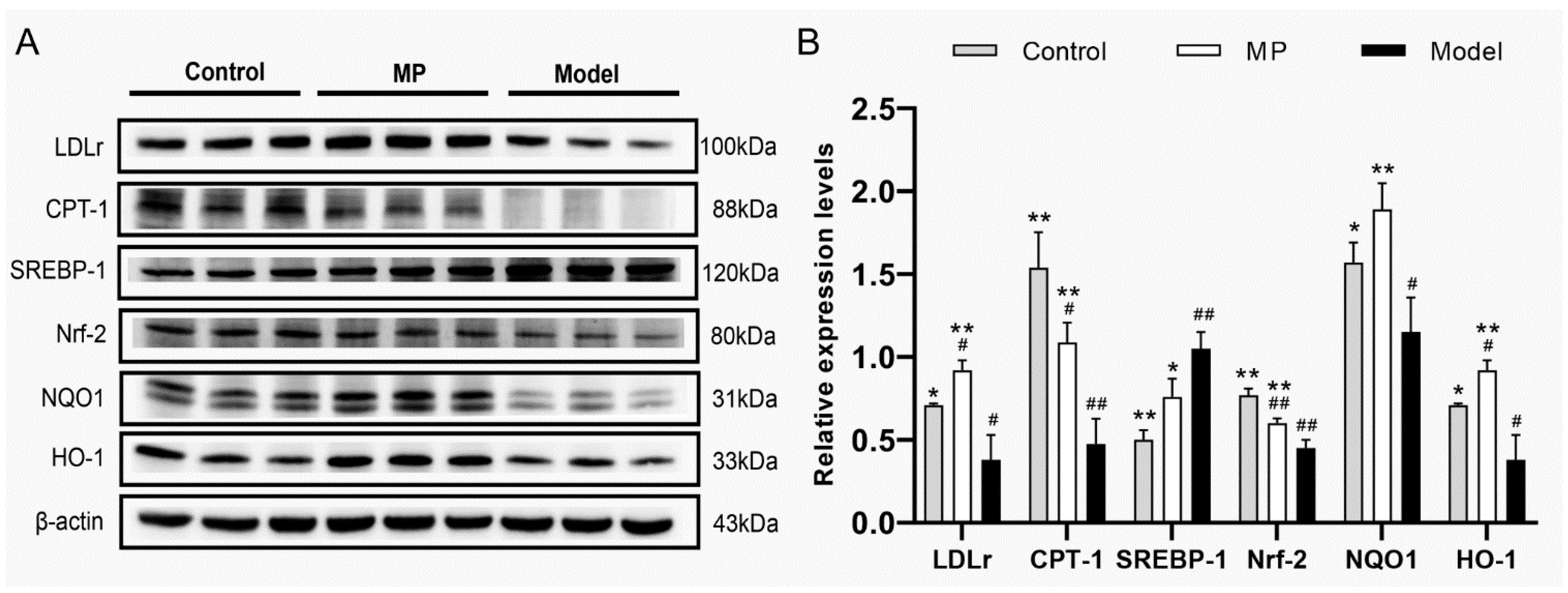
3.9. Effects of MP Intervention on the Protein Expression in Liver
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raghu, R.; Liu, C.T.; Tsai, M.H.; Tang, X.J.; Kalari, K.R.; Subramanian, S. Transcriptome analysis of garlic-induced hepatoprotection against alcoholic fatty liver. J. Agric. Food Chem. 2012, 60, 11104–11119. [Google Scholar] [CrossRef] [PubMed]
- Altamirano, J.; Bataller, R. Alcoholic liver disease: Pathogenesis and new targets for therapy. Nat. Rev. Gastro. Hepat. 2011, 8, 491–501. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, R.S.; Dasarathy, S.; McCullough, A.J. Alcoholic Liver Disease. Off. J. Am. Coll. Gastroenterol. 2010, 105, 14–32. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Y.; Molnar, I.A.; Chen, F. Nature and nurture: Confluence of pathway determinism with metabolic and chemical serendipity diversifies Monascus azaphilone pigments. Nat. Prod. Rep. 2019, 36, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, K.; Chen, F.; Chen, G.; Yu, Y.; Lv, X.; Zhang, W.; Rao, P.; Ni, L. Comparative study on the antioxidant activity of Monascus yellow pigments from two different types of Hongqu-functional qu and coloring qu. Front. Microbiol. 2021, 12, 715295. [Google Scholar] [CrossRef]
- Cheng, C.F.; Pan, T.M. Monascus-fermented red mold dioscorea protects mice against alcohol-induced liver injury, whereas its metabolites ankaflavin and monascin regulate ethanol-induced peroxisome proliferator-activated receptor-γ and sterol regulatory element-binding transcription factor-1 expression in HepG2 cells. J. Sci. Food Agric. 2018, 98, 1889–1898. [Google Scholar]
- Lai, J.R.; Hsu, Y.W.; Pan, T.M.; Lee, C.L. Monascin and ankaflavin of Monascus purpureus prevent alcoholic liver disease through regulating AMPK-mediated lipid metabolism and enhancing both anti-inflammatory and anti-oxidative systems. Molecules 2021, 26, 6301. [Google Scholar] [CrossRef]
- Chen, R.J.; Hung, C.M.; Chen, Y.L.; Wu, M.D.; Yuan, G.F.; Wang, Y.J. Monascuspiloin induces apoptosis and autophagic cell death in human prostate cancer cells via the Akt and AMPK signaling pathways. J. Agric. Food Chem. 2012, 60, 7185–7193. [Google Scholar] [CrossRef]
- Chiu, H.W.; Fang, W.H.; Chen, Y.L.; Wu, M.D.; Yuan, G.F.; Ho, S.Y.; Wang, Y.J. Monascuspiloin enhances the radiation sensitivity of human prostate cancer cells by stimulating endoplasmic reticulum stress and inducing autophagy. PLoS ONE 2012, 7, e40462. [Google Scholar] [CrossRef]
- Szabo, G. Gut–liver axis in alcoholic liver disease. Gastroenterology 2015, 148, 30–36. [Google Scholar] [CrossRef]
- Guo, W.L.; Pan, Y.Y.; Li, L.; Li, T.T.; Liu, B.; Lv, X.C. Ethanol extract of Ganoderma lucidum ameliorates lipid metabolic disorders and modulates the gut microbiota composition in high-fat diet fed rats. Food Funct. 2018, 9, 3419–3431. [Google Scholar] [CrossRef]
- Lv, X.C.; Guo, W.L.; Li, L.; Yu, X.D.; Liu, B. Polysaccharide peptides from Ganoderma lucidum ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet-fed rats. J. Funct. Foods 2019, 57, 48–58. [Google Scholar] [CrossRef]
- Ahmad, M.F. Ganoderma lucidum: Persuasive biologically active constituents and their health endorsement. Biomed. Pharm. 2018, 107, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Pande, A.; Schnabl, B. Microbiome as a therapeutic target in alcohol-related liver disease. J. Hepatol. 2019, 70, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Tian, D.; Liu, Y.; Li, H.; Zhu, J.; Li, M.; Xin, M.; Xia, J. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids A, C2, D, F, DM, X and Y. Eur. J. Med. Chem. 2019, 174, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Li, A.; Jiang, J.; Zhou, L.; Yu, Z.; Lu, H.; Xie, H.; Chen, X.; Shao, L.; Zhang, R.; et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut 2019, 68, 1014–1023. [Google Scholar] [CrossRef]
- Georgia, C.; Tan, W.Y.; PabloOrlicky, B.C.; David, J.O.; Jaya Prakash, G.; Rolando, G.M.; Aalizadeh, R.; Thomaidis, N.S.; Thompson, D.C.; Vasiliou, V. Liver metabolomics identifies bile acid profile changes at early stages of alcoholic liver disease in mice. Chem.-Biol. Interact. 2022, 360, 109931. [Google Scholar]
- Liu, C.T.; Raghu, R.; Lin, S.H.; Wang, S.Y.; Kuo, C.H.; Tseng, Y.J.; Sheen, L.Y. Metabolomics of ginger essential oil against alcoholic fatty liver in mice. J. Agric. Food Chem. 2013, 61, 11231–11240. [Google Scholar] [CrossRef]
- Hsu, Y.W.; Hsu, L.C.; Liang, Y.H.; Kuo, Y.H.; Pan, T.M. Monaphilones A-C, three new antiproliferative azaphilone derivatives from Monascus purpureus NTU. J. Agric. Food Chem. 2010, 58, 8211–8216. [Google Scholar] [CrossRef]
- Cheng, C.F.; Pan, T.M. Protective effect of Monascus-fermented red mold rice against alcoholic liver disease by attenuating oxidative stress and inflammatory response. J. Agric. Food Chem. 2011, 59, 9950–9957. [Google Scholar] [CrossRef]
- Lee, C.L.; Wen, J.Y.; Hsu, Y.W.; Pan, T.M. Monascus-fermented yellow pigments monascin and ankaflavin, showed antiobesity effect via the suppression of differentiation and lipogenesis in obese rats fed a high-fat diet. J. Agric. Food Chem. 2013, 61, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.C.; Liang, Y.H.; Hsu, Y.W.; Kuo, Y.H.; Pan, T.M. Anti-inflammatory properties of yellow and orange pigments from Monascus purpureus NTU. J. Agric. Food Chem. 2013, 61, 2796–2802. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Hsu, W.H.; Huang, T.; Chang, Y.Y.; Hsu, Y.W.; Pan, T.M. Monascin improves diabetes and dyslipidemia by regulating PPARγ and inhibiting lipogenesis in fructose-rich diet-induced C57BL/6 mice. Food Funct. 2013, 4, 950–959. [Google Scholar] [CrossRef]
- Guo, H.; Chou, W.C.; Lai, Y.J.; Liang, K.X.; Tam, J.W.; Brickey, W.J.; Chen, L.; Montgomery, N.D.; Li, X.; Bohannon, L.M.; et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 2020, 370, eaay9097. [Google Scholar] [CrossRef]
- Lin, Y.; Xiao, R.; Luo, G.; Liao, W.; Chen, S.; Han, S.; Liang, S. Multi-omics analysis revealed the association of human gut microbiota composition and metabolic functions with the daily diet of the additive Ficus hirta Vahl. Res. Sq. 2022, 3, 1610765. [Google Scholar]
- Guo, W.L.; Cao, Y.J.; You, S.Z.; Wu, Q.; Zhang, F.; Han, J.Z.; Lv, X.C.; Rao, P.F.; Ai, L.Z.; Ni, L.; et al. Ganoderic acids-rich ethanol extract from Ganoderma lucidum protects against alcoholic liver injury and modulates intestinal microbiota in mice with excessive alcohol intake. Curr. Res. Food Sci. 2022, 5, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Chen, T.H.; Lee, B.H.; Hsu, Y.W.; Pan, T.M. Monascin and ankaflavin act as natural AMPK activators with PPARa agonist activity to down-regulate nonalcoholic steatohepatitis in high-fat diet-fed C57BL/6 mice. Food Chem. Toxicol. 2014, 64, 94–103. [Google Scholar] [CrossRef]
- Song, X.F.; Zhong, L.; Lyu, N.; Liu, F.; Li, B.X.; Hao, Y.N.; Xue, Y.; Li, J.; Feng, Y.; Ma, Y.; et al. Inulin can alleviate metabolism disorders in ob/ob mice by partially restoring leptin-related pathways mediated by gut microbiota. Genom. Proteom. Bioinform. 2019, 17, 64–75. [Google Scholar] [CrossRef]
- Wang, O.; Cheng, Q.; Liu, J.; Wang, Y.; Zhao, L.; Zhou, F.; Ji, B. Hepatoprotective effect of Schisandra chinensis (Turcz.) Baill. lignans and its formula with Rubus idaeus on chronic alcohol-induced liver injury in mice. Food Funct. 2014, 5, 3018–3025. [Google Scholar] [CrossRef]
- Zeng, H.L.; Huang, C.C.; Lin, S.; Zheng, M.J.; Chen, C.J.; Zheng, B.D.; Zhang, Y. Lotus seed resistant starch regulates gut microbiota and increases short-chain fatty acids production and mineral absorption in mice. J. Agric. Food Chem 2017, 65, 9217–9225. [Google Scholar] [CrossRef]
- Deehan, E.C.; Yang, C.; Perez-Muñoz, M.E.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Zhang, Z.X.; Bakal, J.A.; Walter, J. Precision microbiome modulation with discrete dietary fiber structures directs short-chain fatty acid production. Cell Host Microbe 2020, 27, 389–404.e386. [Google Scholar] [CrossRef] [PubMed]
- Swanson, G.R.; Siskin, J.; Gorenz, A.; Shaikh, M.; Raeisi, S.; Fogg, L.; Forsyth, C.; Keshavarzian, A. Disrupted diurnal oscillation of gut-derived short chain fatty acids in shift workers drinking alcohol: Possible mechanism for loss of resiliency of intestinal barrier in disrupted circadian host. Trans. Res. 2020, 221, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, X.X.; Su, C.Y.; Xi, M.; Zhang, X.M.; Jiang, Z.B.; Wang, L.; Hong, B. Butyrate protects against high-fat diet-induced atherosclerosis via up-regulating ABCA1 expression in apolipoprotein E-deficiency mice. Brit. J. Pharmacol. 2020, 177, 1754–1772. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.D.; Cao, Y.N.; Peng, L.X.; Yan, Z.Y.; Zhao, G. Coarse cereals and legume grains exert beneficial effects through their interaction with gut microbiota: A review. J. Agric. Food Chem. 2020, 69, 861–877. [Google Scholar] [CrossRef]
- Liu, X.; Sun, R.Z.; Li, Z.Z.; Xiao, R.X.; Lv, P.F.; Sun, X.R.; Olson, M.A.; Gong, Y.L. Luteolin alleviates non-alcoholic fatty liver disease in rats via restoration of intestinal mucosal barrier damage and microbiota imbalance involving in gut-liver axis. Arch. Biochem. Biophys. 2021, 711, 109019. [Google Scholar] [CrossRef]
- Li, H.L.; Xie, Z.Y.; Zhang, Y.; Liu, Y.; Niu, A.J.; Liu, Y.; Zhang, L.; Guan, L. Rosa rugosa polysaccharide attenuates alcoholic liver disease in mice through the gut-liver axis. Food Biosci. 2021, 44, 101385. [Google Scholar] [CrossRef]
- Tian, Y.; Wen, Z.; Lei, L.; Li, F.; Zhao, J.; Zhi, Q.; Li, F.; Yin, R.; Ming, J. Coreopsis tinctoria flowers extract ameliorates D-galactose induced aging in mice via regulation of Sirt1-Nrf2 signaling pathway. J. Funct. Foods 2019, 60, 103464. [Google Scholar] [CrossRef]
- Chen, P.L.; Lei, S.Z.; Tong, M.Y.; Chang, Q.; Zheng, B.D.; Zhang, Y.; Zeng, H.L. Effect of polysaccharide fractions from Fortunella margarita on the fecal microbiota of mice and SCFA production in vitro. Food Sci. Hum. Well 2022, 11, 97–108. [Google Scholar] [CrossRef]
- Houwenhuyse, S.; Stoks, R.O.; Mukherjee, S.; Decaestecker, E. Locally adapted gut microbiomes mediate host stress tolerance. ISME J. 2021, 15, 2401–2414. [Google Scholar] [CrossRef]
- Gupta, R.S.; Nanda, A.; Khadka, B. Novel molecular, structural and evolutionary characteristics of the phosphoketolases from bifidobacteria and Coriobacteriales. PLoS ONE 2017, 12, e0172176. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.Y.; Yan, Y.Q.; Yin, B.Q.; Zhang, L.; Qin, W.X.; Niu, Y.R.; Tang, M.I.; Zhou, S.; Yan, X.H.; Ma, L. Dietary glycyl-glutamine supplementation ameliorates intestinal integrity, inflammatory response, and oxidative status in association with the gut microbiota in LPS-challenged piglets. Food Funct. 2021, 12, 3539–3551. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Liu, Y.L.; Pan, Y.Y.; Xu, J.X.; Ge, X.D.; Zheng, H.P.; Pang, J.; Liu, B.; Huang, Y. Coumarin-rich Grifola frondosa ethanol extract alleviate lipid metabolism disorders and modulates intestinal flora compositions of high-fat diet rats. J. Funct. Foods 2021, 85, 104649. [Google Scholar]
- D’Oplinter, A.D.W.; Marialetizia, R.; Matthias, V.H.; Delzenne, N.M.; Cani, P.D.; Everard, A. Gut microbes participate in food preference alterations during obesity. Gut Microbes 2021, 13, 1959242. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Z.; Li, R.; Jiang, Y.M.; Zhao, F.; Yu, Z.S.; Wang, Y.Q.; Dong, Z.X.; Liu, P.; Li, X.K. Bifidobacterium breve ATCC15700 pretreatment prevents alcoholic liver disease through modulating gut microbiota in mice exposed to chronic alcohol intake. J. Funct. Foods 2020, 72, 104045. [Google Scholar] [CrossRef]
- Giuffrè, M.; Campigotto, M.; Campisciano, G.; Comar, M.; Crocè, L.S. A story of liver and gut microbes: How does the intestinal flora affect liver disease? A review of the literature. Am. J. Physiol.-Gastrointest. Liver Physiol. 2020, 318, G889–G906. [Google Scholar] [CrossRef]
- Guo, W.L.; Guo, J.B.; Liu, B.Y.; Lu, J.Q.; Chen, M.; Liu, B.; Bai, W.D.; Rao, P.F.; Ni, L.; Lv, X.C. Ganoderic acid A from Ganoderma lucidum ameliorates lipid metabolism and alters gut microbiota composition in hyperlipidemic mice fed a high-fat diet. Food Funct. 2020, 11, 6818–6833. [Google Scholar] [CrossRef]
- Guo, W.L.; Deng, J.C.; Pan, Y.Y.; Xu, J.X.; Hong, J.L.; Shi, F.F.; Liu, G.L.; Qian, M.; Bai, W.D.; Zhang, W.; et al. Hypoglycemic and hypolipidemic activities of grifola frondosa polysaccharides and their relationships with the modulation of intestinal microflora in diabetic mice induced by high-fat diet and streptozotocin. Int. J. Biol. Macromol. 2020, 153, 1231–1240. [Google Scholar] [CrossRef]
- Na, J.; Choi, S.A.; Khan, A.; Huh, J.Y.; Piao, L.; Hwang, I.; Ha, H.; Park, Y.H. Integrative omics reveals metabolic and transcriptomic alteration of nonalcoholic fatty liver disease in catalase knockout mice. Biomol. Ther. 2019, 27, 134. [Google Scholar] [CrossRef]
- Mcilroy, G.D.; Tammireddy, S.R.; Maskrey, B.H.; Grant, L.; Doherty, M.K.; Watson, D.G.; Delibegović, M.; Whitfield, P.D.; Mody, N. Fenretinide mediated retinoic acid receptor signalling and inhibition of ceramide biosynthesis regulates adipogenesis, lipid accumulation, mitochondrial function and nutrient stress signalling in adipocytes and adipose tissue. Biochem. Pharm. 2016, 100, 86–97. [Google Scholar] [CrossRef]
- Nagahora, N.; Yamada, H.; Kikuchi, S.; Hakozaki, M.; Yano, A. Nrf2 activation by 5-lipoxygenase metabolites in human umbilical vascular endothelial cells. Nutrients 2017, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Nityasewaka, P.; Indarto, D.; Suselo, Y.H. Oxonantenine Derives from Annona reticulata is a Potential Candidate of DPP-4 Inhibitor for Diabetes Therapy. Nexus Biomedika 2016, 5, 1–8. [Google Scholar]
- Gopalan-Kriczky, P.; Jensen, D.E.; Lotlikar, P.D. Conjugation of microsome generated and synthetic aflatoxin B1-8, 9 epoxide and styrene oxide to glutathione by purified glutathione S-transferases from hamster and mouse livers. Cancer Lett. 1994, 86, 83–90. [Google Scholar] [CrossRef]
- Huang, Z.R.; Chen, M.; Guo, W.L.; Li, T.T.; Liu, B.; Bai, W.D.; Ai, L.Z.; Rao, P.F.; Rao, L.; Lv, X.C. Monascus purpureus-fermented common buckwheat protects against dyslipidemia and non-alcoholic fatty liver disease through the regulation of liver metabolome and intestinal microbiome. Food Res. Int. 2020, 136, 109511. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Li, Y.; Yue, X.; Cao, Y.; Xiang, F.; Mao, D.; Xiong, Z. Metabonomics of mice intestine in Codonopsis foetens induced apoptosis of intestine cancer cells. Saudi. J. Biol. Sci. 2019, 26, 1003–1010. [Google Scholar] [CrossRef]
- Wang, X.H.; En, H.S.R.; Chen, H.M.; Chang, C.; Mei, L.; Wang, X.L. Study on the mechanism of health protection of Mongolian medicine Polygonatum sibiricum based on metabonomics. Lishizhen Med. Mater. Med. Res. 2016, 27, 2345–2348. [Google Scholar]
- Liu, J.; Li, X.; Wu, J.; Chen, Y.; Li, H.; Tian, Z. Non-diabetic ketoacidosis: A case of alcoholic ketoacidosis accompanied by hyperglycemia. Am. J. Emerg. Med. 2022, 52, 270.e5–270.e8. [Google Scholar] [CrossRef]
- Wang, X.L.; Tan, Z.Q.; Chen, S.D.; Gui, L.; Li, X.C.; Ke, D.; Hou, L.; Leung, J.Y.S. Norethindrone causes cellular and hepatic injury in zebrafish by compromising the metabolic processes associated with antioxidant defence: Insights from metabolomics. Chemosphere 2021, 275, 130049. [Google Scholar] [CrossRef]
- Zuo, H.; Su, X.H.; Jin, Y.X.; Zhang, C.; Wang, L.L.; Yang, L. Transthyretin regulated by Linc00657/MiR-205-5p promoted cholesterol metabolism by inducing SREBP2-HMGCR and inhibiting LXRα-CYP7A. Arch. Med. Res. 2020, 51, 317–326. [Google Scholar] [CrossRef]
- Huang, H.Q.; Jiang, X.J.; Xiao, Z.L.; Yu, L.; Pham, Q.; Sun, J.H.; Chen, P.; Yokoyama, W.; Yu, L.L.; Luo, Y.S.; et al. Red cabbage microgreens lower circulating low-density lipoprotein (LDL), liver cholesterol, and inflammatory cytokines in mice fed a high-fat diet. J. Agric. Food Chem. 2016, 64, 9161–9171. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Chen, L.; Sun, B.; Liu, D.M.; He, Y.C.; Qi, L.S.; Li, G.T.; Han, Z.Q.; Zhan, L.L.; Zhang, S.; et al. LDLR inhibition promotes hepatocellular carcinoma proliferation and metastasis by elevating intracellular cholesterol synthesis through the MEK/ERK signaling pathway. Mol. Met. 2021, 51, 101230. [Google Scholar] [CrossRef]
- Shimano, H. SREBPs: Novel aspects of SREBPs in the regulation of lipid synthesis. FEBS J. 2009, 276, 615. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; French, S.W.; Li, J.; Bardag Gorce, F. Proteasome inhibitor treatment reduced fatty acid, triacylglycerol and cholesterol synthesis. Exp. Mol. Pathol. 2012, 93, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Nido, S.A.; Shituleni, S.A.; Mengistu, B.M.; Liu, Y.; Khan, A.Z.; Gan, F.; Kumbhar, S.; Huang, K. Effects of selenium-enriched probiotics on lipid metabolism, anti-oxidative status, histopathological lesions, and related gene expression in mice fed a high-fat diet. Biol. Trace Elem. Res. 2016, 171, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Zamani Garmsiri, F.; Hashemnia, S.M.R.; Shabani, M.; Bagherieh, M.; Emamgholipour, S.; Meshkani, R. Combination of metformin and genistein alleviates non-alcoholic fatty liver disease in high-fat diet-fed mice. J. Nutr. Biochem. 2021, 87, 108505. [Google Scholar] [CrossRef]
- Jang, Y.J.; Park, B.; Lee, H.W.; Park, H.J.; Koo, H.J.; Kim, B.O.; HwaSohn, E.; Um, S.H.; Pyo, S. Sinigrin attenuates the progression of atherosclerosis in ApoE-/- mice fed a high-cholesterol diet potentially by inhibiting VCAM-1 expression. Chem.-Biol. Interact. 2017, 272, 28–36. [Google Scholar] [CrossRef]
- Faradonbeh, F.A.; Sa, I.I.; Lastuvkova, H.; Cermanova, J.; Hroch, M.; Faistova, H.; Mokry, J.; Nova, Z.; Uher, M.; Nachtigal, P.; et al. Metformin impairs bile acid homeostasis in ethinylestradiol-induced cholestasis in mice. Chem.-Biol. Interact. 2021, 345, 109525. [Google Scholar] [CrossRef]
- Jagannathan, L.; Swaminathan, K.; Kumar, S.M.; Kumar, G.R.; Dey, A. Bio-informatics based analysis of genes implicated in alcohol mediated liver injury. Gene 2012, 494, 130–139. [Google Scholar] [CrossRef]
- Nagaki, M.; Tanaka, M.; Sugiyama, A.; Ohnishi, H.; Moriwaki, H. Interleukin-10 inhibits hepatic injury and tumor necrosis factor-α and interferon-γ mRNA expression induced by staphylococcal enterotoxin B or lipopolysaccharide in galactosamine-sensitized mice. J. Hepatol. 1999, 31, 815–824. [Google Scholar] [CrossRef]
- Ding, L.L.; Li, J.M.; Song, B.L.; Xiao, X.; Zhang, B.F.; Qi, M.; Huang, W.; Yang, L.; Wang, Z. Curcumin rescues high fat diet-induced obesity and insulin sensitivity in mice through regulating SREBP pathway. Toxicol. Appl. Pharm. 2016, 304, 99–109. [Google Scholar] [CrossRef]
- Siddiq, A.; Martin, A. Crocetin exerts hypocholesterolemic effect by inducing LDLR and inhibiting PCSK9 and Sortilin in HepG2 cells. Nutr. Res. 2022, 98, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.Y.; Yu, L.; Zhao, X.L.; Wang, L.T.; Zhao, C.J.; Huang, H.; Zhu, H.L.; Efferth, T.; Gu, C.B.; Fu, Y.J. Seed oil of Rosa roxburghii Tratt against non-alcoholic fatty liver disease in vivo and in vitro through PPARα/PGC-1α-mediated mitochondrial oxidative metabolism. Phytomedicine 2022, 98, 153919. [Google Scholar] [CrossRef] [PubMed]
| Gene | Forward Primer (5′−3′) | Reverse Primer (5′−3′) |
|---|---|---|
| LDLr | ATGCTGGAGATAGAGTGGAGTT | CCGCCAAGATCAAAKAG |
| CYP7A1 | CCTTGGGACGTTTTCCTGCT | GCGCTCTTTGATTTAGAKG |
| SREBP-1c | GCCGGCGCCATGGACGAGCTGG | CAGAKGGCTTCCAGAGAGGAG |
| FASn | CTGCCACAACTCTGAGGACA | TTCGTACCTCCTTGGCAAAC |
| CD36 | ACTTGGGATTGGAGTGGTGATGT | GGATACCTGCAGTTTGAGCCA |
| ACOX1 | GCCTGCTGTGTGGGTATGTCATT | GTCATGGGCGGGTGCAT |
| CPT-1 | TCCATGCATACCAAAGTGGA | TGGTAGGAGAGCAGCACCTT |
| ApoE | AKCCGCTTCTGGGATTACCT | TCAGTGCCGTCAGTTCTTGTG |
| FATP5 | AKTCGGGAGGCAGAAKCT | AGCGGGTCATACAAGTGAGC |
| ADH2 | AACGGTGAAKGTTCCCAAAA | ACGACCCCCAGCCTAATACA |
| ALDH2 | ATCCTCGGCTACATCAAATCG | GTCTTTTACGTCCCCGAACAC |
| Nrf-2 | CCGGGAKCAAKCAGAKA | ACGTTGTCCCCATTTTTGCG |
| NQO1 | AKCCCAGTCTATGCCCCAC | GGCGTGCAAGGGATGATTTC |
| HO-1 | AACAAGCAAKCCCAGTCTATGC | AGGTAGCGGGTATATGCGTGGGCC |
| GSH-Px | GGGACCCTGAGACTTAGAGC | AATCCGTACTAGCGCTCACA |
| SOD1 | TTGGCCGTACAA GGTGG | CGCAATCCCAATCACTCCAC |
| CAT | TCACCCACGATATCACCAGA | AGCTGAGCCTGACTCTCCAG |
| GSH | ACCAGGAAKTGGCAAAK | TCCTCCTTTCTTCCCACCG |
| IL-6 | TTCTCTGGGAAATCGTGGAAA | TGCAAGTGCATCATCGTTGT |
| IL-10 | CAG AGCCACATGCTCCTAGA | GCTTGGCAACCCAAGTAA CC |
| BSEP | TCTGACTCAGTGATTCTTCGCA | CCCATAAACATCAGCCAGTTGT |
| Mouse 18S | AGTCCCTGCCCTTTGTACACA | CGATCCCAGGGCCTCACTA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Zhou, K.; Yang, Z.; Li, J.; Chen, G.; Wu, Q.; Lv, X.; Hu, W.; Rao, P.; Ai, L.; et al. Monascuspiloin from Monascus-Fermented Red Mold Rice Alleviates Alcoholic Liver Injury and Modulates Intestinal Microbiota. Foods 2022, 11, 3048. https://doi.org/10.3390/foods11193048
Wu L, Zhou K, Yang Z, Li J, Chen G, Wu Q, Lv X, Hu W, Rao P, Ai L, et al. Monascuspiloin from Monascus-Fermented Red Mold Rice Alleviates Alcoholic Liver Injury and Modulates Intestinal Microbiota. Foods. 2022; 11(19):3048. https://doi.org/10.3390/foods11193048
Chicago/Turabian StyleWu, Li, Kangxi Zhou, Ziyi Yang, Jiayi Li, Guimei Chen, Qi Wu, Xucong Lv, Wenlin Hu, Pingfan Rao, Lianzhong Ai, and et al. 2022. "Monascuspiloin from Monascus-Fermented Red Mold Rice Alleviates Alcoholic Liver Injury and Modulates Intestinal Microbiota" Foods 11, no. 19: 3048. https://doi.org/10.3390/foods11193048
APA StyleWu, L., Zhou, K., Yang, Z., Li, J., Chen, G., Wu, Q., Lv, X., Hu, W., Rao, P., Ai, L., & Ni, L. (2022). Monascuspiloin from Monascus-Fermented Red Mold Rice Alleviates Alcoholic Liver Injury and Modulates Intestinal Microbiota. Foods, 11(19), 3048. https://doi.org/10.3390/foods11193048







