Method Validation and Assessment of Hazardous Substances and Quality Control Characteristics in Traditional Fruit Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples, Chemicals, and Reagents
2.2. Analysis of pH Value and Titratable Acidity
2.3. Analysis of Cyanide
2.4. Analysis of Total Sulfur Dioxide and Free Sulfur Dioxide
2.5. Analysis of Acetaldehyde
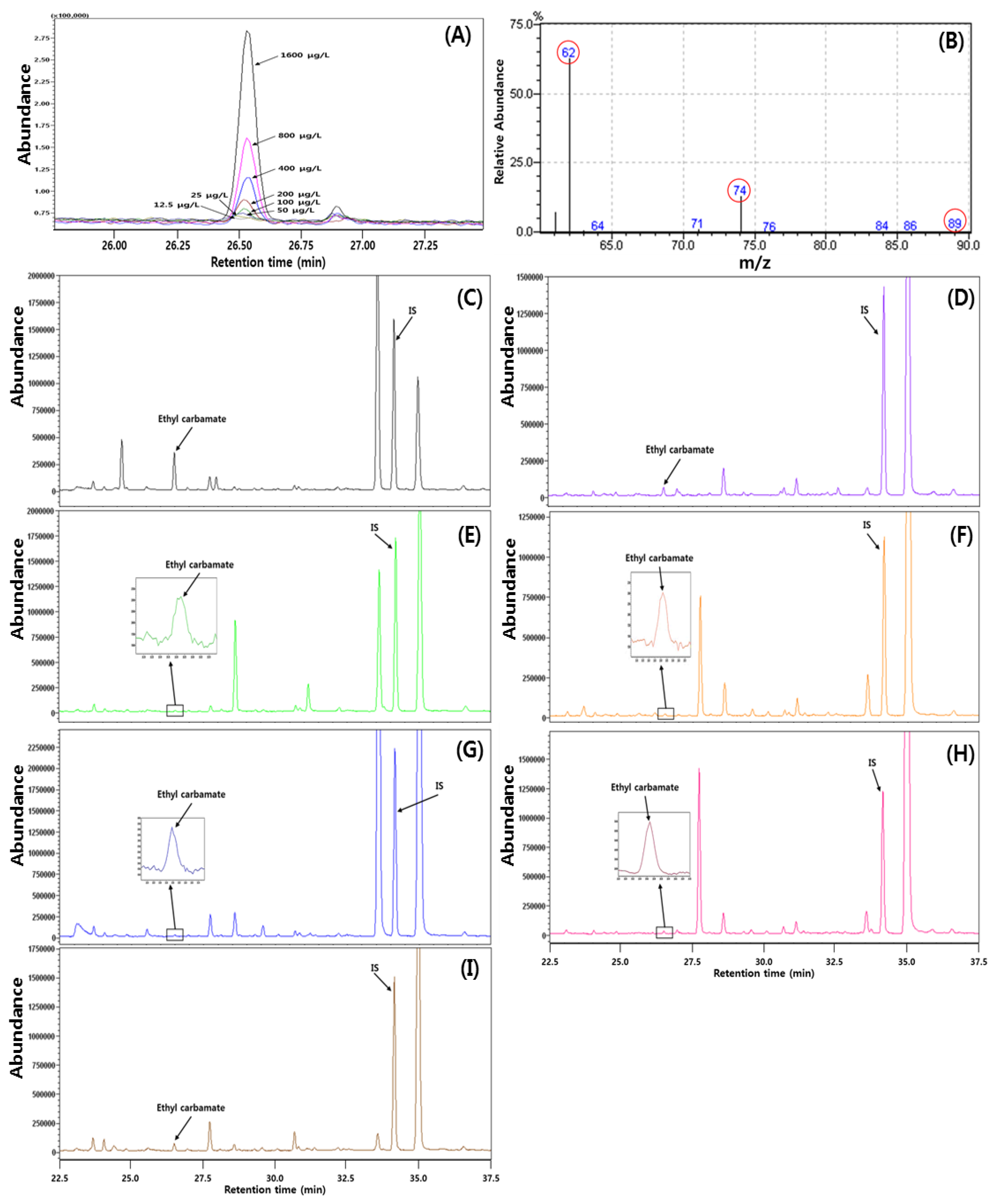
2.6. Analysis of Ethyl Carbamate
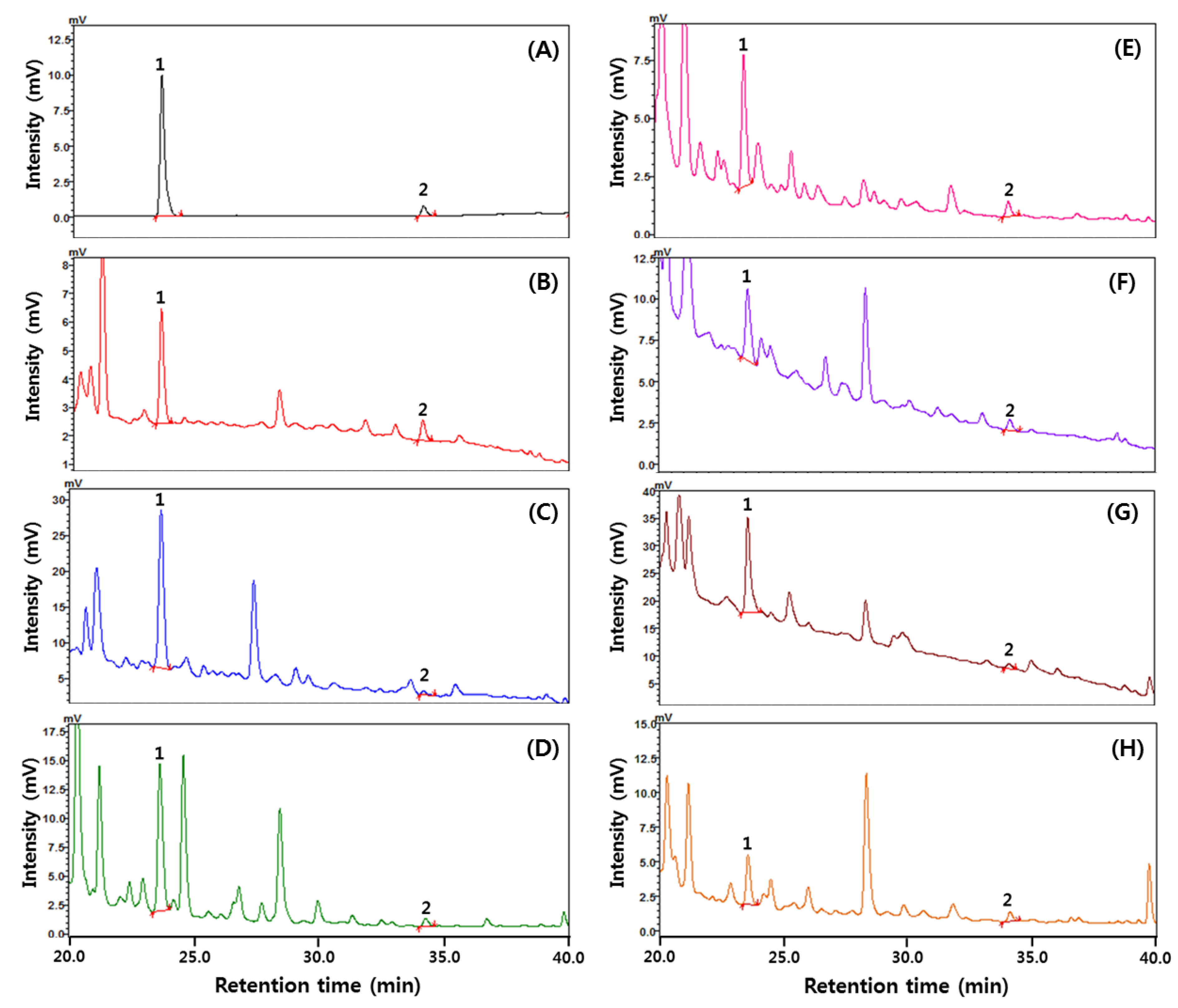
2.7. Analysis of Diacetyl
2.8. Method Validation
2.8.1. Specificity and Linearity
2.8.2. Accuracy and Precision
2.8.3. Sensitivity
2.9. Statistical Analysis
3. Results and Discussion
3.1. pH Level and Titratable Acidity in Fruit Wines
3.2. The Content of Cyanide in Fruit Wines
3.3. The Content of Total and Free Sulfur Dioxide in Fruit Wines
3.4. The Content of Acetaldehyde in Fruit Wines
3.5. The Content of Diacetyl in Fruit Wines
3.6. The Content of Ethyl Carbamate in Fruit Wines
3.7. Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korea Agro-Fisheries & Food Trade Corporation, Food Information Statistics System. Alcoholic Beverages Market Trend Report. 2021. Available online: https://www.atfis.or.kr/home/pdf/view.do?path=/board/202202/807f8c10-9090-4122-9126-e2d599bcaee3.pdf (accessed on 9 September 2022).
- Ministry of Food and Drug Safety. Food Code. Available online: https://www.foodsafetykorea.go.kr/foodcode/01_01.jsp (accessed on 9 September 2022).
- Wei, J.; Zhang, Y.; Yuan, Y.; Dai, L.; Yue, T. Characteristic fruit wine production via reciprocal selection of juice and non-Saccharomyces species. Food Microbiol. 2019, 79, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, M.; Ren, T.; Wang, J.; Niu, C.; Zheng, F.; Li, Q. Effect of Saccharomyces Cerevisiae and non-Saccharomyces strains on alcoholic fermentation behavior and aroma profile of yellow-fleshed peach wine. LWT 2022, 155, 112993. [Google Scholar] [CrossRef]
- Ryu, D.Y.; Koh, E.M. Determination of ethyl carbamate in commercial and homemade Maesilju. J. East Asian Soc. Dietary Life 2015, 25, 309–315. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, J.C.; Hwang, K.T.; Kim, D.H.; Jung, C.M. Quality characteristics of black raspberry wine fermented with different yeasts. J. Korean Soc. Food Sci. Nutr. 2013, 42, 784–791. [Google Scholar] [CrossRef][Green Version]
- Korean Statistical Information Service. List of Fruit Proceeding. Available online: https://kosis.kr/statHtml/statHtml.do?orgId=114&tblId=DT_114_2012_S0077&conn_path=I2 (accessed on 9 September 2022).
- EUR-Lex. 2019/787 of the European Parliament and of the Council of 17 April 2019 on the Definition, Description, Presentation and Labelling of Spirit Drinks, the Use of the Names of Spirit Drinks in the Presentation and Labelling of Other Foodstuffs, the Protection of Geographical Indications for Spirit Drinks, the Use of Ethyl Alcohol and Distillates of Agricultural Origin in Alcoholic Beverages, and Repealing Regulation (EC) No 110/2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R0787&from=pl (accessed on 9 September 2022).
- Parry, C.D.; Patra, J.; Rehm, J. Alcohol consumption and non-Communicable diseases: Epidemiology and policy implications. Addiction 2011, 106, 1718–1724. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Agents Classified by the IARC Monographs. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 9 September 2022).
- World Health Organization. Global Strategy to Reduce the Harmful Use of Alcohol; World Health Organization: Geneva, Switzerland, 2010; pp. 1–44. ISBN 978-92-4-159993-1. [Google Scholar]
- Chung, H.; Yoon, M.K.; Kim, M.H.; Park, S.K.; Lee, J.G.; Kim, Y.S. Volatile hazardous compounds in alcoholic beverages. J. Appl. Biol. Chem. 2012, 55, 141–148. [Google Scholar] [CrossRef][Green Version]
- Nyirenda, K.K. Toxicity potential of cyanogenic glycosides in edible plants. In Medical Toxicology; Erkekoglu, P., Ogawa, T., Eds.; IntechOpen: London, UK, 2021; pp. 1–19. [Google Scholar]
- GB 5009.36–2016; National Standard of the People’s Republic of China: National Food Safety Standard-Determination of Cyanide in Foods. National Health and Family Planning Commission of the People’s Republic of China (NHFPC): Beijing, China, 2016.
- Weber, J.V.; Sharypov, V.I. Ethyl carbamate in foods and beverages: A Review. Environ. Chem. Lett. 2008, 7, 233–247. [Google Scholar] [CrossRef]
- Jiao, Z.; Dong, Y.; Chen, Q. Ethyl carbamate in fermented beverages: Presence, analytical chemistry, formation mechanism, and mitigation proposals. Compr. Rev. Food Sci. Food Saf. 2014, 13, 611–626. [Google Scholar] [CrossRef]
- EUR-Lex. Commission Recommendation (EU) 2016/22 of 7 January 2016 on the Prevention and Reduction of Ethyl Carbamate Contamination in Stone Fruit Spirits and Stone Fruit Marc Spirits, Repealing Recommendation 2010/133/EU (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32016H0022 (accessed on 17 September 2022).
- Ministry of Food and Drug Safety. Ethyl Carbamate Reduction Manual for Alcoholic Beverages; MFDS: Cheongju-si, Korea, 2011.
- Yoon, H.S.; Park, J.M.; Park, H.J.; Jeong, C.W.; Choi, W.I.; Park, J.H.; Kim, S.D. Quality characteristics of Korean domestic commercial white wines. Korean J. Food Nutr. 2016, 29, 538–546. [Google Scholar] [CrossRef]
- Shin, K.S.; Lee, J.H. Acetaldehyde contents and quality characteristics of commercial alcoholic beverages. Food Sci. Biotechnol. 2019, 28, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Wee, H.J.; Lee, S.B.; Choi, K.T.; Ham, J.Y.; Yeo, S.H.; Park, H.D. Characteristics of freeze-concentrated apple cider fermented using mixed culture of non-Saccharomyces and Saccharomyces cerevisiae fermivin. Korean J. Food Preserv. 2018, 25, 730–741. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersberg, MD, USA, 2010. [Google Scholar]
- Qin, Y.; Duan, B.; Shin, J.A.; So, H.J.; Hong, E.S.; Jeong, H.G.; Lee, J.H.; Lee, K.T. Effect of fermentation on cyanide and ethyl carbamate contents in cassava flour and evaluation of their mass balance during lab-scale continuous distillation. Foods 2021, 10, 1089. [Google Scholar] [CrossRef] [PubMed]
- Megazyme Ltd. Total and Free Sulfite Assay Kit (Liquid Ready) Assay Protocol. Available online: https://www.megazyme.com/total-and-free-sulfite-assay-kit (accessed on 17 September 2022).
- Megazyme Ltd. Total Sulfite Assay Kit (Liquid Ready) Assay Protocol. Available online: https://www.megazyme.com/total-sulfite-assay-kit (accessed on 17 September 2022).
- Megazyme Ltd. Acetaldehyde Assay Kit Assay Protocol. Available online: https://www.megazyme.com/acetaldehyde-assay-kit (accessed on 17 September 2022).
- The International Organization of Vine and Wine. Method for the Determination of A-Dicarbonyl Compounds of Wine by HPLC after Derivatization by 1,2-Diaminobenzene (OIV-Oeno 386A-2010); OIV: Paris, France, 2010. [Google Scholar]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Harmonised Guideline. Validation of analytical procedures Q2 (R2). In Proceedings of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland, 24 March 2022; pp. 1–39. [Google Scholar]
- National Institute for Occupational Safety & Health. Guidelines for Air Sampling and Analytical Method Development and Evaluation; NIOSH: Atlanta, GA, USA, 1995.
- Shim, K.H.; Sung, N.K.; Choi, J.S.; Kang, K.S. Changes in major components of Japanese Apricot during ripening. J. Korean Soc. Food Nutr. 1989, 18, 101–108. [Google Scholar]
- Lee, S.J.; Ahn, B.M. Changes in physicochemical characteristics of black raspberry wines from different regions during fermentation. Korean J. Food Sci. Technol. 2009, 41, 662–667. [Google Scholar]
- Lee, H.L.; Youn, K.S. Quality characteristics of cold-air and infrared-dried peaches. Korean J. Food Preserv. 2012, 19, 485–491. [Google Scholar] [CrossRef]
- Bang, H.Y.; Cho, S.D.; Kim, D.M.; Kim, G.H. Comparison of antioxidative activities of fuji apples parts according to production region. J. Korean Soc. Food Sci. Nutr. 2015, 44, 557–563. [Google Scholar] [CrossRef]
- Song, T.H.; Kim, C.J. Effect of cellulose coatings on postharvest storage qualities of Plums (Prunus salicina L.). J. KOPAST 1998, 4, 45–57. [Google Scholar]
- Kim, K.H.; Han, G.D. Wine Making using Campbell early grape with different yeasts. Korean J. Microbiol. Biotechnol. 2011, 39, 43–48. [Google Scholar]
- Davis, C.R.; Wibowo, D.J.; Lee, T.H.; Fleet, G.H. Growth and metabolism of lactic acid bacteria during and after malolactic fermentation of wines at different pH. AEM 1986, 51, 539–545. [Google Scholar] [CrossRef]
- Fischer, U.; Noble, A.C. The Effect of ethanol, catechin concentration, and pH on sourness and bitterness of wine. Am. J. Enol. Vitic. 1994, 45, 6–10. [Google Scholar]
- Park, H.S. Characteristics of peach wine with different commercial yeast strains. J. East Asian Soc. Dietary Life 2010, 20, 531–535. [Google Scholar]
- Ye, M.; Yue, T.; Yuan, Y.; Li, Z. Application of FT-NIR spectroscopy to apple wine for rapid simultaneous determination of soluble solids content, pH, total acidity, and total ester content. Food Bioprocess Technol. 2014, 7, 3055–3062. [Google Scholar] [CrossRef]
- Miljić, U.; Puškaš, V.; Velićanski, A.; Mašković, P.; Cvetković, D.; Vujić, J. Chemical composition andin vitroantimicrobial and cytotoxic activities of plum (Prunus domestica L.) wine. J. Inst. Brew. 2016, 122, 342–349. [Google Scholar] [CrossRef]
- Moon, J.S.; Kong, T.I.; Cheong, C. Characteristics of fermentation and aging by different adding of brewing water in Korean traditional Cheongju. J. Korea Acad.-Ind. Coop. Soc. 2015, 16, 5468–5475. [Google Scholar] [CrossRef]
- Park, W.M.; Park, H.G.; Rhee, S.J.; Kang, K.I.; Lee, C.H.; Yoon, K.E. Properties of wine from domestic grape, Vitis labrusca cultivar. Campbell’s Early, fermented by carbonic maceration vinification process. Korean J. Food Sci. Technol. 2004, 36, 773–778. [Google Scholar]
- Sung, N.H.; Jo, Y.J.; Park, C.W.; Woo, S.M.; Jang, S.Y.; Choi, I.W.; Jeong, Y.J. Characteristics of alcohol fermentation in citrus hydrolysated through narirutin addition. Korean J. Food Preserv. 2012, 19, 153–158. [Google Scholar] [CrossRef]
- Torija, M.J.; Beltran, G.; Novo, M.; Poblet, M.; Rozès, N.; Mas, A.; Guillamón, J.M. Effect of organic acids and nitrogen source on alcoholic fermentation: Study of their buffering capacity. J. Agric. Food Chem. 2003, 51, 916–922. [Google Scholar] [CrossRef]
- Theron, M.M.; Lues, J.F.R. Organic acids and meat preservation: A review. Food Rev. Int. 2007, 23, 141–158. [Google Scholar] [CrossRef]
- Egekeze, J.O.; Oehme, F.W. Cyanides and their toxicity: A literature review. Vet. Q. 1980, 2, 104–114. [Google Scholar] [CrossRef]
- Cho, H.J.; Do, B.K.; Shim, S.M.; Kwon, H.J.; Lee, D.H.; Nah, A.H.; Choi, Y.J.; Lee, S.Y. Determination of cyanogenic compounds in edible plants by ion chromatography. Toxicol. Res. 2013, 29, 143–147. [Google Scholar] [CrossRef]
- Cho, J.W.; Kim, B.Y.; Choi, S.J.; Jeong, J.B.; Kim, H.S. Changes in amygdalin contents of Maesil (Prunus mume) wine according to preparation steps and its characteristics. Korean J. Food Sci. Technol. 2019, 51, 42–47. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. Transition of phenolics and cyanogenic glycosides from apricot and cherry fruit kernels into liqueur. Food Chem. 2016, 203, 483–490. [Google Scholar] [CrossRef]
- Herzan, J.; Prokes, K.; Baron, M.; Kumsta, M.; Pavlousek, P.; Sochor, J. Study of carbonyl compounds in white wine production. Food Sci. Nutr. 2020, 8, 5850–5859. [Google Scholar] [CrossRef] [PubMed]
- Abramovic, H.; Kosmerl, T.; Poklar Ulrih, N.; Cigic, B. Contribution of SO2 to antioxidant potential of white wine. Food Chem. 2015, 174, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Kim, I.Y.; Ko, J.Y.; Yim, S.B.; Jeong, Y.H. Physicochemical characteristics and acceptability of commercial low-priced French wines. J. Korean Soc. Food Sci. Nutr. 2010, 39, 1666–1671. [Google Scholar] [CrossRef]
- Santos, M.C.; Nunes, C.; Cappelle, J.; Gonçalves, F.J.; Rodrigues, A.; Saraiva, J.A.; Coimbra, M.A. Effect of high pressure treatments on the physicochemical properties of a sulfur dioxide-free red wine. Food Chem. 2013, 141, 2558–2566. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Food and Drug Safety. Food Additives Code. Available online: https://www.foodsafetykorea.go.kr/foodcode/04_02_01.jsp?idx=2 (accessed on 9 September 2022).
- Cho, Y.S.; Kim, J.J.; Jeon, G.G.; Chung, M.S.; Joo, Y.S.; Lee, K.W. Total SO2 levels and risk assessment of wine and fruit wine consumed in South Korea. Food Control 2021, 127, 108124. [Google Scholar] [CrossRef]
- Miyake, T.; Shibamoto, T. Quantitative analysis of acetaldehyde in foods and beverages. J. Agric. Food Chem. 1993, 41, 1968–1970. [Google Scholar] [CrossRef]
- Osborne, J.P.; Mira de Orduna, R.; Pilone, G.J.; Liu, S.Q. Acetaldehyde metabolism by wine lactic and bacteria. FEMS Microbiol. Lett. 2000, 191, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Jackowetz, N.; Li, E.; Mira de Orduña, R. Sulfur dioxide content of wines: The role of wine making and carbonyl compounds. Res. Focus 2011, 7, 1–7. [Google Scholar]
- Sheridan, M.K.; Elias, R.J. Reaction of Acetaldehyde with wine flavonoids in the presence of sulfur dioxide. J. Agric. Food Chem. 2016, 64, 8615–8624. [Google Scholar] [CrossRef] [PubMed]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavor. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Henschke, P.A. The ‘buttery’ attribute of wine-diacetyl-desirability, spoilage and beyond. Int. J. Food Microbiol. 2004, 96, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Lee, K.T.; Shin, J.A. Analytical method validation and monitoring of diacetyl in liquors from Korean market. Food Sci. Biotechnol. 2017, 26, 893–899. [Google Scholar] [CrossRef]
- Ramos, R.M.; Pacheco, J.G.; Gonçalves, L.M.; Valente, I.M.; Rodrigues, J.A.; Barros, A.A. Determination of free and total diacetyl in wine by HPLC–UV using gas-diffusion microextraction and pre-column derivatization. Food Control 2012, 24, 220–224. [Google Scholar] [CrossRef]
- Kim, D.H.; Jang, H.S.; Choi, G.I.; Kim, H.J.; Kim, H.J.; Kim, H.L.; Kim, K.S. Determination of Residue Levels of Ethyl Carbamate in Alcoholic Beverages by Gas Chromatography/Tandem Mass Spectrometry (GC/MS/MS). J. Food Hyg. Saf. 2013, 28, 63–68. [Google Scholar] [CrossRef]
- Choi, B.Y.; Koh, E.M. Changes of ethyl carbamate and its precursors in maesil (Prunus mume) extract during one-year fermentation. Food Chem. 2016, 209, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y.; Koh, E.M. Effect of fruit thermal processing on ethyl carbamate content in maesil (Prunus mume) liqueur. Food Sci. Biotechnol. 2021, 30, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Korea Consumer Agency. A Report on the Monitoring Results of Ethyl Carbamate in Maesil Wine. Available online: https://www.kca.go.kr/smartconsumer/synapviewer.do?menukey=7301&fno=10006461&bid=00000146&did=1001105217 (accessed on 9 September 2022).
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R. Amygdalin content of seeds, kernels and food products commercially-available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists. AOAC Guidelines for Single Laboratory Validationo of Chemical Methods for Dietary Supplements and Botanicals; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2002; pp. 1–38. [Google Scholar]
- Ministry of Food and Drug Safety. Guidelines for Standard Procedures for Preparation of Method Validation for Food. Available online: https://www.mfds.go.kr/brd/m_1060/view.do?seq=12920&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=69 (accessed on 9 September 2022).
| Wine Type | No. | Alcohol Content (%) (1) | pH | Titratable Acidity (g/L) (2) | |||
|---|---|---|---|---|---|---|---|
| pH | Ave ± SD | Titratable Acidity | Ave ± SD | ||||
| Traditional Korean fruit wine | Maesil wine | 1 | 12.0 | 3.14 ± 0.01 A (3) | 3.12 ± 0.05 cd (4) | 4.68 ± 0.01 A | 5.35 ± 0.64 ab |
| 2 | 14.0 | 3.05 ± 0.01 B | 5.41 ± 0.08 B | ||||
| 3 | 14.0 | 3.15 ± 0.00 A | 5.95 ± 0.14 C | ||||
| Black raspberry wine | 1 | 12.0 | 3.05 ± 0.01 A | 3.01 ± 0.04 d | 7.90 ± 0.22 A | 6.68 ± 1.83 ab | |
| 2 | 15.0 | 3.03 ± 0.01 A | 4.58 ± 0.08 B | ||||
| 3 | 13.0 | 2.96 ± 0.01 B | 7.57 ± 0.20 A | ||||
| Peach wine | 1 | 12.0 | 3.81 ± 0.01 A | 3.76 ± 0.06 a | 6.74 ± 0.30 C | 7.98 ± 1.18 a | |
| 2 | 12.0 | 3.78 ± 0.01 B | 9.10 ± 0.04 A | ||||
| 3 | 7.0 | 3.70 ± 0.01 C | 8.09 ± 0.28 B | ||||
| Apple wine | 1 | 16.0 | 3.84 ± 0.01 A | 3.70 ± 0.12 ab | 2.01 ± 0.01 C | 4.70 ± 2.40 b | |
| 2 | 12.0 | 3.61 ± 0.01 C | 6.61 ± 0.04 A | ||||
| 3 | 12.0 | 3.65 ± 0.01 B | 5.49 ± 0.06 B | ||||
| Plum wine | 1 | 12.0 | 3.40 ± 0.01 | 3.61 ± 0.31 ab | 9.08 ± 0.12 * | 7.84 ± 1.76 a | |
| 2 | 12.0 | 3.83 ± 0.01 *(5) | 6.60 ± 0.08 | ||||
| Imported grape wine | Red grape wine | 1 | 14.0 | 3.50 ± 0.00 B | 3.52 ± 0.11 b | 6.76 ± 0.18 A | 6.12 ± 0.67 ab |
| 2 | 13.0 | 3.42 ± 0.00C | 6.17 ± 0.14 B | ||||
| 3 | 13.0 | 3.64 ± 0.01 A | 5.42 ± 0.14 C | ||||
| White grape wine | 1 | 14.0 | 3.25 ± 0.01 C | 3.31 ± 0.05 c | 6.65 ± 0.21A | 6.43 ± 0.21 ab | |
| 2 | 14.0 | 3.34 ± 0.01 A | 6.24 ± 0.15 B | ||||
| 3 | 13.0 | 3.33 ± 0.01 B | 6.42 ± 0.01 AB | ||||
| Wine Type | No. | Cyanide Content (mg/L) | ||
|---|---|---|---|---|
| Cyanide | Ave ± SD | |||
| Traditional Korean fruit wine | Maesil wine | 1 | 0.04 ± 0.01 B(1) | 0.06 ± 0.04 b(2) |
| 2 | 0.10 ± 0.02 A | |||
| 3 | 0.04 ± 0.01 B | |||
| Black raspberry wine | 1 | 0.03 ± 0.02 B | 0.08 ± 0.06 b | |
| 2 | 0.15 ± 0.04 A | |||
| 3 | 0.06 ± 0.02 B | |||
| Peach wine | 1 | 0.04 ± 0.02 A | 0.06 ± 0.03 b | |
| 2 | 0.09 ± 0.06 A | |||
| 3 | 0.07 ± 0.02 A | |||
| Apple wine | 1 | 0.12 ± 0.02 A | 0.13 ± 0.00 b | |
| 2 | 0.13 ± 0.04 A | |||
| 3 | 0.13 ± 0.02 A | |||
| Plum wine | 1 | 0.11 ± 0.05 NS(3) | 0.08 ± 0.04 b | |
| 2 | 0.05 ± 0.01 | |||
| Imported grape wine | Red grape wine | 1 | 0.54 ± 0.03 A | 0.35 ± 0.029 a |
| 2 | 0.50 ± 0.04 A | |||
| 3 | 0.02 ± 0.02 B | |||
| White grape wine | 1 | 0.03 ± 0.03 A | 0.02 ± 0.00 b | |
| 2 | 0.03 ± 0.01 A | |||
| 3 | 0.01 ± 0.02 A | |||
| Wine Type | No. | Sulfur Dioxide (mg/L) | Acetaldehyde (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TSO2 | Ave ± SD | FSO2 | Ave ± SD | Titration (1) | Ave ± SD | Enzymatic (2) | Ave ± SD | |||
| Traditional Korean fruit wine | Maesil wine | 1 | 7.54 ± 0.20 | 7.54 ± 0.00 a(5) | - (3) | - | 24.30 ± 0.48 B(4) | 26.01 ± 11.65 ab, NS(6) | 23.55 ± 0.70 A | 17.40 ± 9.61 a6 |
| 2 | - | - | 38.42 ± 1.21 A | 22.34 ± 0.98 A | ||||||
| 3 | - | - | 15.31 ± 0.56 C | 6.32 ± 0.16 B | ||||||
| Black raspberry wine | 1 | - | - | - | - | 14.26 ± 0.73 C | 22.44 ± 10.55 ab,NS | 5.03 ± 0.28 C | 8.36 ± 4.48 a | |
| 2 | - | - | 18.72 ± 1.10 B | 6.60 ± 0.16 B | ||||||
| 3 | - | - | 34.35 ± 1.00 A | 13.45 ± 0.32 A | ||||||
| Peach wine | 1 | 97.88 ± 0.26 B | 148.86 ± 138.08 a | 0.17 ± 0.00 C | 8.89 ± 13.20 a | 42.48 ± 0.73 A | 19.80 ± 19.78 ab,NS | 71.88 ± 2.49 B | 64.91 ± 47.34 a | |
| 2 | 39.94 ± 0.63 C | 2.42 ± 0.10 B | 10.81 ± 0.95 B | 14.47 ± 1.00 C | ||||||
| 3 | 302.75 ± 3.10 A | 24.08 ± 0.68 A | 6.12 ± 0.45 C | 108.38 ± 0.83 A | ||||||
| Apple wine | 1 | - | 146.67 ± 84.70 a | - | 17.50 ± 0.00 a | 16.97 ± 1.66 B | 15.06 ± 11.28 b,NS | 5.12 ± 0.16 C | 54.13 ± 45.34 a | |
| 2 | 144.95 ± 3.49 NS(7) | - | 25.26 ± 0.28 A | 94.56 ± 2.05 A | ||||||
| 3 | 148.38 ± 1.97 | 17.50 ± 0.39 | 2.94 ± 0.28 C | 62.71 ± 3.53 B | ||||||
| Plum wine | 1 | 14.18 ± 0.70 | 65.88 ± 73.12 a | 0.51 ± 0.00 | 0.56 ± 0.08 a | 19.04 ± 0.55 | 46.86 ± 39.34 a,NS | 5.86 ± 0.00 $ | 32.76 ± 38.04 a | |
| 2 | 117.58 ± 1.03 $(7) | 0.62 ± 0.10 NS | 74.68 ± 1.00 $ | 59.66 ± 2.52 | ||||||
| Imported grape wine | Red grape wine | 1 | 2.70 ± 0.16 B | 7.81 ± 4.44 a | 2.42 ± 0.19 B | 2.66 ± 0.57 a | 22.55 ± 0.73 A | 20.80 ± 1.93 ab,*(6) | 3.92 ± 0.56 C | 10.77 ± 7.36 a |
| 2 | 10.69 ± 0.62 A | 2.25 ± 0.26 B | 21.11 ± 0.73 B | 18.55 ± 0.98 A | ||||||
| 3 | 10.04 ± 0.84 A | 3.32 ± 0.26 A | 18.72 ± 0.28 C | 9.84 ± 0.42 B | ||||||
| White grape wine | 1 | 121.33 ± 0.75 A | 99.84 ± 20.77 a | 14.18 ± 0.61 A | 6.19 ± 6.92 a | 4.67 ± 0.51 C | 8.97 ± 4.16 b | 45.12 ± 0.58 B | 44.69 ± 7.32 a,* | |
| 2 | 98.30 ± 0.64 B | 2.03 ± 0.45 B | 9.26 ± 0.13 B | 51.79 ± 1.43 A | ||||||
| 3 | 79.88 ± 0.48 C | 2.36 ± 0.29 B | 12.98 ± 0.83 A | 37.16 ± 1.58 C | ||||||
| Wine Type | No. | Diacetyl (mg/L) | Ethyl Carbamate (μg/kg) | |||
|---|---|---|---|---|---|---|
| Diacetyl | Ave ± SD | Ethyl Carbamate | Ave ± SD | |||
| Traditional Korean fruit wine | Maesil wine | 1 | 0.72 ± 0.04 A(1) | 0.41 ± 0.27 a(2) | 109.37 ± 5.71 C | 259.69 ± 209.03 a |
| 2 | 0.27 ± 0.00 B | 498.39 ± 9.21 A | ||||
| 3 | 0.25 ± 0.00 B | 171.31 ± 6.22 B | ||||
| Black raspberry wine | 1 | 5.89 ± 0.27 A | 2.67 ± 2.79 a | 20.80 ± 3.07 B | 23.56 ± 3.64 b | |
| 2 | 0.87 ± 0.04 C | 27.69 ± 2.94 A | ||||
| 3 | 1.25 ± 0.12 B | 22.19 ± 0.26 B | ||||
| Peach wine | 1 | 2.68 ± 0.22 A | 1.41 ± 1.15 a | 7.45 ± 0.36 A | 6.06 ± 1.71 b | |
| 2 | 0.44 ± 0.03 C | 4.14 ± 0.65 B | ||||
| 3 | 1.11 ± 0.02 B | 6.58 ± 0.54 A | ||||
| Apple wine | 1 | 0.39 ± 0.03 C | 0.66 ± 0.34 a | 42.66 ± 3.73 A | 17.32 ± 21.99 b | |
| 2 | 1.03 ± 0.08 A | 6.06 ± 0.32 B | ||||
| 3 | 0.56 ± 0.01 B | 3.24 ± 0.31 B | ||||
| Plum wine | 1 | 2.19 ± 0.13 *(3) | 1.46 ± 1.03 a | 5.82 ± 0.35 * | 5.22 ± 0.85 b | |
| 2 | 0.73 ± 0.08 | 4.62 ± 0.09 | ||||
| Imported grape wine | Red grape wine | 1 | 1.89 ± 0.08 C | 2.95 ± 1.19 a | 8.64 ± 1.51 B | 11.35 ± 5.55 b |
| 2 | 2.72 ± 0.02 B | 17.73 ± 0.88 A | ||||
| 3 | 4.24 ± 0.44 A | 7.67 ± 0.69 B | ||||
| White grape wine | 1 | 0.25 ± 0.00 B | 0.31 ± 0.15 a | 21.34 ± 1.97 A | 16.24 ± 5.33 b | |
| 2 | 0.19 ± 0.02 C | 16.69 ± 1.24 B | ||||
| 3 | 0.48 ± 0.03 A | 10.70 ± 0.84 C | ||||
| Wine Type | Titration Method | Enzymatic Assay | |||
|---|---|---|---|---|---|
| Acetaldehyde | Recovery Rate (%) | RSD (%) (1) | Recovery Rate (%) | RSD (%) | |
| Maesil wine (2) (No.2) | +10 mg/L | 106.87 ± 6.94 | 1.41 | 100.92 ± 5.78 | 1.92 |
| +50 mg/L | 107.35 ± 2.29 | 1.24 | 103.52 ± 3.26 | 2.27 | |
| +100 mg/L | 108.01 ± 1.20 | 0.82 | 100.93 ± 1.95 | 1.61 | |
| Apple wine (No.1) | +10 mg/L | 108.30 ± 4.54 | 1.26 | 101.86 ± 4.84 | 3.49 |
| +50 mg/L | 110.66 ± 2.40 | 1.49 | 99.26 ± 3.85 | 3.61 | |
| +100 mg/L | 108.61 ± 3.35 | 2.50 | 105.28 ± 5.71 | 5.24 | |
| White grape wine (No.1) | +10 mg/L | 105.33 ± 4.54 | 2.99 | 100.93 ± 5.78 | 1.08 |
| +50 mg/L | 107.34 ± 2.40 | 2.06 | 102.96 ± 2.25 | 1.18 | |
| +100 mg/L | 108.77 ± 2.05 | 1.81 | 106.67 ± 1.63 | 1.08 | |
| Diacetyl | Ethyl Carbamate | |||||
|---|---|---|---|---|---|---|
| Instrument | HPLC-UV | GC-MS-SIM | ||||
| Sensitivity | LOD (1) | 0.0007 mg/L | 0.323 μg/L | |||
| LOQ (2) | 0.003 mg/L | 1.067 μg/L | ||||
| Linearity | Calibration curve | Y = 13.686x +1.3621 | Y = 0.0016x +0.004 | |||
| Range of linearity | 0.098–19.620 mg/L | 0.39–1600.00 μg/L | ||||
| Coefficient regression (R2) | 0.9983 | 0.9999 | ||||
| Diacetyl | Ethyl carbamate | |||||
| Recovery rate (%) | RSD (%) (3) | Recovery rate (%) | RSD (%) | |||
| Accuracy Precision | Apple wine | Apple wine | ||||
| +1 mg/L | 105.51 ± 4.21 | 2.02 | +10 μg/L | 115.40 ± 10.32 | 1.01 | |
| +5 mg/L | 101.15 ± 4.94 | 4.06 | +50 μg/L | 113.03 ± 2.28 | 0.70 | |
| +10 mg/L | 109.18 ± 4.65 | 3.89 | +100 μg/L | 104.49 ± 2.13 | 0.88 | |
| White grape wine | White grape wine | |||||
| +1 mg/L | 99.24 ± 2.63 | 2.10 | +10 μg/L | 107.83 ± 3.95 | 4.54 | |
| +5 mg/L | 109.75 ± 4.10 | 3.43 | +50 μg/L | 111.79 ± 9.50 | 6.13 | |
| +10 mg/L | 108.66 ± 3.95 | 3.55 | +100 μg/L | 106.33 ± 5.81 | 1.22 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, C.-W.; Chang, H.-J.; Lee, J.-H. Method Validation and Assessment of Hazardous Substances and Quality Control Characteristics in Traditional Fruit Wines. Foods 2022, 11, 3047. https://doi.org/10.3390/foods11193047
Baek C-W, Chang H-J, Lee J-H. Method Validation and Assessment of Hazardous Substances and Quality Control Characteristics in Traditional Fruit Wines. Foods. 2022; 11(19):3047. https://doi.org/10.3390/foods11193047
Chicago/Turabian StyleBaek, Chae-Wan, Hyeon-Jun Chang, and Jeung-Hee Lee. 2022. "Method Validation and Assessment of Hazardous Substances and Quality Control Characteristics in Traditional Fruit Wines" Foods 11, no. 19: 3047. https://doi.org/10.3390/foods11193047
APA StyleBaek, C.-W., Chang, H.-J., & Lee, J.-H. (2022). Method Validation and Assessment of Hazardous Substances and Quality Control Characteristics in Traditional Fruit Wines. Foods, 11(19), 3047. https://doi.org/10.3390/foods11193047





