Biostimulation of Plectranthus amboinicus (Lour.) Spreng. with Different Yeast Strains: Morphological Performance, Productivity, Phenotypic Plasticity, and Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location and Materials Source
2.2. Yeast Culturing
2.3. Experimental Design and Layout
2.4. Growth Parameters
2.5. Essential Oil Extraction
2.6. Biochemical Analysis
2.6.1. Total Carbohydrates, Ash, and Protein Content
2.6.2. Radical Scavenging (DPPH Assay)
2.6.3. Ferric Reducing Antioxidant Potential (FRAP)
2.6.4. Nutrients Estimation
2.7. Statistical Analysis
3. Results
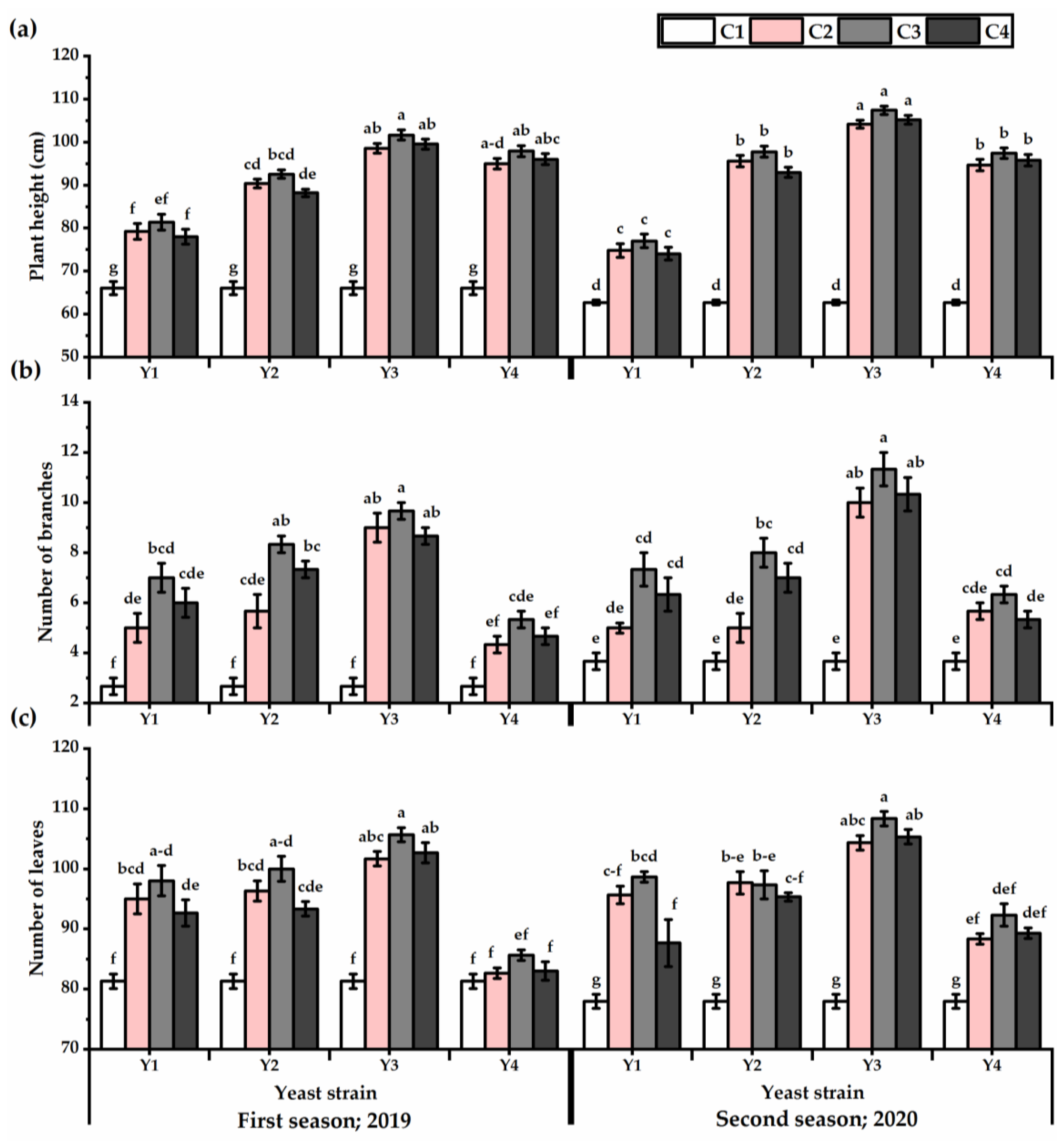
3.1. Vegetative Growth
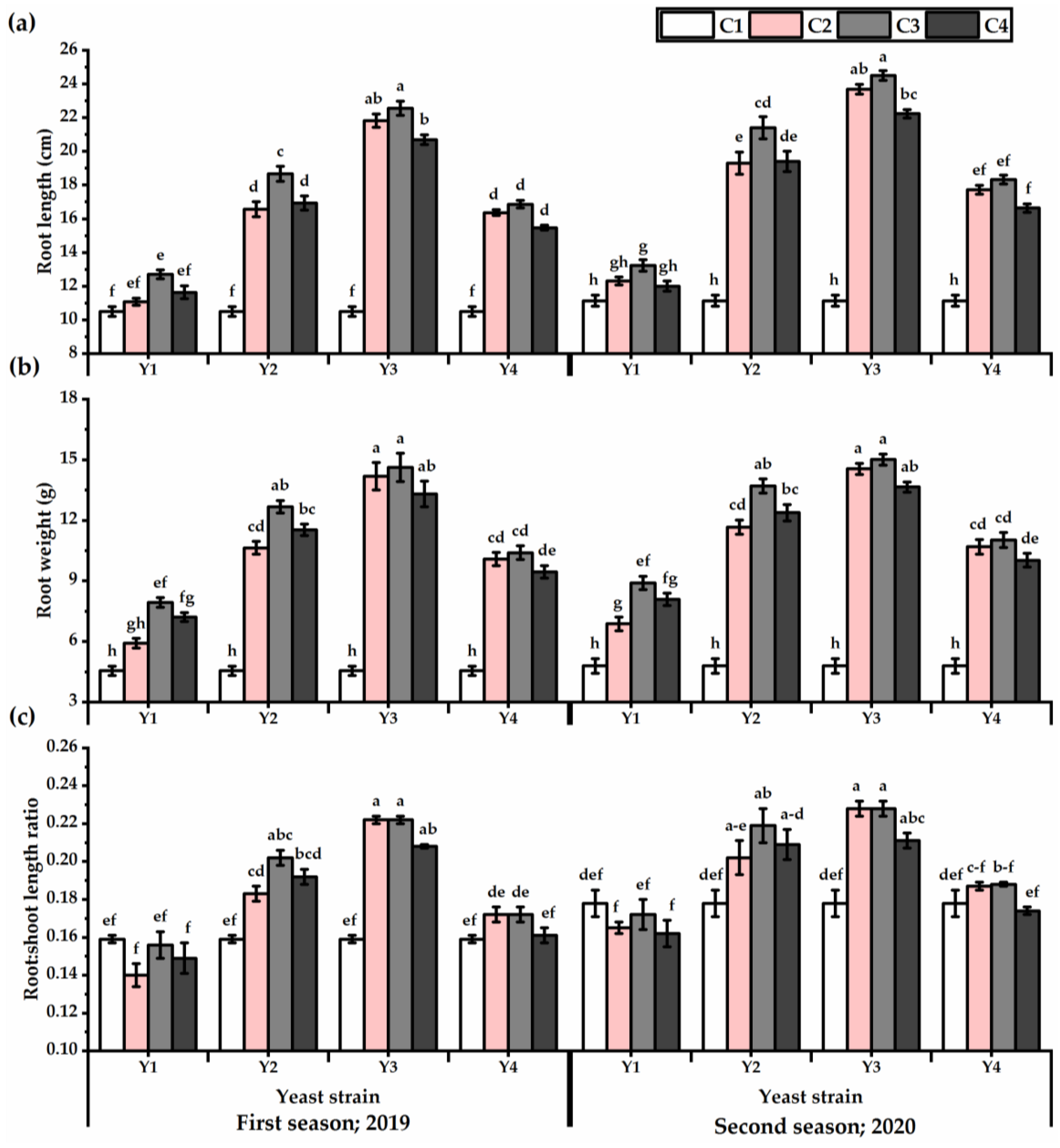
3.2. Root Traits
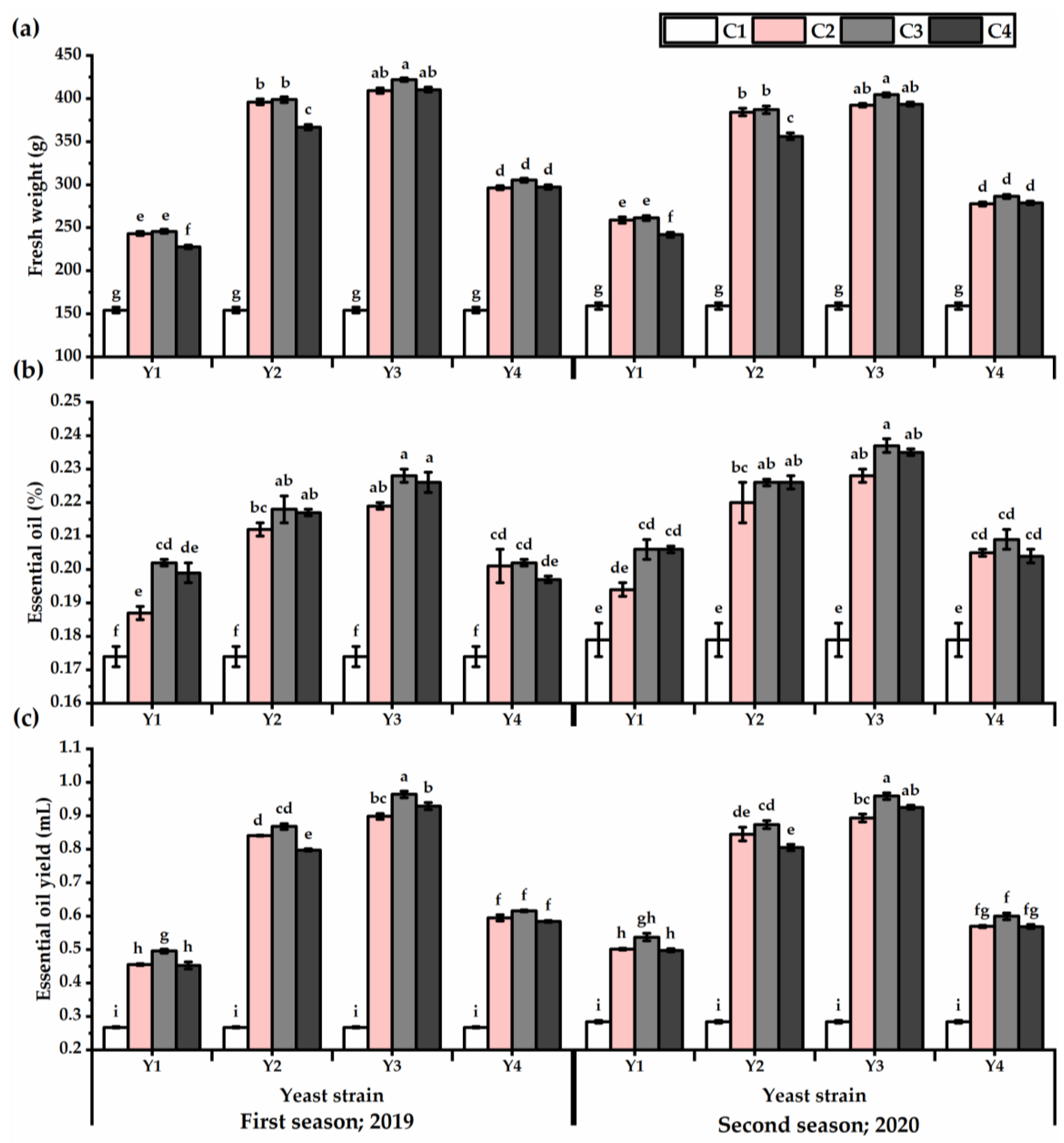
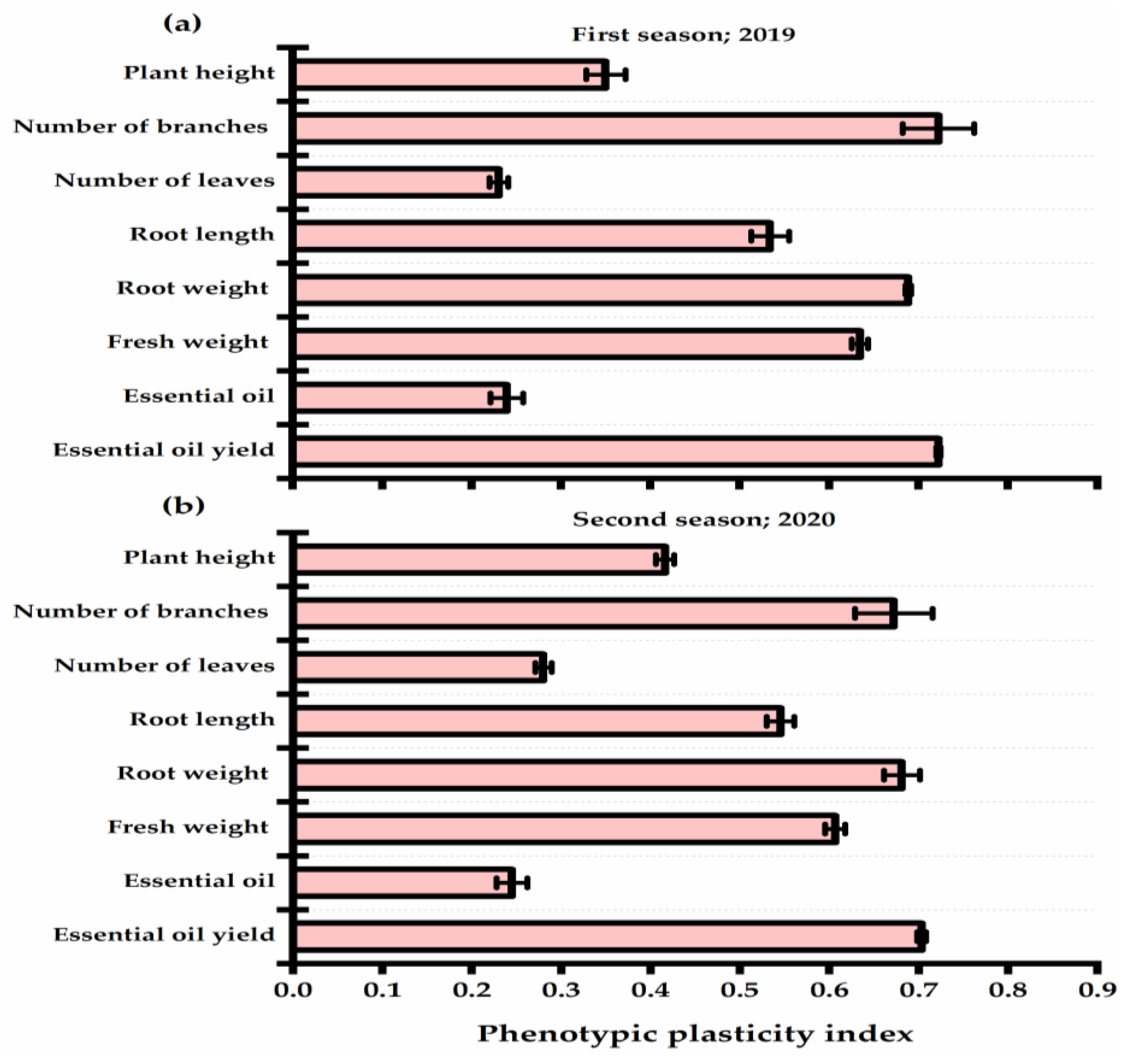
3.3. Yield Attributes and Phenotypic Plasticity
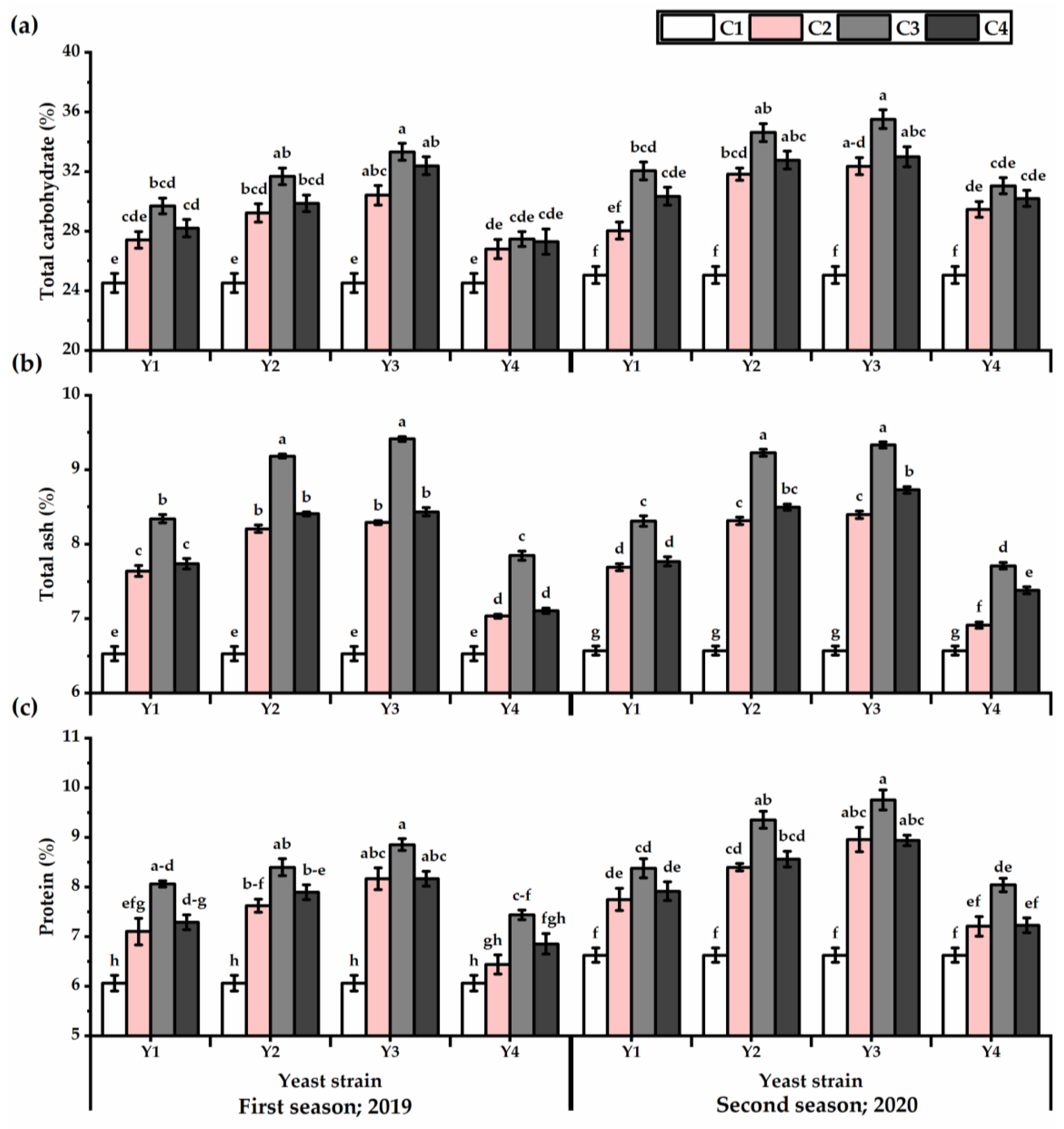
3.4. Carbohydrates, Ash, and Protein
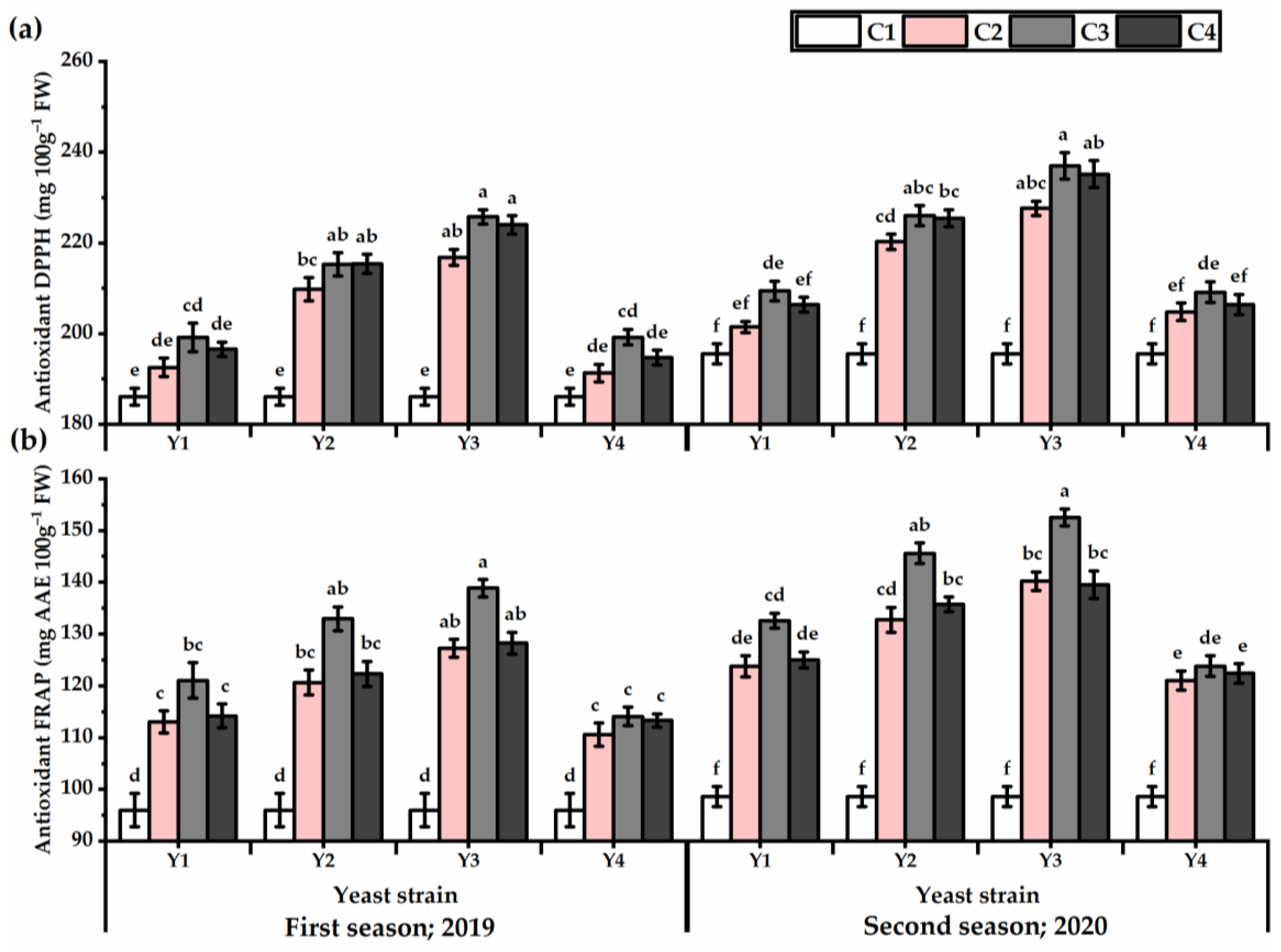
3.5. DPPH and FRAP
3.6. Nutrients Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, P.; Jaiswal, D.K.; Sinha, R.K. Climate change: Impact on agricultural production and sustainable mitigation. In Global Climate Change; Singh, S., Singh, P., Rangabhashiyam, S., Srivastava, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 151–174. [Google Scholar]
- El-Serafy, R.S.; El-Sheshtawy, A.A.; Abd El-Razek, U.A.; Abd El-Hakim, A.F.; Hasham, M.M.A.; Sami, R.; Khojah, E.; Al-Mushhin, A.A.M. Growth, yield, quality, and phytochemical behavior of three cultivars of quinoa in response to moringa and Azolla extracts under organic farming conditions. Agronomy 2021, 11, 2186. [Google Scholar] [CrossRef]
- El-Serafy, R.S.; El-Sheshtawy, A.A.; Dahab, A.A.; Al-Ashkar, I. Can yeast extract and chitosan-oligosaccharide improve fruit yield and modify the pharmaceutical active ingredients of organic fennel? Ind. Crops Prod. 2021, 173, 114130. [Google Scholar] [CrossRef]
- Rosa-Magri, M.M.; Avansini, S.H.; Lopes-Assad, M.L.; Tauk-Tornisielo, S.M.; Ceccato-Antonini, S.R. Release of potassium from rock powder by the yeast Torulaspora globosa. Braz. Arch. Biol. Technol. 2012, 55, 577–582. [Google Scholar] [CrossRef]
- El-Maraghy, S.S.; Tohamy, T.A.; Hussein, K.A. Expression of SidD gene and physiological characterization of the rhizosphere plant growth-promoting yeasts. Heliyon 2020, 6, e04384. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Fell, J.W. Yeast systematics and phylogeny—Implications of molecular identification methods for studies in ecology. In Biodiversity and Ecophysiology of Yeasts; Péter, G., Rosa, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 11–30. [Google Scholar]
- Botha, A. The importance and ecology of yeasts in soil. Soil Biol. Biochem. 2011, 43, 1–8. [Google Scholar] [CrossRef]
- Amprayn, K.O.; Rose, M.T.; Kecskés, M.; Pereg, L.; Nguyen, H.T.; Kennedy, I.R. Plant growth promoting characteristics of soil yeast (Candida tropicalis HY) and its effectiveness for promoting rice growth. Appl. Soil Ecol. 2012, 61, 295–299. [Google Scholar] [CrossRef]
- Silambarasan, S.; Vangnai, A.S. Plant-growth promoting Candida sp. AVGB4 with capability of 4-nitroaniline biodegradation under drought stress. Ecotoxicol. Environ. Saf. 2017, 139, 472–480. [Google Scholar] [CrossRef]
- Cloete, K.J.; Valentine, A.J.; Stander, M.A.; Blomerus, L.M.; Botha, A. Evidence of symbiosis between the soil yeast Cryptococcus laurentii and a Sclerophyllous medicinal shrub, Agathosma betulina (Berg.). Pillans. Microb. Ecol. 2009, 57, 624–632. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, K.P. Enriching vermicompost by nitrogen fixing and phosphate solubilizing bacteria. Bioresour. Technol. 2001, 76, 173–175. [Google Scholar] [CrossRef]
- Sansone, G.; Rezza, I.; Calvente, V.; Benuzzi, D.; de Tosetti, M.I.S. Control of Botrytis cinerea strains resistant to iprodione in apple with rhodotorulic acid and yeasts. Postharvest Biol. Technol. 2005, 35, 245–251. [Google Scholar] [CrossRef]
- Nassar, A.H.; El-Tarabily, K.A.; Sivasithamparam, K. Promotion of plant growth by an auxin-producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots. Biol. Fertil. Soils 2005, 42, 97–108. [Google Scholar] [CrossRef]
- Diouf, D.; Diop, T.A.; Ndoye, I. Actinorhizal, mycorrhizal and rhizobial symbioses: How much do we know? Afr. J. Biotechnol. 2003, 2, 1–7. [Google Scholar]
- El-Tarabily, K.A.; Sivasithamparam, K. Potential of yeasts as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Mycoscience 2006, 47, 25–35. [Google Scholar] [CrossRef]
- Saad-Allah, K.M.; Fetouh, M.I.; Elhaak, M.A. Induction of milk thistle (Silybum marianum L. Gaertn) growth and phytochemicals production by natural stimulants. J. Appl. Res. Med. Arom. Plants 2017, 6, 101–110. [Google Scholar] [CrossRef]
- Abdel-Moneim, M.M.; El-Mazny, M.Y.; Abdel-Mageed, Y.T.; Moustala, Y.M.M.; Yamani, S.H.S. Effect of some natural antioxidants on the productivity and storage ability of Egyptian onion grown in sandy soil. In Proceedings of the Minia 2nd International Conference for Agriculture and Irrigation in the Nile Basin Countries, Minia, Egypt, 23–25 March 2015; pp. 411–429. [Google Scholar]
- Mukherjee, S.; Sen, S.K. Exploration of novel rhizospheric yeast isolate as fertilizing soil inoculant for improvement of maize cultivation. J. Sci. Food Agric. 2015, 95, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, L.V.; Brazhnikova, Y.V.; Berzhanova, R.Z.; Mukasheva, T.D. Plant growth-promoting and antifungal activity of yeasts from dark chestnut soil. Microbiol. Res. 2015, 175, 78–83. [Google Scholar] [CrossRef]
- Arumugam, G.; Swamy, M.K.; Sinniah, U.R. Plectranthus amboinicus (Lour.) Spreng: Botanical, phytochemical, pharmacological and nutritional significance. Molecules 2016, 21, 369. [Google Scholar] [CrossRef]
- Castillo, R.A.M.; Gonzalez, V.P. Plecthranthus amboinicus (Lour.) Spreng. Rev. Cuba. Plant Med. 1999, 4, 110–115. [Google Scholar]
- Singh, G.; Singh, O.P.; Prasad, Y.R.; de Lamposona, M.P.; Catalan, C. Studies on essential oils. Part 33. Chemical and insecticidal investigations on leaf oil of Coleus amboinicus (Lour). Flavour Fragr. J. 2002, 17, 440–442. [Google Scholar] [CrossRef]
- Murthy, P.S.; Ramalakshmi, K.; Srinivas, P. Fungitoxic activity of Indian borage (Plectranthus amboinicus) volatiles. Food Chem. 2009, 114, 1014–1018. [Google Scholar] [CrossRef]
- Senthilkumar, A.; Venkatesalu, V. Chemical composition and larvicidal activity of the essential oil of Plectranthus amboinicus (Lour.) Spreng against Anopheles stephensi: A malarial vector mosquito. Parasitol. Res. 2010, 107, 1275–1278. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, T.B.; Braga, M.A.; de Oliveira, F.F.M.; Santiago, G.M.P.; Carvalho, C.B.M.; Cabral, P.B.; Santiago, T.M.; Sousa, J.S.; Barros, E.B.; do Nascimento, R.F.; et al. Effect of subinihibitory and inhibitory concentrations of Plectranthus amboinicus (Lour.) Spreng essential oil on Klebsiella pneumoniae. Phytomedicine 2012, 19, 962–968. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, P.; Negi, P.S. Antioxidant and antibacterial activities in the leaf extracts of Indian borage (Plectranthus amboinicus). Food Nutr. Sci. 2012, 3, 146–152. [Google Scholar]
- Valera, D.; Rivas, R.; Avila, J.L.; Aubert, L.; Amelot, M.A.; Usubillaga, A.M. The essential oil of Coleus amboinicus Lour., chemical composition and evaluation of insect antifeedant effects. Ciencia 2003, 11, 113–118. [Google Scholar]
- Koba, K.; Grade, D.; Sanda, K.; Raynaud, C.; Chaumont, J.P. Chemical composition and antimicrobial properties of the leaf essential oil of Coleus aromaticus Benth. from Cambodia. Int. J. Essent. Oil Ther. 2007, 1, 16–20. [Google Scholar]
- El-Hawary, S.S.; El-Sofany, R.H.; Abdel-Monem, A.R.; Ashour, R.S.; Sleem, A.A. Seasonal variation in the composition of Plectranthus amboinicus (Lour.) Spreng essential oil and its biological activities. Am. J. Essent. Oil Nat. Prod. 2013, 1, 11–18. [Google Scholar]
- Erny, S.M.N.; Razali, M.; Mirfat, A.H.S.; Mohd Shukri, M.A. Antimicrobial activity and bioactive evaluation of Plectranthus amboinicus essential oil. Am. J. Res. Commun. 2014, 2, 121–127. [Google Scholar]
- El-Gohary, A.E.; Amer, H.M.; Salem, S.H.; Hussein, M.S. Foliar application of selenium and humic acid changes yield, essential oil, and chemical composition of Plectranthus amboinicus (Lour.) plant and its antimicrobial effects. Egypt. Pharm. J. 2019, 18, 365–367. [Google Scholar]
- Muniandy, K.; Hassan, Z.; Isa, M.H. The action of Coleus aromaticus as a potential wound healing agent in experimentally induced diabetic mice. PERINTIS E-J. 2014, 4, 1–30. [Google Scholar]
- Saraswati, S.; Jatinder, K.K.; Avinash, K.N. Analytical techniques for phytochemicals screening and bioactivities of some Coleus species: A review. J. Pharm. Sci. Res. 2016, 8, 227–237. [Google Scholar]
- Grayer, R.J.; Eckert, M.R.; Lever, A.; Veitch, N.C.; Kite, G.C.; Paton, A.J. Distribution of exudate flavonoids in the genus Plectranthus. Biochem. Syst. Ecol. 2010, 38, 335–341. [Google Scholar] [CrossRef]
- Lonhienne, T.; Mason, M.G.; Ragan, M.A.; Hugenholtz, P.; Schmidt, S.; Paungfoo Lonhienne, C. Yeast as a biofertilizer alters plant growth and morphology. Crop Sci. 2014, 54, 785–790. [Google Scholar] [CrossRef]
- Singh, T.; Sharma, U.; Agrawal, V. Isolation and optimization of plumbagin production in root callus of Plumbago zeylanica L. augmented with chitosan and yeast extract. Ind. Crops Prod. 2020, 151, 112446. [Google Scholar] [CrossRef]
- Difco Manual. Dehydrated Culture Media and Reagents for Microbiology; Laboratories Incorporated Detroit: Detroit, MI, USA, 1984; Volume 48232, p. 1027. [Google Scholar]
- Valladares, F.; Wright, S.J.; Lasso, E.; Kitajima, K.; Pearcy, R.W. Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecological 2000, 81, 1925–1936. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Mohamady, M.A.; Fernández-López, J.; ElRazik, K.A.; Omer, E.A.; Pérez-Alvarez, J.A.; Sendra, E. In vitro antioxidant and antibacterial activities of essentials oils obtained from Egyptian aromatic plants. Food Control 2011, 22, 1715–1722. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Jones, D.B. Factors for Converting Percentages of Nitrogen in Foods and Feeds into Percentages of Protein; USDA Circular Series; US Department of Agriculture: Washington, DC, USA, 1931; Volume 183, pp. 1–21.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Badhani, A.; Sakalani, S.; Mishra, A.P. Variation in biochemical’s and antioxidant activity of some wild edible fruits of Uttarakhand. Rep. Opin. 2011, 3, 1–10. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Jackson, W.A. Nitrate acquisition and assimilation by higher plants: Processes in the root system. In Nitrogen in the Environment; Nielsen, D.R., MacDonald, J.G., Eds.; Academic Press: New York, NY, USA, 1978; Volume 2. [Google Scholar]
- Black, C.A.; Evans, D.D.; Ensminger, L.E. Methods of Soil Analysis; American Society of Agronomy: Madison, WI, USA, 1965. [Google Scholar]
- Jackson, B.L.J.; During, C.M. Studies of slowly available potassium in soils of New Zealand. I. Effects of leaching, temperature and potassium depletion on the equilibrium concentration of potassium in solution. Plant Soil 1979, 51, 197–204. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989. [Google Scholar]
- Hernández-Fernández, M.; Cordero-Bueso, G.; Ruiz-Muñoz, M.; Cantoral, J.M. Culturable Yeasts as Biofertilizers and Biopesticides for a Sustainable Agriculture: A Comprehensive Review. Plants 2021, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Puglielli, G.; Crescente, M.F.; Frattaroli, A.R.; Gratani, L. Morphological, anatomical and physiological leaf trait plasticity of Sesleria nitida (Poaceae) in open vs shaded conditions. Pol. J. Ecol. 2015, 63, 10–22. [Google Scholar]
- Sheha, A.M.; Abou El-Enin, M.M.; El-Hashash, E.F.; Rady, M.M.; El-Serafy, R.S.; Shaaban, A. The productivity and overall benefits of faba bean-sugar beet intercropping systems interacted with foliar-applied nutrients. J. Plant Nutr. 2022, 1–18. [Google Scholar] [CrossRef]
- Fu, S.F.; Wei, J.Y.; Chen, H.W.; Liu, Y.Y.; Lu, H.Y.; Chou, J.Y. Indole-3-acetic acid: A widespread physiological code in interactions of fungi with other organisms. Plant Signal. Behav. 2015, 10, e1048052. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.P.; Hunter, A.; Kashpur, O.; Normanly, J. Aberrant synthesis of indole-3-acetic acid in Saccharomyces cerevisiae triggers morphogenic transition, a virulence trait of pathogenic fungi. Genetics 2010, 185, 211–220. [Google Scholar] [CrossRef]
- El-Tarabily, K.A. Suppression of Rhizoctonia solani diseases of sugar beet by antagonistic and plant growth-promoting yeasts. J. Appl. Microbiol. 2004, 96, 69–75. [Google Scholar] [CrossRef] [PubMed]
- French, R.J.; Buirchell, B.J. Lupin: The largest grain legume crop in Western Australia, its adaptation and improvement through plant breeding. Aust. J. Agric. Res. 2005, 56, 1169–1180. [Google Scholar] [CrossRef]
- Sannazzaro, A.I.; Ruiz, O.A.; Albertó, E.O.; Menéndez, A.B. Alleviation of salt stress in Lotus glaber by Glomus intraradices. Plant Soil 2006, 285, 279–287. [Google Scholar] [CrossRef]
- Alwhib, M.S.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; Soliman, D.W.K.; Wirth, S.; Egamberdieva, D. Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J. Integr. Agric. 2017, 16, 1751–1757. [Google Scholar]
- Sharma, I. Phytopathogenic fungi and their biocontrol applications. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nanotechnology; Sharma, V.K., Shah, M.P., Kumar, A., Eds.; Fungal Diversity of Sustainable Agriculture; Academic Press: Cambridge, MA, USA, 2021; Volume 1, pp. 155–188. [Google Scholar]
- Atteya, A.K.G.; El-Serafy, R.S.; El-Zabalawy, K.M.; Elhakem, A.; Genaidy, E.A.E. Brassinolide maximized the fruit and oil yield, induced the secondary metabolites, and stimulated linoleic acid synthesis of Opuntia ficus-indica oil. Horticulturae 2022, 8, 452. [Google Scholar] [CrossRef]
- Dixon, G.R.; Tilston, E.L. Soil Microbiology and Sustainable Crop Production; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Pérez-Montaño, F.; Alías-Villegas, C.; Bellogín, R.A.; del Cerro, P.; Espuny, M.R.; Jiménez-Guerrero, I.; López-Baena, F.J.; Ollero, F.J.; Cubo, T. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Streletskii, R.A.; Kachalkin, A.V.; Glushakova, A.M.; Yurkov, A.M.; Demin, V.V. Yeasts producing zeatin. PeerJ 2019, 7, e6474. [Google Scholar] [CrossRef] [PubMed]
- El-Mehy, A.A.; El-Gendy, H.M.; Aioub, A.A.A.; Mahmoud, S.F.; Abdel-Gawad, S.; Elesawy, A.E.; Elnahal, A.S.M. Response of faba bean to intercropping, biological and chemical control against broomrape and root rot diseases. Saudi J. Biol. Sci. 2022, 29, 3482–3493. [Google Scholar] [CrossRef] [PubMed]
- Osugi, A.; Sakakibara, H. Q&A: How do plants respond to cytokinins and what is their importance? BMC Boil. 2015, 13, 102. [Google Scholar]
- Agamy, R.; Hashem, M.; Alamri, S. Effect of soil amendment with yeasts as bio-fertilizers on the growth and productivity of sugar beet. Afr. J. Agric. Res. 2013, 8, 46–56. [Google Scholar]
- Akhtyamova, N.; Sattarova, R.K. Endophytic yeast Rhodotorula rubra strain TG-1: Antagonistic protection activities. Biochem. Physiol. 2013, 2, 1000104. [Google Scholar]
- Rakib, A.A.; Athab, M.A.; Matny, O.N. Management of potato virus Y (PVY) in potato by some biocontrol agents under field conditions. Adv. Environ. Biol. 2013, 7, 441–444. [Google Scholar]
- Sen, D.; Paul, K.; Saha, C.; Mukherjee, G.; Nag, M.; Ghosh, S.; Das, A.; Seal, A.; Tripathy, S. A unique life-strategy of an endophytic yeast Rhodotorula mucilaginosa JGTA-S1—A comparative genomics viewpoint. DNA Res. 2019, 26, 131–146. [Google Scholar] [CrossRef]
- Mou, H.; Lu, J.; Zhu, S.; Lin, C.; Tian, G.; Xu, X.; Zhao, W. Transcriptomic analysis of Paulownia infected by paulownia witches’-broom Phytoplasma. PLoS ONE 2013, 8, e77217. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Price, N.P.J.; Ray, K.J.; Kuo, T.M. Production of sophorolipid biosurfactants by multiple species of the Starmerella (Candida) bombicola yeast clade. FEMS Microbiol. Lett. 2010, 311, 140–146. [Google Scholar] [CrossRef]
- Desai, S.N.; Farris, F.F.; Ray, S.D. Lipid Peroxidation, Encyclopedia of Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 89–93. [Google Scholar]
- Arnold, P.A.; Kruuk, L.E.B.; Nicotra, A.B. How to analyse plant phenotypic plasticity in response to a changing climate. New Phytol. 2019, 222, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- El-Serafy, R.S. Phenotypic plasticity, biomass allocation, and biochemical analysis of cordyline seedlings in response to oligo-Chitosan foliar spray. J. Soil Sci. Plant Nutr. 2020, 20, 1503–1514. [Google Scholar] [CrossRef]
- Hill, J.O.; Simpson, R.J.; Moore, A.D.; Chapman, D.F. Morphology and response of roots of pasture species to phosphorus and nitrogen nutrition. Plant Soil 2006, 286, 7–19. [Google Scholar] [CrossRef]
- Bossdorf, O.; Lipowsky, A.; Prati, D. Selection of readapted populations allowed Senecio inaequidens to invade Central Europe. Divers. Distrib. 2008, 14, 676–685. [Google Scholar] [CrossRef]
- Atteya, A.K.; El Gendy, A.E.N.G. Impact of actosol and yeast extract on productivity and essential oil constituents of Zinnia elegans plants. Biosci. Res. 2018, 15, 1542–1558. [Google Scholar]
- Złotek, U.; Szymanowska, U.; Rybczynska-Tkaczyk, K.; Jakubczyk, A. Effect of jasmonic acid, yeast extract elicitation, and drying methods on the main bioactive compounds and consumer quality of lovage (Levisticum Officinale Koch). Foods 2020, 9, 323. [Google Scholar] [CrossRef]
- Taha, R.S.; Seleiman, M.F.; Alhammad, B.A.; Alkahtani, J.; Alwahibi, M.S.; Mahdi, A.H.A. Activated yeast extract enhances growth, anatomical structure, and productivity of Lupinus termis L. plants under actual salinity conditions. Agronomy 2021, 11, 74. [Google Scholar] [CrossRef]
- Abbas, S.M. The influence of biostimulants on the growth and on the biochemical composition of Vicia faba cv. Giza 3 beans. Rom. Biotechnol. Lett. 2013, 18, 8061–8068. [Google Scholar]
- Hammad, S.A.R.; Ali, O.A.M. Physiological and biochemical studies on drought tolerance of wheat plants by application of amino acids and yeast extract. Ann. Agric. Sci. 2014, 59, 133–145. [Google Scholar] [CrossRef] [Green Version]
- Neseim, M.R.; Amin, A.Y.; El-Mohammady, M.M.S. Effect of potassium applied with foliar spray of yeast on sugar beet growth and yield under drought stress. Glob. Adv. Res. J. Agric. Sci. 2014, 3, 211–222. [Google Scholar]
- Medani, R.A.; Taha, R.S. Improving growth and yield of caraway (Carum carvi L.) plants by decapitation and/or active dry yeast application. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 47–60. [Google Scholar]
- Abdallah, M.M.S.; El Habbasha, S.F.; El Sebai, T. Comparison of yeast extract and Nicotinaminde foliar applications effect on quinoa plants grown under sandy soil condition. Int. J. PharmTech Res. 2016, 9, 24–32. [Google Scholar]
- Sugiah, S.; Balia, R.L.; Utama, G.L. The potential of mannoprotein extracted from candida apicola cell wall as emulsification agent. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural. Dev. 2019, 19, 335–340. [Google Scholar]
- Chandra, P.; Arora, D.S. Antioxidant activity of fungi isolated from soil of different areas of Punjab, India. J. Appl. Nat. Sci. 2009, 1, 123–128. [Google Scholar] [CrossRef]
- Rios, M.F.; Pajan, C.M.G.; Galan, R.H.; Sanchez, A.J.M.; Callado, I.G. Synthesis and free radical scavenging activity of a novel metabolite from the fungus Colletotrichum gloeosporioides. Bioorg. Med. Chem. Lett. 2006, 16, 5836–5839. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Oyebanji, E.; Fowora, M.; Aiyeolemi, A.; Orabuchi, C.; Akinnawo, B.; Adekunle, A.A. Extracts of endophytic fungi from leaves of selected Nigerian ethnomedicinal plants exhibited antioxidant activity. BMC Complement. Med. Ther. 2021, 21, 98. [Google Scholar] [CrossRef]
- Atteya, A.K.; Albalawi, A.N.; El-Serafy, R.S.; Albalawi, K.N.; Bayomy, H.M.; Genaidy, E.A.E. Response of Moringa oleifera seeds and fixed oil production to vermicompost and NPK fertilizers under calcareous soil conditions. Plants 2021, 10, 1998. [Google Scholar] [CrossRef]
- Scervino, J.M.; Mesa, M.P.; Monica, I.D.; Recchi, M.; Moreno, N.S.; Godeas, A. Soil fungal isolates produce different organic acid patterns involved in phosphate salts solubilization. Biol. Fertil. Soil 2010, 46, 755–763. [Google Scholar] [CrossRef]
- Millan, A.F.-S.; Farran, I.; Larraya, L.; Ancin, M.; Arregui, L.M.; Veramendi, J. Plant growth-promoting traits of yeasts isolated from Spanish vineyards: Benefits for seedling development. Microbiol. Res. 2020, 237, 126480. [Google Scholar] [CrossRef]
- Mohamed, H.M.; El-Homosy, R.F.; Abd-Ellatef, A.-E.H.; Salh, F.M.; Hussein, M.Y. Identification of yeast strains isolated from agricultural soils for releasing potassium-bearing minerals. Geomicrobiol. J. 2017, 34, 261–266. [Google Scholar] [CrossRef]
- Ahmed, M.E.M. Response of garlic plants (Allium sativum L.) to foliar application of some bio-stimulants. Egypt. J. Hortic. 2015, 42, 613–625. [Google Scholar]
- Ahmed, A.A.; El-Baky, M.A.; Zaki, M.F.; El-Aal, F.S.A. Effect of foliar application of active yeast extract and zinc on growth, yield and quality of potato plant (Solanum tuberosum L.). J. Appl. Sci. Res. 2011, 7, 2479–2488. [Google Scholar]
- Mahmoud, H.I.; Azzaz, N.A.; Khalifa, Y.A.; Mahmoud, M.A.; Fakhry, G. Effect of foliar application with active yeast extract and benzyladenine on some vegetative growth criteria and chemical composition of lupine (Lupinus termis L.) plants. Minia J. Agric. Res. Dev. 2016, 36, 193–214. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshallash, K.S.; Mohamed, M.F.; Dahab, A.A.; Abd El-Salam, H.S.; El-Serafy, R.S. Biostimulation of Plectranthus amboinicus (Lour.) Spreng. with Different Yeast Strains: Morphological Performance, Productivity, Phenotypic Plasticity, and Antioxidant Activity. Horticulturae 2022, 8, 887. https://doi.org/10.3390/horticulturae8100887
Alshallash KS, Mohamed MF, Dahab AA, Abd El-Salam HS, El-Serafy RS. Biostimulation of Plectranthus amboinicus (Lour.) Spreng. with Different Yeast Strains: Morphological Performance, Productivity, Phenotypic Plasticity, and Antioxidant Activity. Horticulturae. 2022; 8(10):887. https://doi.org/10.3390/horticulturae8100887
Chicago/Turabian StyleAlshallash, Khalid S., Mohamed F. Mohamed, Abeer A. Dahab, Hemat S. Abd El-Salam, and Rasha S. El-Serafy. 2022. "Biostimulation of Plectranthus amboinicus (Lour.) Spreng. with Different Yeast Strains: Morphological Performance, Productivity, Phenotypic Plasticity, and Antioxidant Activity" Horticulturae 8, no. 10: 887. https://doi.org/10.3390/horticulturae8100887




