X-ray Diffraction, Micro-Raman and X-ray Photoemission Spectroscopic Investigations for Hydrothermally Obtained Hybrid Compounds of Delafossite CuGaO2 and Wurtzite ZnO
Abstract
:1. Introduction
2. Materials and Method
2.1. Preparation of CuGaO2 Powder
2.2. Preparation of CuGaO2/ZnO Hybrids
2.3. Characterization
3. Results and Discussion
3.1. Structural Analysis of CuGaO2 and CuGaO2/ZnO Hybrids
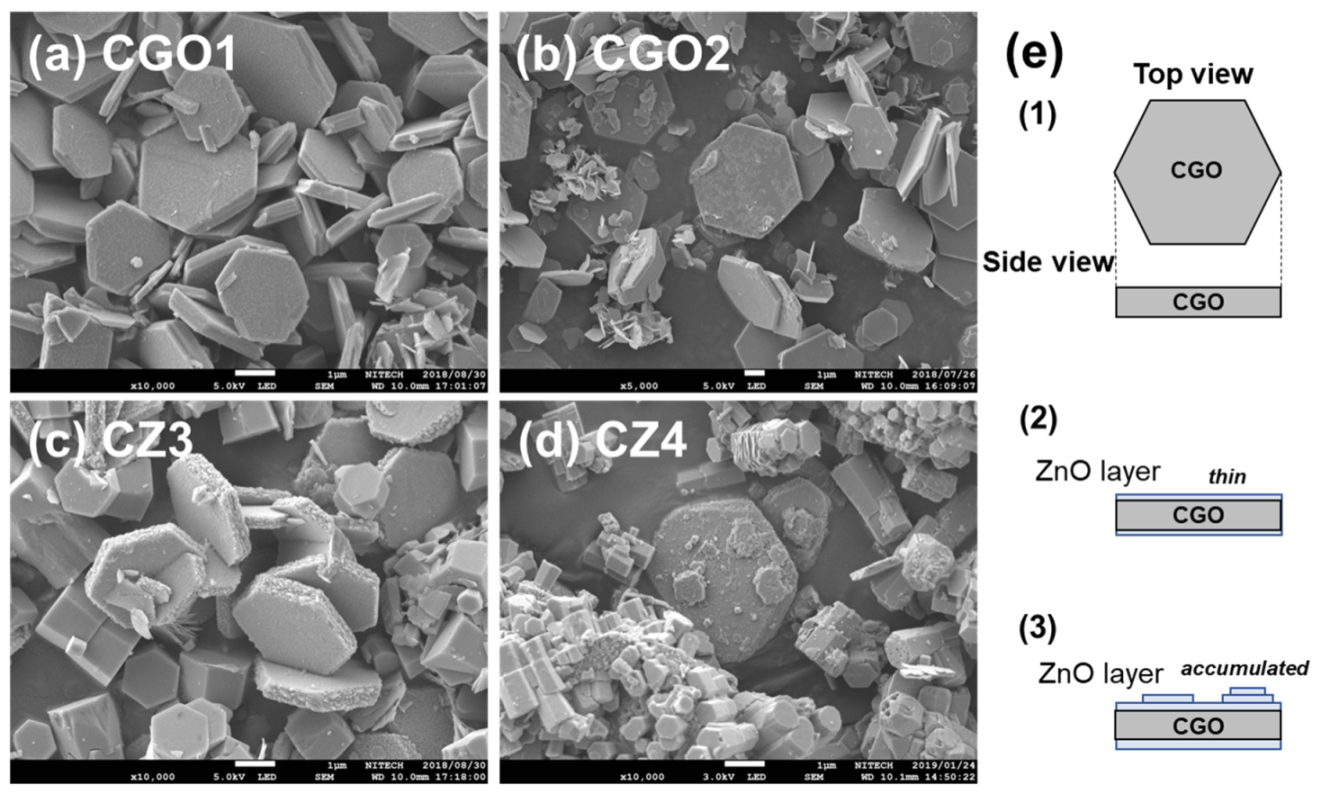
3.2. SEM Observation of CuGaO2 and CuGaO2/ZnO Hybrids
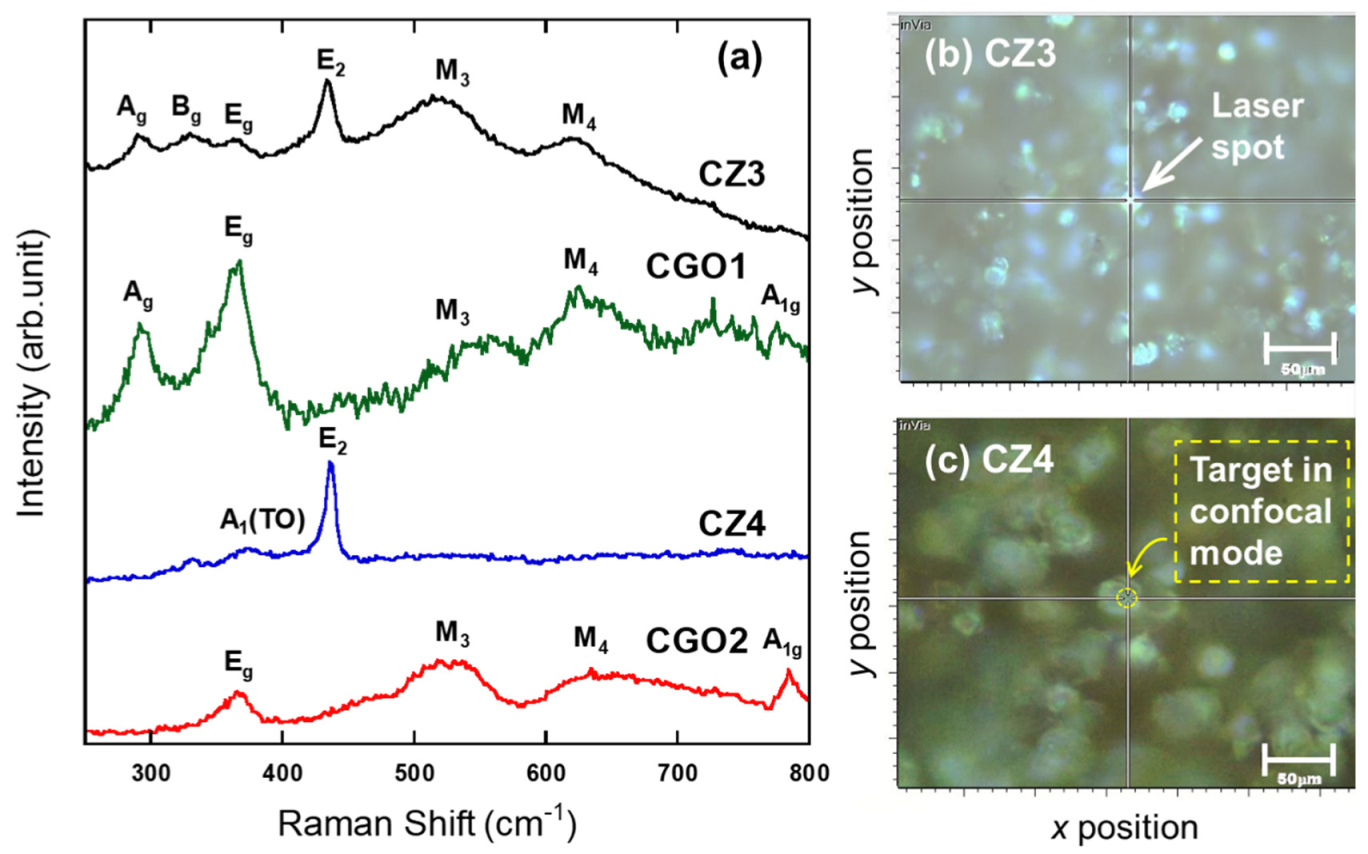
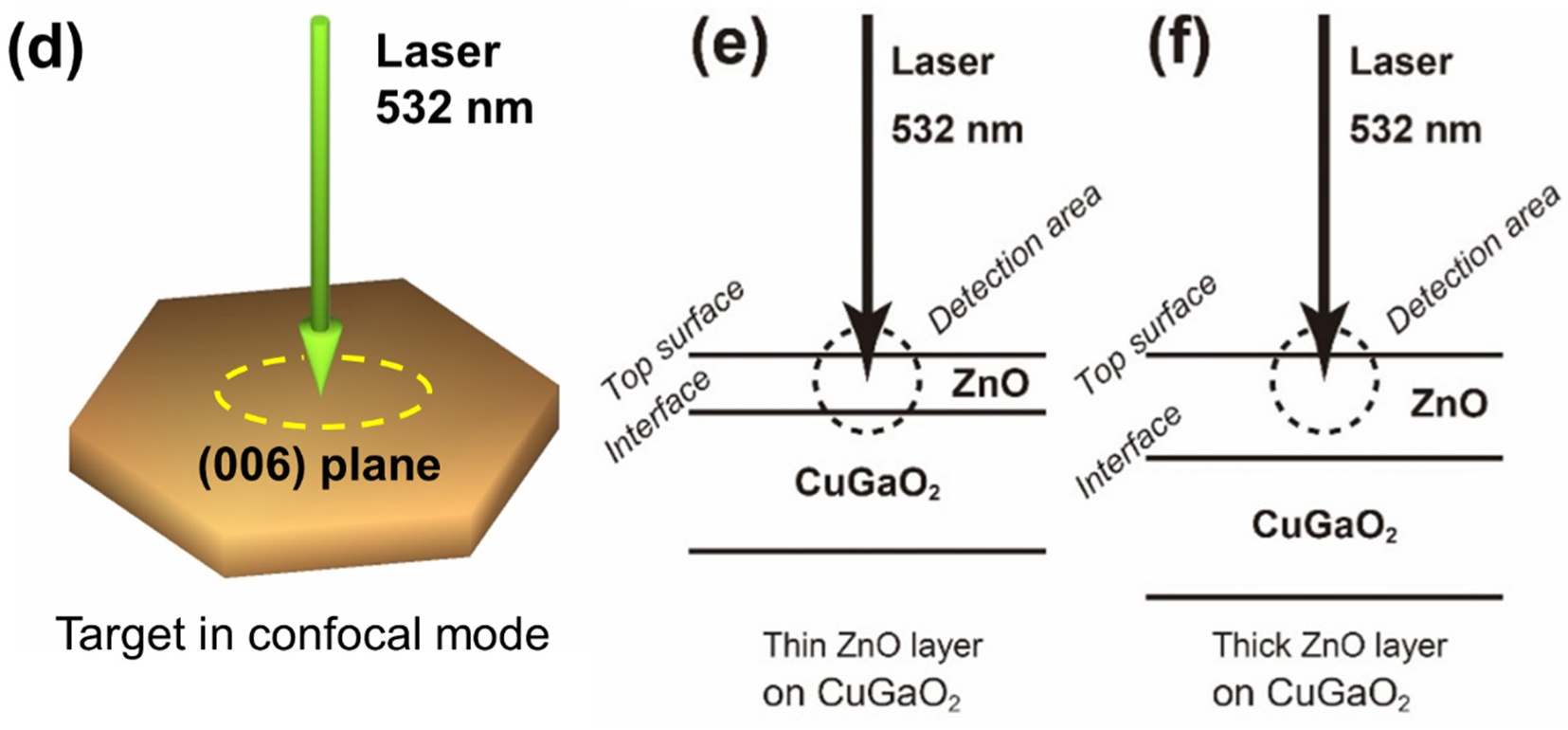
3.3. Micro-Raman Investigation
3.4. Unique Reaction in the Boundary between CuGaO2 and ZnO in CZ3
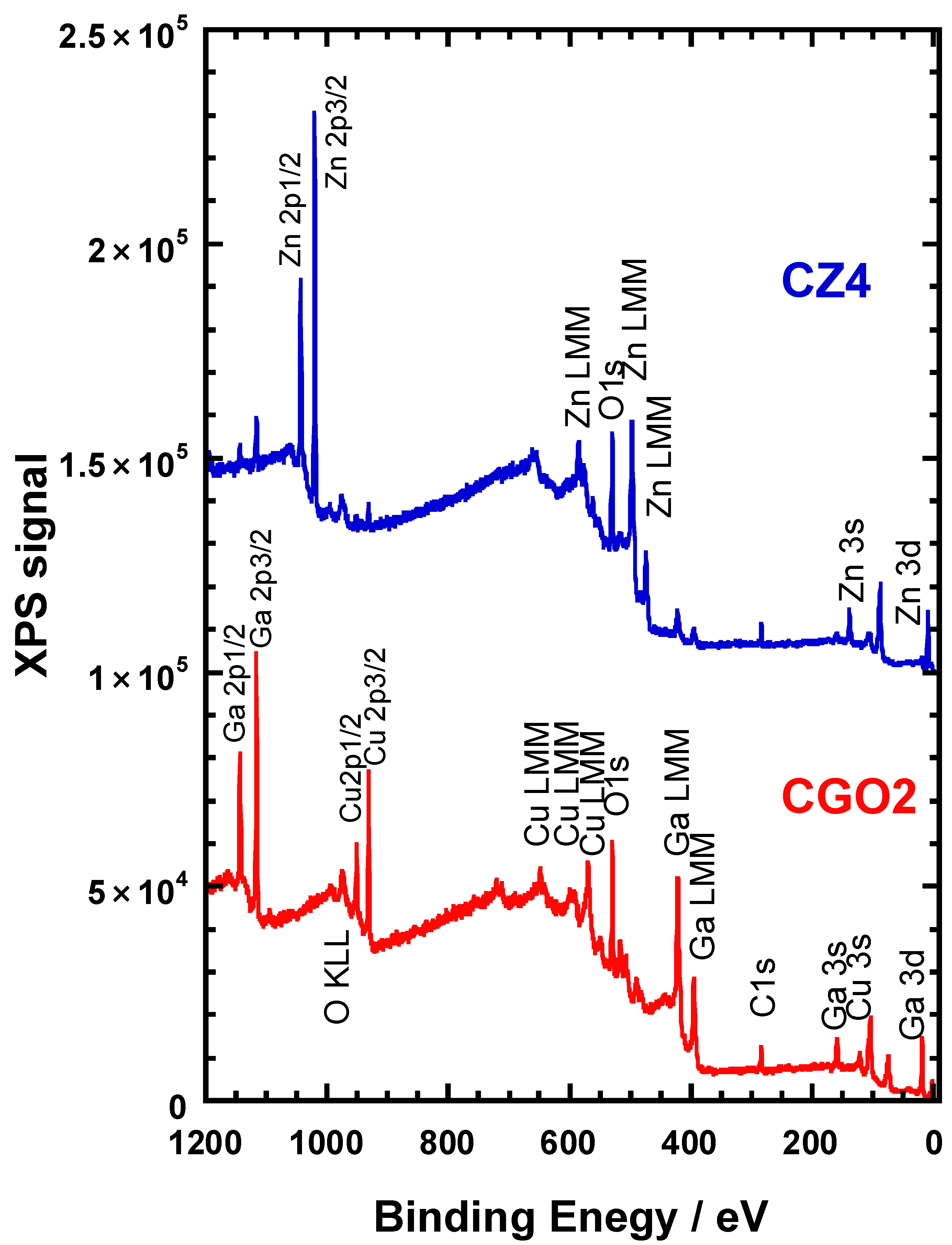
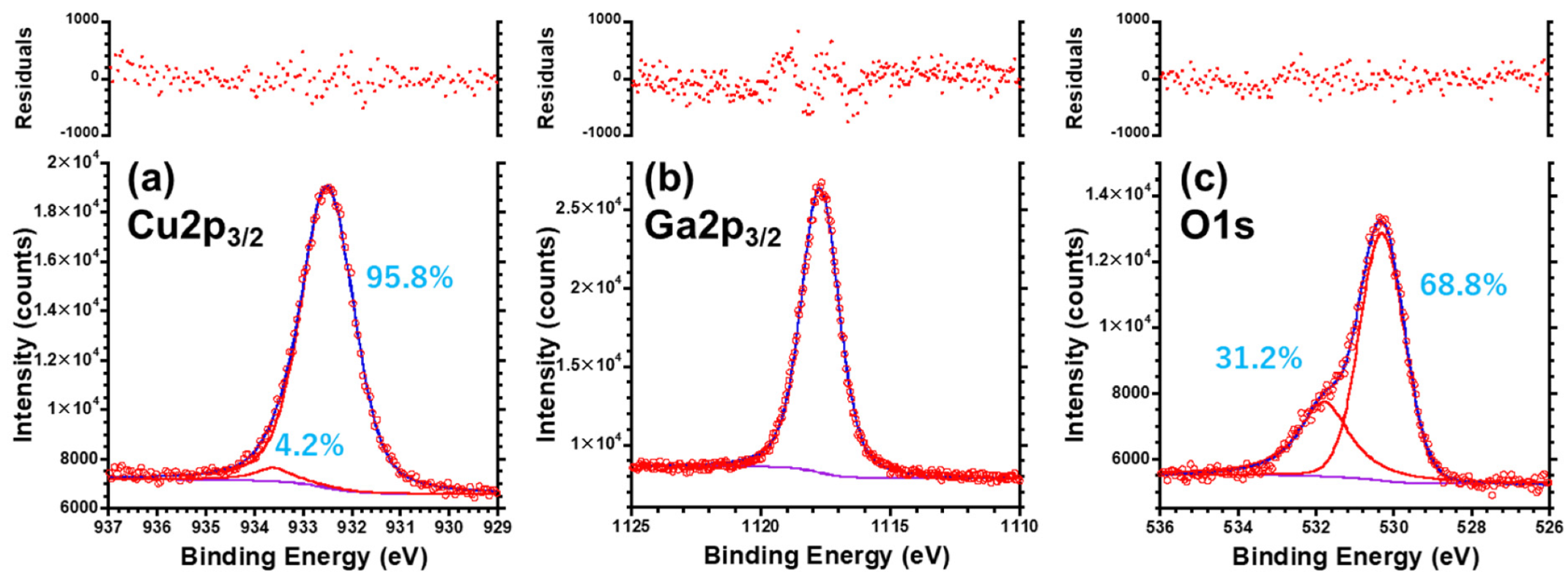
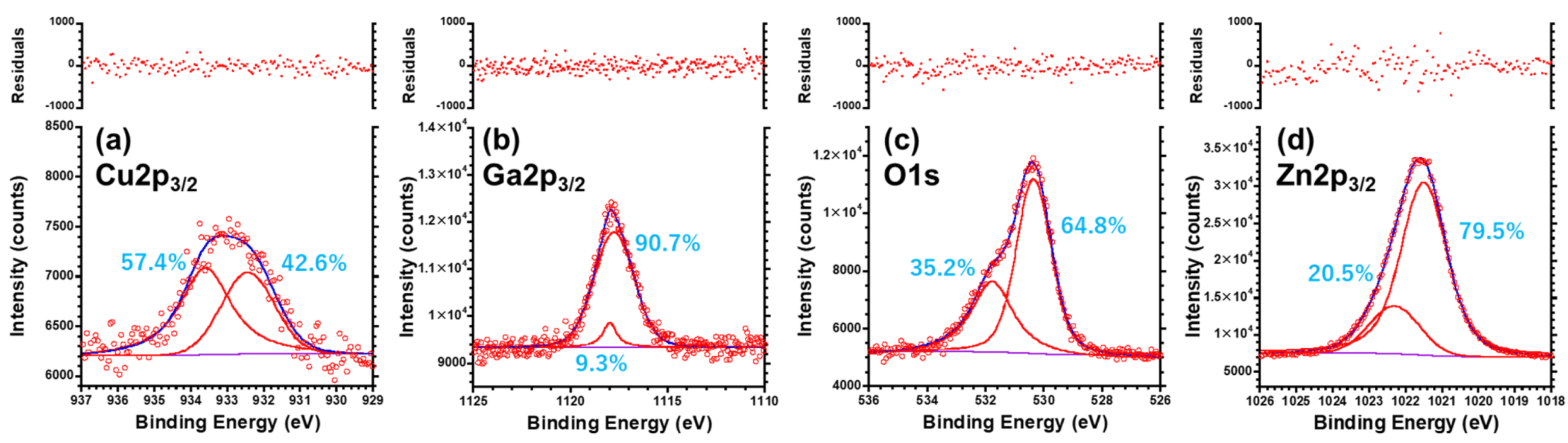
3.5. XPS Analysis for the CuGaO2 Base Crystal and CuGaO2/ZnO Hybrid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Davies, R.H.; Worsley, D. Water purification by semiconductor photocatalysis. Chem. Soc. Rev. 1993, 22, 417–425. [Google Scholar] [CrossRef]
- Yu, M.; Natu, G.; Ji, Z.; Wu, Y. p-Type dye-sensitized solar cells based on delafossite CuGaO2 nanoplates with saturation photovoltages exceeding 460 mV. J. Phys. Chem. Lett. 2012, 3, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fang, W.; Yu, C.; Zhou, W.; Zhu, L.; Xie, Y. Ag-based semiconductor photocatalysts in environmental purification. Appl. Surf. Sci. 2015, 358, 46–56. [Google Scholar] [CrossRef]
- Ke, J.; Younis, M.A.; Kong, Y.; Zhou, H.; Liu, J.; Lei, L.; Hou, Y. Nanostructured ternary metal tungstate-based photocatalysts for environmental purification and solar water splitting: A review. Nano-Micro Lett. 2018, 10, 69. [Google Scholar] [CrossRef]
- Zhao, R.D.; Zhang, Y.M.; Liu, Q.L.; Zhao, Z.Y. Effects of the preparation process on the photocatalytic performance of delafossite CuCrO2. Inorg. Chem. 2020, 59, 16679–16689. [Google Scholar] [CrossRef]
- Kumar, M.; Zhao, H.; Persson, C. Study of band-structure, optical properties and native defects in AIBIIIO2 (AI=Cu or Ag, BIII=Al, Ga or In) delafossite. Semicond. Sci. Technol. 2013, 28, 065003. [Google Scholar] [CrossRef]
- Bredar, A.R.C.; Blanchet, M.D.; Comes, R.B.; Farnum, B.H. Evidence and influence of copper vacancies in p-type CuGaO2 mesoporous films. ACS Appl. Energy Mater. 2019, 2, 19–28. [Google Scholar] [CrossRef]
- Choi, M.U.; Hayakawa, T. Hydrothermal synthesis and photocatalytic properties of CuGaO2/ZnO hexagonal platelet hybrids. Mater. Res. Bull. 2019, 113, 84–89. [Google Scholar] [CrossRef]
- Wang, J.; Ibarra, V.; Barrera, D.; Xu, L.; Lee, Y.J.; Hsu, J.W.P. Solution synthesized p-type copper gallium oxide nanoplates as hole transport layer for organic photovoltaic devices. J. Phys. Chem. Lett. 2015, 6, 1071–1075. [Google Scholar] [CrossRef]
- Suzuki, I.; Nagatani, H.; Kita, M.; Iguchi, Y.; Sato, C.; Yanagi, H.; Ohashi, N.; Omata, T. First-principles study of CuGaO2 polymorphs: Delafossite α-CuGaO2 and wurtzite β-CuGaO2. Inorg. Chem. 2016, 55, 7610–7616. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xiong, D.; Wang, H.; Zhang, W.; Zeng, X.; Ming, L.; Chen, W.; Xu, X.; Cui, J.; Wang, M.; et al. Remarkable photocurrent of p-type dye-sensitized solar cell achieved by size controlled CuGaO2 nanoplates. J. Mater. Chem. A 2014, 2, 2968–2976. [Google Scholar] [CrossRef]
- Ehara, T. Preparation of CuGaO2 thin film by a sol-gel method using two kinds of metal source combination. J. Mater. Sci. Chem. Eng. 2018, 6, 68–78. [Google Scholar]
- Xu, Y.; Ma, G.; Wang, G.; Shi, L.; Zhang, H.; Jin, L.; Ma, X.; Zou, Y.; Yin, J.; Li, Y. Interface state luminescence and sub-bandgap absorption based on CuGaO2 nanoplates/ZnO nanowires heterostructure arrays. Phys. Status Solidi B 2018, 255, 1800391. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Bernstein, J.L. Remeasurement of the structure of hexagonal ZnO. Acta Cryst. B 1969, 25, 1233–1236. [Google Scholar] [CrossRef]
- Zhang, Z.; Yates, J.T., Jr. Band bending in semiconductors: Chemical and physical consequences at surfaces and interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Pouran, S.R. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Dong, S.; Feng, J.; Fan, M.; Pi, Y.; Hu, L.; Han, X.; Liu, M.; Sun, J.; Sun, J. Recent developments in heterogeneous photocatalytic water treatment using visible light responsive photocatalysts: A review. RSC Adv. 2015, 5, 14610. [Google Scholar] [CrossRef]
- Han, M.; Lu, Y.; Liu, Y.; Hu, Z.; Chen, G.; Jiang, K.; Zhang, J.; Li, W. Raman scattering measurements of phonon anharmonicity in the delafossite CuGa1−xCrxO2 (0 ≤ x ≤ 1) films. J. Raman Spectrosc. 2020, 51, 851–859. [Google Scholar] [CrossRef]
- Wang, H.; Mann, C.K.; Vickers, T.J. Effect of powder properties on the intensity of Raman scattering by crystalline solids. Appl. Spectrosc. 2002, 56, 1538–1544. [Google Scholar] [CrossRef]
- Wirths, S.; Tiedemann, A.T.; Ikonic, Z.; Harrison, P.; Holländer, B.; Stoica, T.; Mussler, G.; Myronov, M.; Hartmann, J.M.; Grützmacher, D.; et al. Band engineering and growth of tensile strained Ge/(Si) GeSn heterostructures for tunnel field effect transistors. Appl. Phys. Lett. 2013, 102, 192103. [Google Scholar] [CrossRef] [Green Version]
- Laurentius, L.; Stoyanov, S.R.; Gusarov, S.; Kovalenko, A.; Du, R.; Lopinski, G.P.; McDermott, M.T. Diazonium-derived aryl films on gold nanoparticles: Evidence for a carbon-gold covalent bond. ACS Nano 2011, 5, 4219–4227. [Google Scholar] [CrossRef] [PubMed]
- Bood, J.; Brackmann, C.; Bengtsson, P.-E.; Aldén, M. Multipoint temperature and oxygen-concentration measurements using rotational coherent anti-Stokes Raman spectroscopy. Opt. Lett. 2000, 25, 1535–1537. [Google Scholar] [CrossRef]
- Han, M.J.; Jiang, K.; Zhang, J.Z.; Li, Y.W.; Hu, Z.G.; Chu, J.H. Temperature dependent phonon evolutions and optical properties of highly c-axis oriented CuGaO2 semiconductor films grown by the sol-gel method. Appl. Phys. Lett. 2011, 99, 131104. [Google Scholar]
- Ahmed, J.; Mao, Y. Synthesis, characterization and electrocatalytic properties of Delafossite CuGaO2. J. Solid State Chem. 2016, 242, 77–85. [Google Scholar] [CrossRef]
- Debbichi, L.; de Lucas, M.C.M.; Pierson, J.F.; Krüger, P. Vibrational properties of CuO and Cu4O3 from first-principles calculations, and Raman and infrared spectroscopy. J. Phys. Chem. C 2012, 116, 10232–10237. [Google Scholar] [CrossRef]
- Prabhakaran, G.; Murugan, R. Room temperature ferromagnetic properties of Cu2O microcrystals. J. Alloys Compd. 2013, 579, 572–575. [Google Scholar] [CrossRef]
- Pellicer-Porres, J.; Segura, A.; Martínez, E.; Saitta, A.M.; Polian, A.; Chervin, J.C.; Canny, B. Vibrational properties of delafossite CuGaO2 at ambient and high pressures. Phys. Rev. B 2005, 72, 064301. [Google Scholar] [CrossRef]
- Gao, H.; Zeng, X.; Guo, Q.; Yang, Z.; Deng, Y.; Li, H.; Xiong, D. P-type transparent conducting characteristics of delafossite Ca doped CuScO2 prepared by hydrothermal synthesis. Dalton Trans. 2021, 50, 5262–5268. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Sakthinathan, S.; Yu, C.-L.; Wang, C.-C.; Chiu, T.-W.; Han, Q. Preparation and characterization of delafossite CuCrO2 film on flexible substrate. Ceram. Int. 2021, 47, 23234–23239. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Zhang, P.; Sun, X.; Zheng, H.; Zhang, W. Preparation and characterization of solution-processed nanocrystalline p-Type CuAlO2 thin-film transistors. Nanoscale Res. Lett. 2018, 13, 259. [Google Scholar] [CrossRef]
- Wu, C.; Qiu, L.; Li, S.; Guo, D.; Li, P.; Wang, S.; Du, P.; Chen, Z.; Liu, A.; Wang, X.; et al. High Sensitive and Stable Solution-Processed All Inorganic Self-powered Solar-blind Photodetector based on CuMO2/Ga2O3 p-n heterojunction. Mater. Today Phys. 2021, 17, 100335. [Google Scholar] [CrossRef]
- He, C.; Guo, D.; Chen, K.; Wang, S.; Shen, J.; Zhao, N.; Liu, A.; Zheng, Y.; Li, P.; Wu, Z.; et al. α-Ga2O3 Nanorod Array–Cu2O Microsphere p–n Junctions for Self-Powered Spectrum-Distinguishable Photodetectors. ACS Appl. Nano Mater. 2019, 2, 4095–4103. [Google Scholar] [CrossRef]
- Guo, D.; Chen, K.; Wang, S.; Wu, F.; Liu, A.; Li, C.; Li, P.; Tan, C.; Tang, W. Self-Powered Solar-Blind Photodetectors Based on α/β Phase Junction of Ga2O3. Phys. Rev. Applied. 2020, 13, 024051. [Google Scholar] [CrossRef]
- Srinivasan, R.; Chavillon, B.; Doussier-Brochard, C.; Cario, L.; Paris, M.; Gautron, E.; Deniard, P.; Odobel, F.; Jobic, S. Tuning the size and color of the p-type wide band gap delafossite semiconductor CuGaO2 with ethylene glycol assisted hydrothermal synthesis. J. Mater. Chem. 2008, 18, 5647–5653. [Google Scholar] [CrossRef]
- Izumi, F.; Momma, K. Three-dimensional visualization in powder diffraction. Solid State Phenom. 2007, 130, 15–20. [Google Scholar] [CrossRef]
- Huang, J.; Krishna, U.R.; Lemberger, M.; Jank, M.P.M.; Ryssel, H.; Frey, L. Impact of forming gas annealing on ZnO-TFTs. In Proceedings of the 2010 10th IEEE International Conference on Solid-State and Integrated Circuit Technology, Shanghai, China, 1–4 November 2010; pp. 1548–1550. [Google Scholar]
- Gingasu, D.; Mindru, I.; Patron, L.; Marinescu, G.; Tuna, F.; Preda, S.; Calderon-Moreno, J.M.; Andronescu, C. Synthesis of CuGa2O4 nanoparticles by precursor and self-propagating combustion method. Ceram. Int. 2012, 38, 6739–6751. [Google Scholar] [CrossRef]
- Materials Project: Mp-1541943. Available online: https://materialsproject.org/materials/mp-1541943/ (accessed on 22 September 2021).
- Hautier, G.; Fischer, C.C.; Jain, A.; Mueller, T.; Ceder, G. Finding nature’s missing ternary oxide compounds using machine learning and density functional theory. Chem. Mater. 2010, 22, 3762–3767. [Google Scholar] [CrossRef]
- Samson, D.O.; Makama, E.K. Effect of Concentration on Morphological, Optical and Electrical Properties of Copper Doped Zinc Oxide Thin Films Deposited by Electrostatic Spray Pyrolysis (ESP) Technique. J. Mater. Sci. Res. Rev. 2018, 1, 24–39. [Google Scholar]
- Stoica, M.; Faghaninia, A.; Sun, X.; Lo, C.S. Computational design of p-type transparent conductors for photovoltaic applications. In Proceedings of the IEEE 40th Conference on Photovoltaic Specialists (PVSC), Denver, CO, USA, 8–13 June 2014; pp. 260–265. [Google Scholar] [CrossRef]
- Stoica, M.; Lo, C.S. P-type Zinc Oxide Spinels: Application to Transparent Conductors and Spintronics. New J. Phys. 2014, 16, 055011. [Google Scholar] [CrossRef]
- Singstock, N.R.; Bartel, C.J.; Holder, A.M.; Musgrave, C.B. High-Throughput Analysis of Materials for Chemical Looping Processes. Adv. Energy Mater. 2020, 10, 2000685. [Google Scholar] [CrossRef]
- Choi, M.; Yagi, S.; Ohta, Y.; Kido, K.; Hayakawa, T. Estimation of delafossite p-type CuGaO2/ZnO hybrids as semiconductor photocatalyst by controlling particle size. J. Phys. Chem. Solids 2021, 150, 109845. [Google Scholar] [CrossRef]
- Shu, G.; Dai, B.; Ralchenko, V.G.; Khomich, A.A.; Ashkinazi, E.E.; Bolshakov, A.P.; Bokova-Sirosh, S.N.; Liu, K.; Zhao, J.; Han, J.; et al. Epitaxial growth of mosaic diamond: Mapping of stress and defects in crystal junction with a confocal Raman spectroscopy. J. Cryst. Growth 2017, 463, 19–26. [Google Scholar] [CrossRef]
- Decremps, F.; Pellicer-Porres, J.; Saitta, A.M.; Chervin, J.C.; Polian, A. High-pressure Raman spectroscopy study of wurtzite ZnO. Phys. Rev. B 2002, 65, 092101. [Google Scholar] [CrossRef]
- Litvinchuk, A.P.; Möller, A.; Debbichi, L.; Krüger, P.; Lliev, M.N.; Gospodinov, M.M. Second-ordeRaman scattering in CuO. J. Phys. Condens. Matter 2013, 25, 105402. [Google Scholar] [CrossRef]
- Cuscó, R.; Alarcón-Lladó, E.; Ibáñez, J.; Artús, L.; Jiménez, J.; Wang, B.; Callahan, M.J. Temperature dependence of Raman scattering in ZnO. Phys. Rev. B 2007, 75, 165202. [Google Scholar] [CrossRef]
- Makhova, L.; Wett, D.; Lorenz, M.; Konovalov, I. X-ray spectroscopic investigation of forbidden direct transitions in CuGaO2 and CuInO2. Phys. Status Solidi 2006, 11, 2861–2866. [Google Scholar] [CrossRef]
- Ramana, C.V.; Rubio, E.J.; Barraza, C.D.; Gallardo, A.M.; McPeak, S.; Kotru, S.; Grant, J.T. Chemical bonding, optical constants, and electrical resistivity of sputter-deposited gallium oxide thin films. J. Appl. Phys. 2014, 115, 043508. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Wood, B.J. Systematic XPS study of gallium-substituted boehmite. J. Mater. Sci. 2016, 51, 5436–5444. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Davar, F.; Khansari, A. Nanosphericals and nanobundles of ZnO: Synthesis and characterization. J. Alloys Compds. 2011, 509, 61–65. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Daud, A.R.; Tabet, N.; Al-Douri, Y. XPS and optical studies of different morphologies of ZnO nanostructures prepared by microwave methods. Ceram. Int. 2013, 39, 2283–2292. [Google Scholar] [CrossRef]
- Poulston, S.; Parlett, P.M.; Stone, P.; Bowker, M. Surface oxidation and reduction of CuO and Cu2O studied using XPS and XAES. Surf. Interface Anal. 1996, 24, 811–820. [Google Scholar] [CrossRef]
- Castle, J.E. A unified scale for the Auger parameter: Methodology and benefits. Suf. Interface Anal. 2022, 54, 455–464. [Google Scholar] [CrossRef]
- Deroubaix, G.; Marcus, P. X-ray photoelectron spectroscopy analysis of copper and zinc oxides and sulphides. Surf. Interface Anal. 1992, 18, 39–46. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides, and hydroxides: Sc, Ti, V., Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Schön, G. Auger and direct electron spectra in X-ray photoelectron studies of zinc, zinc oxide, gallium and gallium oxide. J. Electron Spectrosc. Relat. Phenom. 1973, 2, 75–86. [Google Scholar] [CrossRef]
- Borque, J.L.; Biesinger, M.C.; Baines, K.M. Chemical state determination of molecular gallium compounds using XPS. Dalton Trans. 2016, 45, 7678–7696. [Google Scholar] [CrossRef]
- Bera, S.; Dhara, S.; Velmurugan, S.; Tyagi, A.K. Analysis on binding energy and Auger parameter for estimating size and stoichiometry of ZnO nanorods. Int. J. Spect. 2012, 2012, 371092. [Google Scholar] [CrossRef]
- Dake, L.S.; Baer, D.R.; Zachra, J.M. Auger parameter measurements of zinc compounds relevant to zinc transport in the environment. Surf. Interface Anal. 1989, 14, 14–71. [Google Scholar] [CrossRef]
- Islam, M.N.; Ghosh, T.B.; Chopra, K.L.; Acharya, H.N. XPS and X-ray diffraction studies of aluminum-doped zinc oxide transparent conducting films. Thin Solid Films 1996, 280, 20–25. [Google Scholar] [CrossRef]
- Jesen, I.J.T.; Gorantla, S.; Løvvik, O.M.; Gan, J.; Nguyen, P.D.; Monakhov, E.; Svensson, B.G.; Gunnæs, A.E.; Diplas, S. Interface phenomena in magnetron sputtered Cu2O/ZnO heterostructures. J. Phys. Condens. Matter. 2017, 29, 435002. [Google Scholar] [CrossRef] [PubMed]
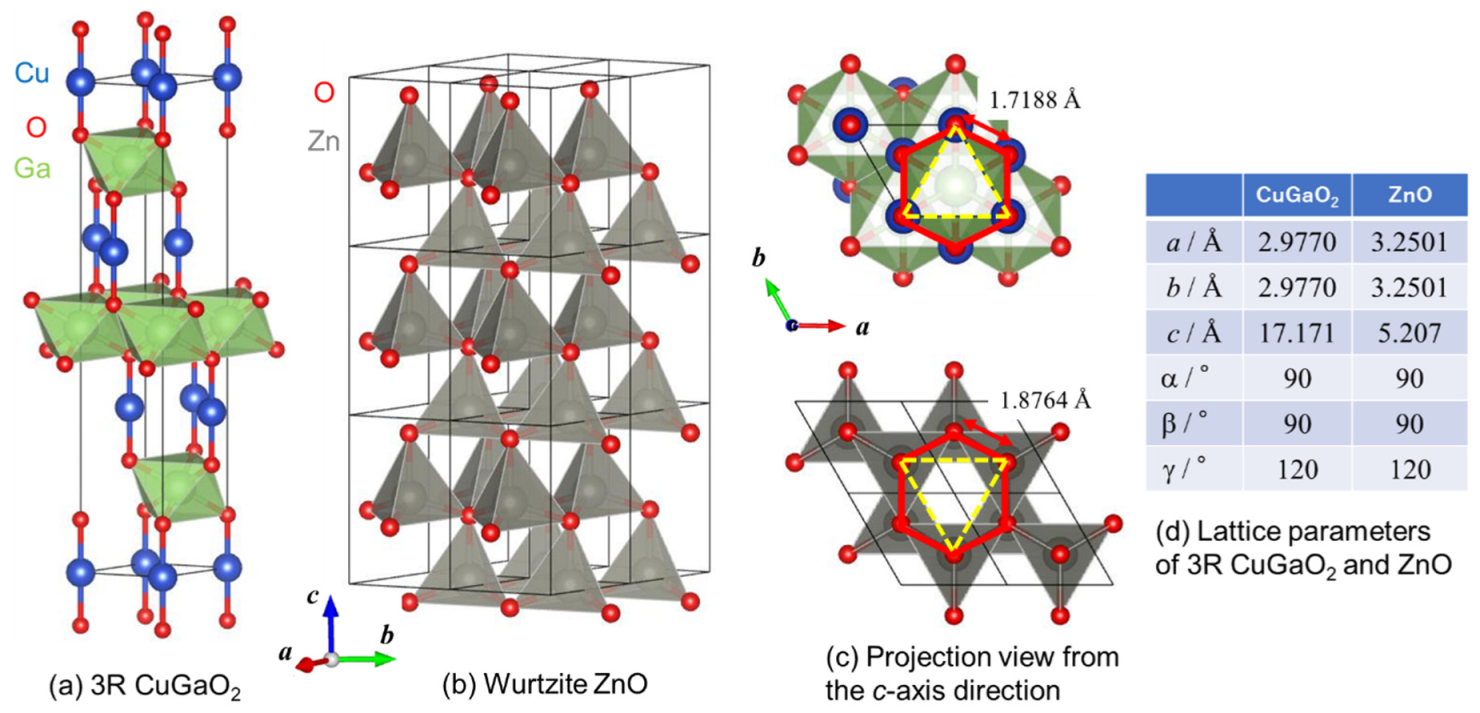
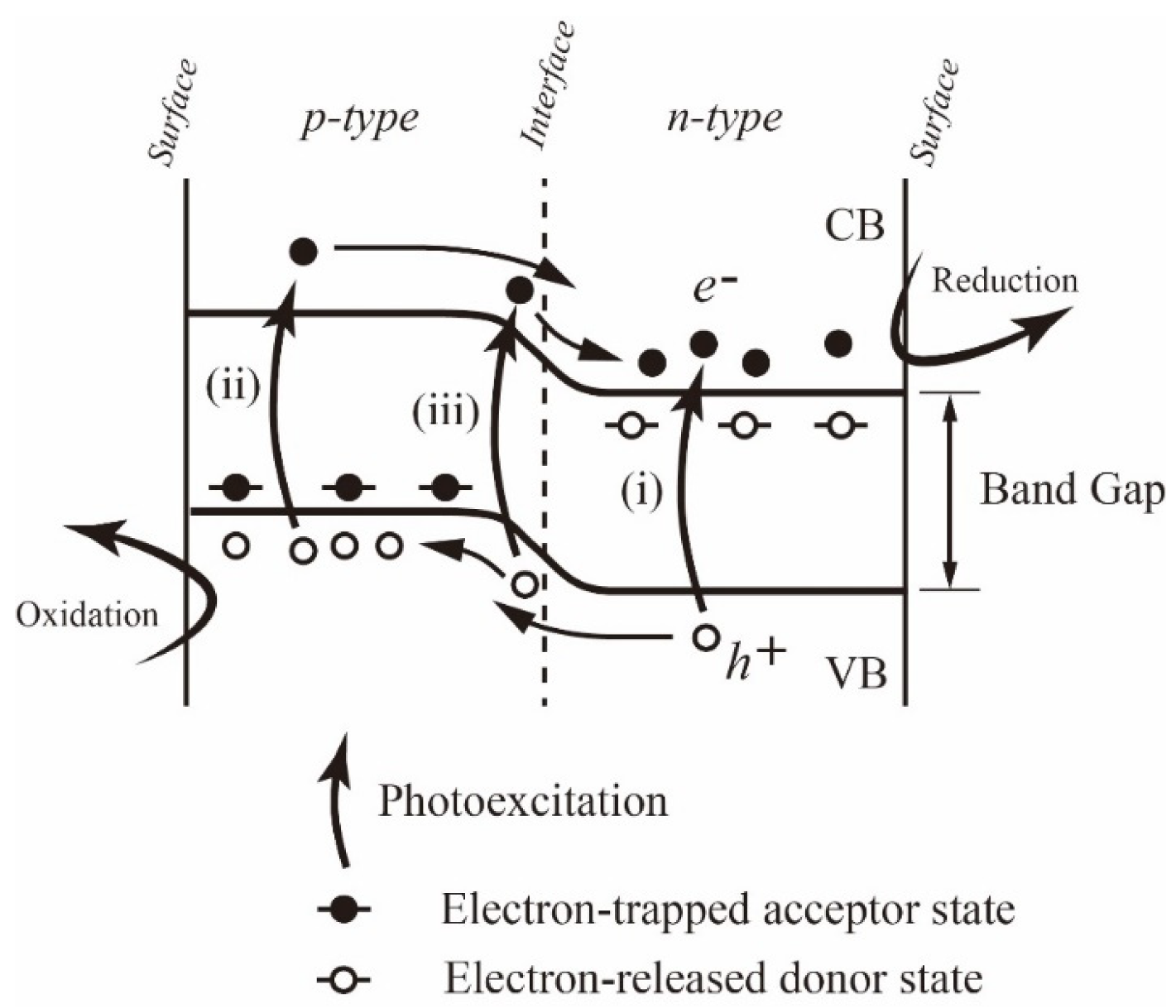
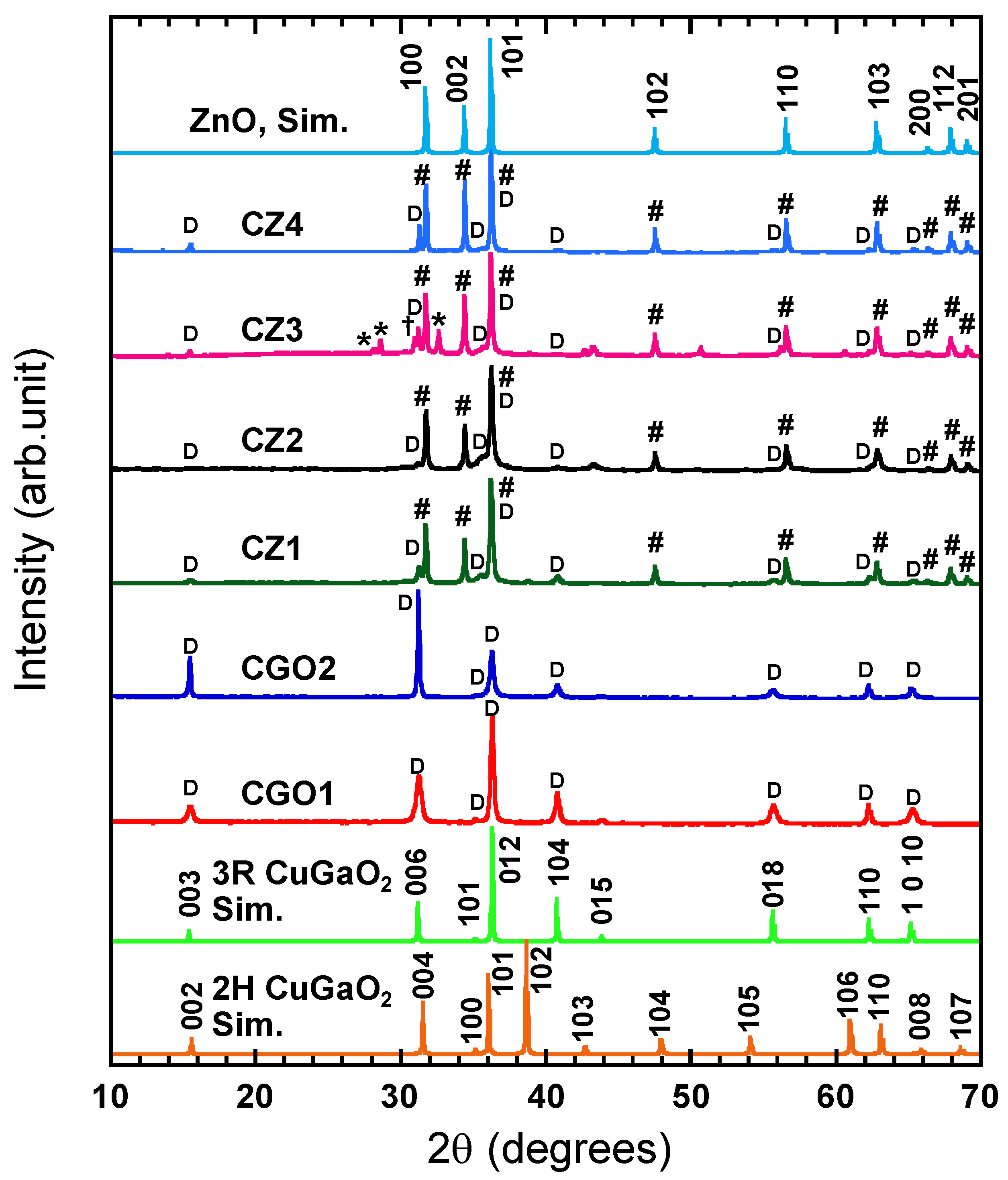
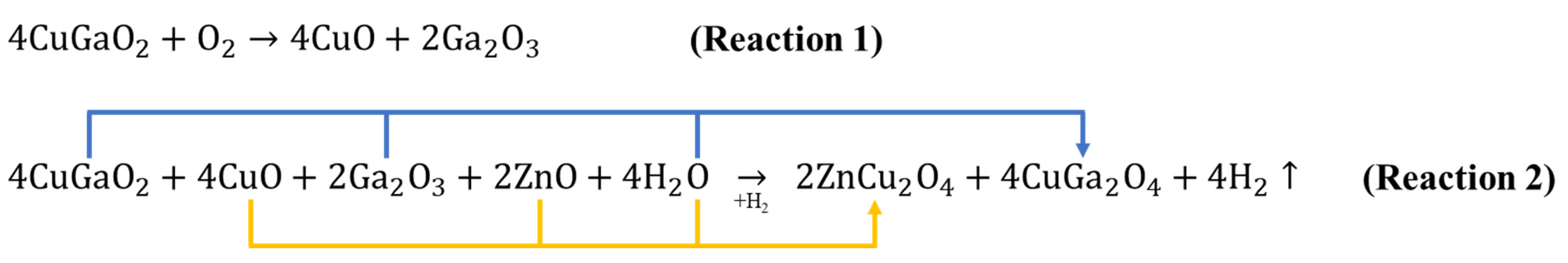
| Sample Name | CuGaO2 Used | Mass of Zn(ac)2·2H2O | Nominal [Zn]/[Cu] Ratio |
|---|---|---|---|
| CZ1 | CGO1 | 0.18 g | 2.75 |
| CZ2 | CGO1 | 0.36 g | 5.5 |
| CZ3 | CGO1 | 0.72 g | 11 |
| CZ4 | CGO2 | 1.08 g | 16.5 |
| Sample | XPS CL | Peak Position/eV | Width/eV |
|---|---|---|---|
| CGO2 | 932.5 | 1.2 | |
| 952.3 | 1.4 | ||
| 1117.8 | 1.7 | ||
| 1144.7 | 1.6 | ||
| 530.3 | 1.5 | ||
| CZ4 | 932.69 | 2.4 | |
| 952.6 | 2.3 | ||
| 1117.8 | 2.3 | ||
| 1144.8 | 2.4 | ||
| 1021.7 | 1.7 | ||
| 1044.8 | 1.8 | ||
| 530.3 | 1.8 |
| Peak Position/eV | Area (%) | Width/eV | Shape (=Lw/Gw) | Gw (%) | Lw (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CGO2 | ||||||||||
| Cu+ | 932.51 | 0.01 | 95.8 | 1.5 | 1.28 | 0.05 | 0.46 | 68.5 | 31.5 | |
| Cu2+ | 933.60 | Fixed | 4.2 | 1.1 | 0.97 | 0.22 | 8.0 × 103 | 0.0 | 100.0 | |
| Ga#1 | 1117.74 | 0.01 | 100.0 | - | 1.71 | 0.05 | 0.50 | 66.3 | 33.7 | |
| O#1 | 530.32 | 0.01 | 68.8 | 1.7 | 1.35 | 0.04 | 0.10 | 90.9 | 9.1 | |
| O#2 | 531.80 | 0.03 | 31.2 | 2.4 | 1.55 | 0.36 | 4.41 | 18.5 | 81.5 | |
| CZ4 | ||||||||||
| Cu+ | 932.44 | 0.22 | 42.6 | 14.5 | 1.72 | 0.82 | 0.00 | 99.9 | 0.1 | |
| Cu2+ | 933.60 | 0.18 | 58.4 | 16.1 | 1.71 | 1.09 | 4.24 | 16.4 | 81.6 | |
| Ga#1 | 1117.76 | 0.02 | 90.7 | 9.5 | 2.41 | 0.34 | 0.19 | 84.0 | 16.0 | |
| Ga#2 | 1117.98 | 0.05 | 9.3 | 7.7 | 0.84 | 0.39 | 1.66 × 103 | 0.1 | 98.9 | |
| O#1 | 530.36 | 0.01 | 64.8 | 2.1 | 1.40 | 0.11 | 0.33 | 75.4 | 24.6 | |
| O#2 | 531.78 | 0.04 | 35.2 | 3.0 | 1.77 | 0.33 | 37.3 | 2.6 | 97.4 | |
| Zn#1 | 1021.51 | 0.04 | 79.5 | 10.3 | 1.49 | 0.14 | 0.66 | 60.4 | 39.6 | |
| Zn#2 | 1022.31 | 0.29 | 20.5 | 10.2 | 1.64 | 0.39 | 3.8 × 10−4 | 100.0 | 0.0 | |
| Substance | /eV | Cu LMM/eV | /eV | References |
|---|---|---|---|---|
| CGO2 | 932.5 | 916.6 | 1849.1 | This work |
| Cu(metal) | 932.7 | 918.4 | 1851.1 | [55] |
| Cu2O | 932.4 | 916.5 | 1848.9 | [55,57,58] |
| CuO | 933.6 | 917.8 | 1851.4 | [55,57] |
| Cu(OH)2 | 934.7 | 916.2 | 1850.9 | [58] |
| Substance | /eV | Ga LMM/eV | /eV | References |
|---|---|---|---|---|
| CGO2 | 1117.8 | 1090.9 | 2208.7 | this work |
| CZ4 | 1117.8 | 1090.3 | 2208.1 | this work |
| Ga(metal) | 1116.5 | 1068.0 | 2184.5 | [59,60] |
| Ga2O3 | 1117.8 | 1062.6 | 2180.4 | [51,60] |
| Substance | /eV | Zn LMM/eV | /eV | References |
|---|---|---|---|---|
| CZ4 | 1021.7 | 988.4 | 2010.1 | this work |
| Zn(metal) | 1021.8 | 992.1 | 2013.9 | [56,57] |
| ZnO | 1022.1 | 989.4 | 2010.3 | [57,62] |
| CuZn(alloy) | 1021.5 | 992.4 | 2013.9 | [57] |
| AZO(4at%Al) | 1022.0 | 987.3 | 2009.4 | [63] |
| AZO(2at%Al) on Cu2O | 1021.8 | 988.2 | 2010.0 | [64] |
| AZO(2at%Al) on CuO | 1022.2 | 987.9 | 2010.1 | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.; Brabec, C.; Hayakawa, T. X-ray Diffraction, Micro-Raman and X-ray Photoemission Spectroscopic Investigations for Hydrothermally Obtained Hybrid Compounds of Delafossite CuGaO2 and Wurtzite ZnO. Ceramics 2022, 5, 655-672. https://doi.org/10.3390/ceramics5040048
Choi M, Brabec C, Hayakawa T. X-ray Diffraction, Micro-Raman and X-ray Photoemission Spectroscopic Investigations for Hydrothermally Obtained Hybrid Compounds of Delafossite CuGaO2 and Wurtzite ZnO. Ceramics. 2022; 5(4):655-672. https://doi.org/10.3390/ceramics5040048
Chicago/Turabian StyleChoi, Minuk, Christoph Brabec, and Tomokatsu Hayakawa. 2022. "X-ray Diffraction, Micro-Raman and X-ray Photoemission Spectroscopic Investigations for Hydrothermally Obtained Hybrid Compounds of Delafossite CuGaO2 and Wurtzite ZnO" Ceramics 5, no. 4: 655-672. https://doi.org/10.3390/ceramics5040048
APA StyleChoi, M., Brabec, C., & Hayakawa, T. (2022). X-ray Diffraction, Micro-Raman and X-ray Photoemission Spectroscopic Investigations for Hydrothermally Obtained Hybrid Compounds of Delafossite CuGaO2 and Wurtzite ZnO. Ceramics, 5(4), 655-672. https://doi.org/10.3390/ceramics5040048






