Ecofriendly High NIR Reflectance Ceramic Pigments Based on Rare Earths Compared with Classical Chromophores Prepared by DPC Method
Abstract
1. Introduction
- -
- Category A deals with pigments suspended in glass matrixes which require the highest degree of heat stability and chemical resistance to withstand the attack of molten glass.
- -
- Category B deals with pigments suspended in plastics and other polymers, which require only moderate heat stability.
- -
- Category C deals with pigments suspended in liquid vehicles, which require little, or no heat stability.
1.1. Cool Pigments
- (a)
- Absence of absorbent ions in the NIR range (700–2500 nm.): e.g., Co2+ in a tetrahedral environment shows absorbance in the 1200–1600 nm (associated to the d-d transitions bands 4A2(F) → 4T1(F) at 1400 nm and 4A2(F) → 4T2(F) at 1600 nm), avoiding NIR reflectance in this NIR range, but an alternative for the blue colour in glazes is complicated [10].
- (b)
- Therefore, a good cool pigment shows semiconductor behaviour with high band gap: e.g., TiO2 (2.4 eV for anatase polymorph) or CdS, ZnO and SrTiO3 (3.4 eV). They show intense charge transfer bands located at the UV region that block UV radiation, which provides protection to the organic binders.
- (c)
- High thermal emittance. A good cool pigment shows the ability to release heat that it has absorbed and then its temperature decreases [11]. The emittance index of solids is high (e.g., ceramics 0.9, asphalt 0.88), but it is low for conductive metals (e.g., Ag 0.02, Al 0.03, Cu 0.04).
- (a)
- High refractive index that allows for achieving high scattering efficiency of light when the pigment is added to a matrix with a very different refractive index. The scattering efficiency increases with the difference of the refractive index pigment-medium. For example, TiO2 (anatase and rutile polymorphs) shows a high refractive index (2.55 and 2.73 respectively), therefore, the pigments based on rutile structure such as the yellow-orange of Cr-Sb rutile or yellow of Ni-Sb rutile are two of the best classical cool pigments. Related pigments such as Cr-pseudobrookite (Cr-Fe2TiO5) [12], Cr-armalcolite (Cr-(MgFe2Ti3O5) [13] or Ni-geikielite (Ni-MgTiO3) [14] based on titanates also show high NIR reflectance.
- (b)
- For an efficient scattering efficiency of light, the particle size should be about half the wavelength of the light; for NIR light (700–2500 nm), the particle diameter should be between 350–1250 nm. The particle shape and packing status are other important factors, in addition to the particle size. It is important to note that in ceramic applications, the particle size should be the minimum to avoid the attack of the media (glazes or ceramic stoneware); its size is between 0.1–5 µm and small particles can be dissolved by the melting glazes, decreasing the tinting strength of the pigment, and big particles decrease the density of the colour centres with the same result.
1.2. Rare Earths and Cool Pigments
- (a)
- For blue pigments, Smith et al. [20,21] synthesized by ceramic route at 1200 °C the solid solution Mn-YInO3 with perovskite structure (YIn0.8Mn0.2O3 powder shows L*a*b* = 34.1/11.7/−44.4 and R = 34%, and 20% in vinyl paint 77.9/−2.9/−19.3 with RNIR = 62%). Sheethu et al. and Zhang et al. [22,23] describe blue pigments based on the solid solutions of rare earths in wesselsite SrCuSi4O10 (Sr0.3La0.7Cu0.3Li0.7Si4O10 powder, which shows L*a*b* = 49.6/10.4/−51.7 and RNIR = 66% obtained by the ceramic route at 950 °C/16 h, whereas Sr0.8Eu0.2Cu0.3Si4O10 shows L*a*b* = 46.6/−4.6/−28.1 and RNIR = 72.3 synthesized by the citrate route at 900 °C/2 h).
- (b)
- For magenta-brown shades, García et al. and Llusar et al. [18,19] describe the application of brown-red pigments based on Ce1−xMxO2 solid solutions (M = Pr,Tb) showing RNIR = 80% for x = 0.05 in glazes. Vishnu et al. [24] report brown pigments by solid state reaction at 1500 °C/18 h based on the solid solution Pr-Y2Ce2O7 with fluorite structure (Y2Ce1.7Pr0.3O7 powder shows L*a*b* = 32.6/18.1/12 and RNIR = 58%) and yellow pigments from Mo-Y2Ce2O7 solid solutions (Y2Ce1.5Mo0.5O7 powder shows L*a*b* = 90.2/−4.5/62.4 and RNIR = 81%); later, Raj et al. [25] report a brown pigment doping with Tb as the same structure. Jovani et al. [26] report a brown pigment based on Y2−xTbxZr2−yFeyO7−δ (L*a*b* = 64/34/33 for 4 wt.% addition in a conventional frit of powder x = 0.35).
- (c)
- For yellow pigments, Gargori et al. [27] report the preparation of a yellow pigment based on the codoped V5+,Ca2+-Y2Sn2O7 pyrochlore (CaxY2−xVySn2−yO7) solid solutions at 1200 °C/6 h (the powder x = y = 0.16 shows L*a*b*= 85.7/1.1/34 and 5 wt.% enamelled in a double firing frit at 1000 °C shows L*a*b* = 79.3/2.6/35 with RNIR = 81%). Liu et al. [28] develop by citrate route at 700 °C/6 h high NIR reflectance yellow pigments based on Al-LaFeO3 (LaAlxFe1−xO3) perovskite solid solutions; at low x the colour is brown and at x > 0.7 it is yellow (L*a*b* = 71.2/2.8/10.0 with RNIR = 57%). Although the authors do not evaluate the NIR reflectance, the (Ca1−x−yEuxZny)2Al2SiO7+δ (0 ≤ x ≤ 0.15; 0 ≤ y ≤ 0.07) solid solutions with gehlenite structure (Ca2Al2SiO7) show high NIR reflectance, and (Ca0.87Eu0.10Zn0.03)2Al2SiO7+δ pigment exhibited the most vivid yellow colour (L*a*b* = 74/0/76) [29]. The origin of the yellow colour is the 4f-5d transition of divalent Eu2+ ion, and codoping with Zn enhances the yellowness by increasing the Eu2+ amount in the samples. Schildhammer et al. report a yellow pigment based on Yb6Mo2O15 crystallizing in a trigonal structure by ceramic method at 1350 °C/20 h with the colour coordinates L*a*b* = 88.7/6.6/55 and RNIR = 94%. Colour variation from pale yellow to brick-red was realized by partial substitution of Yb3+ or Mo6+ with various metal ions [30].
- (d)
- Obtaining black pigments based on rare earths as chromophores is very complicated given that the origin of the colour, as discussed above, is based on 4f-5d transitions or on the generation of additional charge transfer bands induced by entrance of rare earths ions in the network, because the absorptions associated with 4f transitions are sharp and weak. Thus, rare earth chromophores only absorb in the blue-green range producing yellow or brown colours; blues are due to traditional chromophores in the rare earth network such as Mn3+ in trigonal bipyramid [20,21], which could be obtained in other free-rare earth structures [31], or to weakly allowed bands associated to Cu2+ [22,23] that connect with ancient pigments such as the Egyptian Blue Family [32,33]. Therefore, to obtain a whole absorbent pigment of the visible range is virtually impossible using rare earths as chromophores, but there are black or dark brown pigments involving rare earth lattices: Gargori et al. describe a black pigment based on perovskite CrNdO3, and a green pigment that turns into black when it is modified by fluorides [14], probably associated to the high concentration of vacancies introduced by the dopants [34] (adding 2 wt.% BaF2 and 8 wt.% MgF2 at 1100 °C/3 h the powder shows L*a*b* = 43.4/2.4/4.0 but low RNIR = 4%). Likewise, the undoped YInO3 shows a black colour although the authors [20,21] only measure the NIR reflectance of the Mn3+ doped blue pigment.
1.3. Dry Powder Coating (DPC) Method
2. Materials and Methods
2.1. Samples Preparation
- (a)
- (b)
- Red-brown of Pr-CeO2 cerianite (fluorite structure, cubic, Space Group Fm3m) [18]. In total, 50 g of the composition Ce0.95Pr0.05O2 was prepared from oxides (Pr6O11, CeO2).
- (c)
- Yellow of Mo-Y2Ce2O7 yttrium cerate (fluorite structure, cubic, Space Group Fm3m) [24]. A total of 50 g of the composition Y2Ce1.5Mo0.5O7 was prepared from (NH4)2MoO4 and oxides (Y2O3, CeO2).
- (d)
- Black-brown of Sr4Mn2CuO9 (hexagonal perovskite, Space Group P321) [37]. The structure of this pigment consists of octahedra and trigonal prisms that form a chain structure by face-sharing. The Mn4+ ions occupy octahedral sites and Cu2+ ions randomly fill the centre of the square faces of the trigonal prisms. A total of 50 g of the composition Sr4Mn2CuO9 were prepared from carbonates (SrCO3) and oxides (MnO2, CuO).
- (a’)
- Blue of Co-Zn2SiO4 willemite-decorated ZnO (trigonal, Space Group R-3) [38]. A total of 50 g of the composition [Co0.6Zn1.4SiO4]·0.2ZnO was prepared from oxides (Co3O4, ZnO, quartz SiO2).
- (b’)
- Red-brown of Cr-ZnFe2O4 Franklinite-decorated ZnO (CPMA number 13-37-7, spinel, cubic, Space Group Fd3m) [39]. In total, 50 g of the composition [ZnFe1.8Cr0.2O4]. ZnO was prepared from oxides (Cr2O3, Fe2O3 hematite, ZnO).
- (c’)
- Yellow of Ni,Sb-TiO2-decorated TiO2 (CPMA number 11-15-4, tetragonal, Space Group P42/mnm) [40]. A total of 50 g of the composition [Ni0.25Sb0.25Ti0.5O2]. TiO2 was prepared from oxides (Sb2O3, TiO2 anatase, NiO).
- (d’)
2.2. Samples Characterization
2.3. Application of Pigments
3. Aims
4. Results and Discussion
4.1. X-ray Diffraction (XRD) and SEM-EDX Characterization
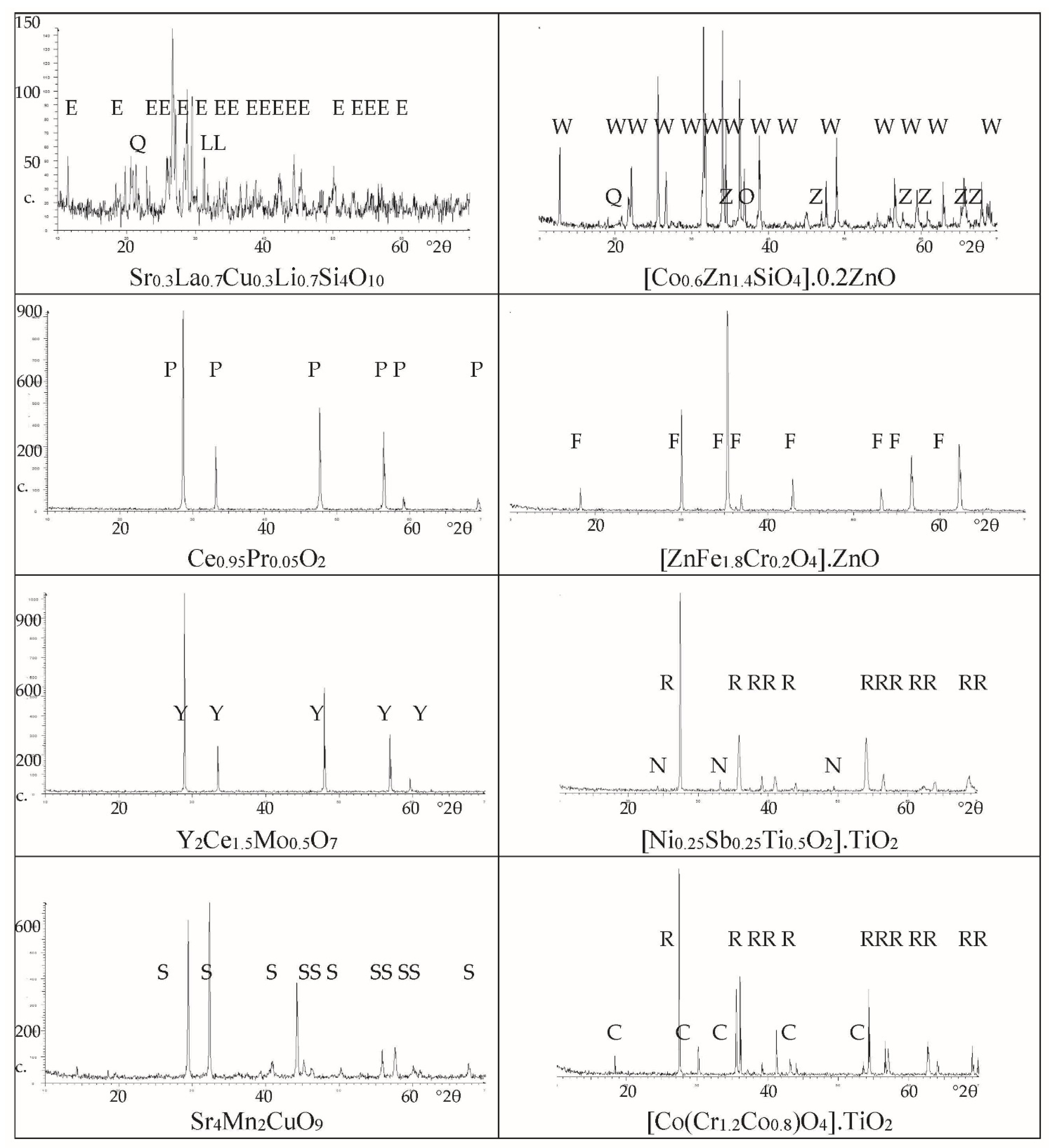
- (a)
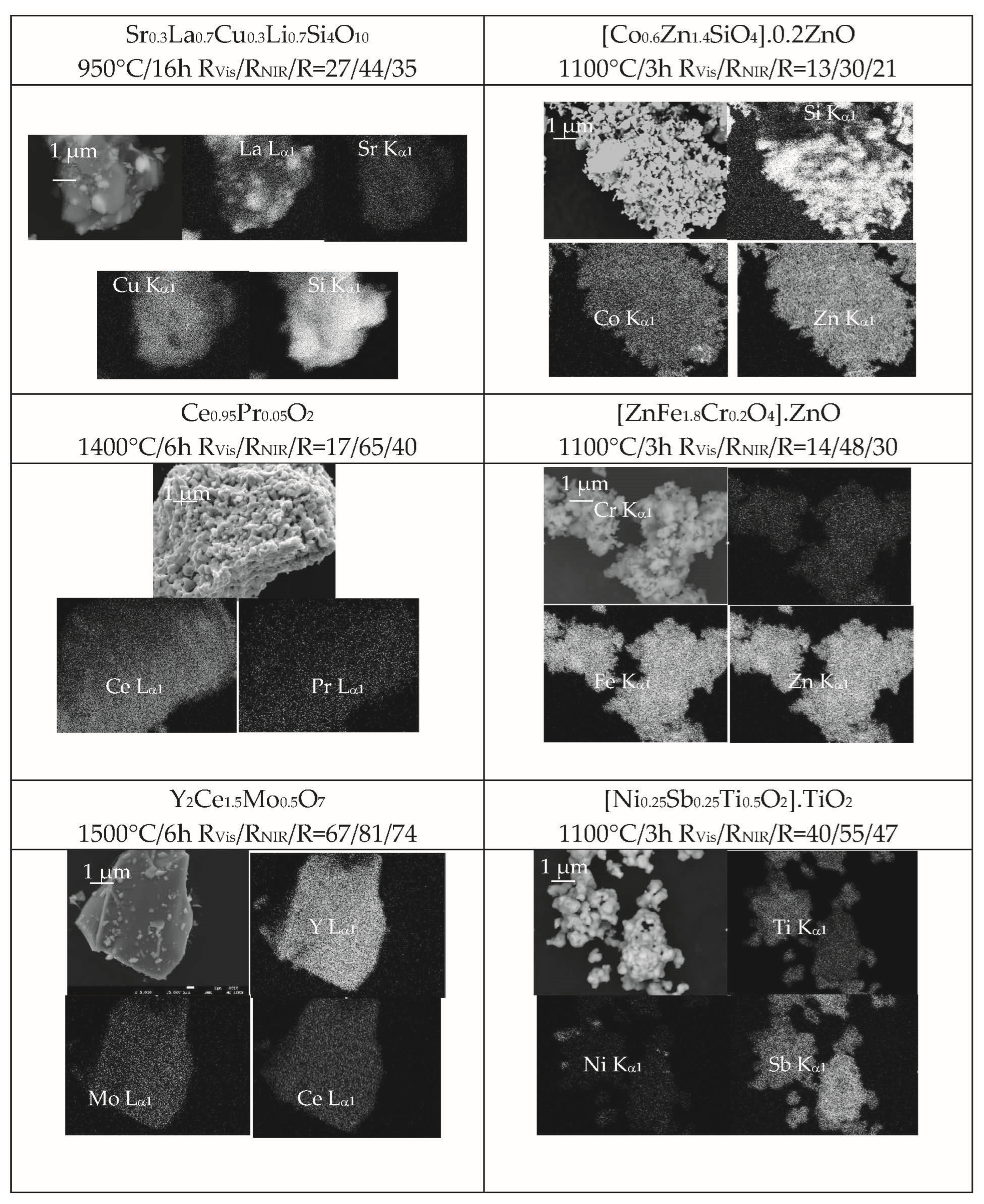
- In the case of blue pigments, XRD shows the crystallization of wesselsite SrCuSi4O10 (E in Figure 2) as the main crystalline phase, although several weak peaks associated to residual quartz (Q) and La2O3 (L) are detected. For the classical Co-willemite-decorated ZnO, willemite and ZnO are the main phases, but weak XRD peaks of unreacted quartz and Co3O4 are also detected. The SEM images (Figure 3) show that the La,Li-wesselsite blue powder presents aggregates of 10 μm of mean size highly sintered, and are sometimes decorated by little orthohedrons with particle sizes between 0.2–1 μm. The microstructure of Co-willemite blue pigment is homogeneous, showing aggregates between 5–25 μm integrated with fine spherical particles of around 1 μm of particle size. The SEM-EDX results in Figure 4 are in agreement with XRD observations: the La Lα1 signal show the presence of unreacted lanthanum oxide in the La,Li-wesselsite blue powder, whereas unreacted quartz along with a little Co inhomogeneity are also detected in the mapping of the Co-willemite powder.
- (b)
- Regarding red-brown pigments, cerianite (P) is the only crystalline phase detected for Pr-cerianite red-brown pigment, and franklinite (F) along ZnO (Z) in the case of Cr-franklinite ZnO decorated pigment (Figure 2). The microstructure of Pr-cerianite pigment is homogeneous, showing aggregates between 1–10 μm in size, integrated by homogeneous and finer particles of 0.5 μm in particle size (Figure 3); similarly, the Cr-franklinite powders show high homogeneity with aggregates between 0.5–3 μm, constituted by fine spherical particles of 0.3 μm of particle size. The SEM-EDX results in Figure 4 agree with the above observations, and all cation EDX signals are homogeneous in the corresponding composition mappings.
- (c)
- As for the yellow pigments, the yttrium cerate Y2Ce2O7 is the single crystalline phase (Y) detected by XRD (Figure 2). In the case of Ni,Sb-TiO2 decorated rutile, only very weak peaks of residual NiTiO3 (N) are detected accompanying the main rutile phase (R). Highly sintered particles 10–50 μm-sized are observed by SEM in the case of the yellow of Mo-yttrium cerate pigment (Figure 3), whereas in the case of Ni,Sb-rutile, the microstructure consists of aggregates between 1–5 μm-sized, integrated by finer spherical particles of 0.2–2 μm in particle size. The SEM-EDX results in Figure 4 correlate well with the above observations, since all cation EDX signals are homogeneous in the case of Mo-yttrium cerate yellow pigment, whereas the Ni Kα1 signal show very scarce inhomogeneities associated with the presence of residual NiTiO3 detected by XRD.
- (d)
- Finally, concerning the studied black pigments, the hexagonal perovskite Sr4Mn2CuO9 (S) is the only crystalline phase detected for the Sr4Mn2CuO9 black-brown pigment (Figure 2). Co-chromite CoCr2O4 (C) and intense peaks of rutile (R) are detected in the case of Co(Cr,Co)2O4-decorated rutile black-green pigment. Regarding morphology, homogeneous microstructures are observed by SEM images (Figure 3) in both cases; the Sr4Mn2CuO9 black-brown pigment consists of aggregates between 2–10 μm-sized integrated by fine spherical particles of 1 μm. In the case of the Co(Cr,Co)2O4-decorated rutile black-green pigment, fine aggregates between 1–5 μm-sized are observed with two kinds of particles: i.e., cubic particles of 1 μm and spherical particles of 0.2 μm. The SEM-EDX results in Figure 4 agree with the above observations, and all cation EDX signals are homogeneous in the case of Sr4Mn2CuO9 black-brown pigment, whereas the Ti Kα1 signal corroborates the presence of a rutile phase associated with the largest cubic particles (around 1 μm) observed in SEM images in the case of the Co(Cr,Co)2O4- rutile decorated black- green pigment.
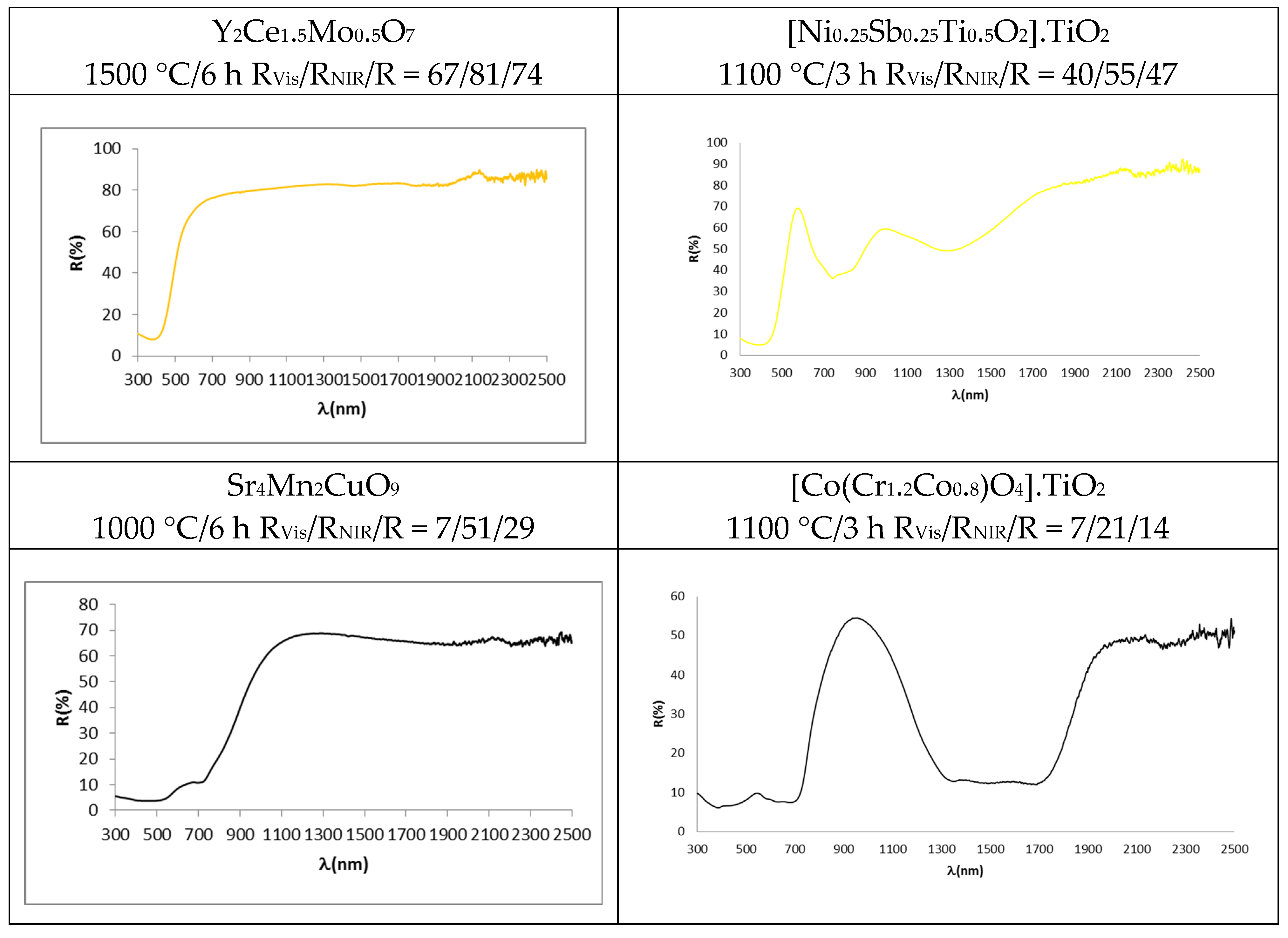
4.2. UV-Vis-NIR Reflectance Spectra
- (a)
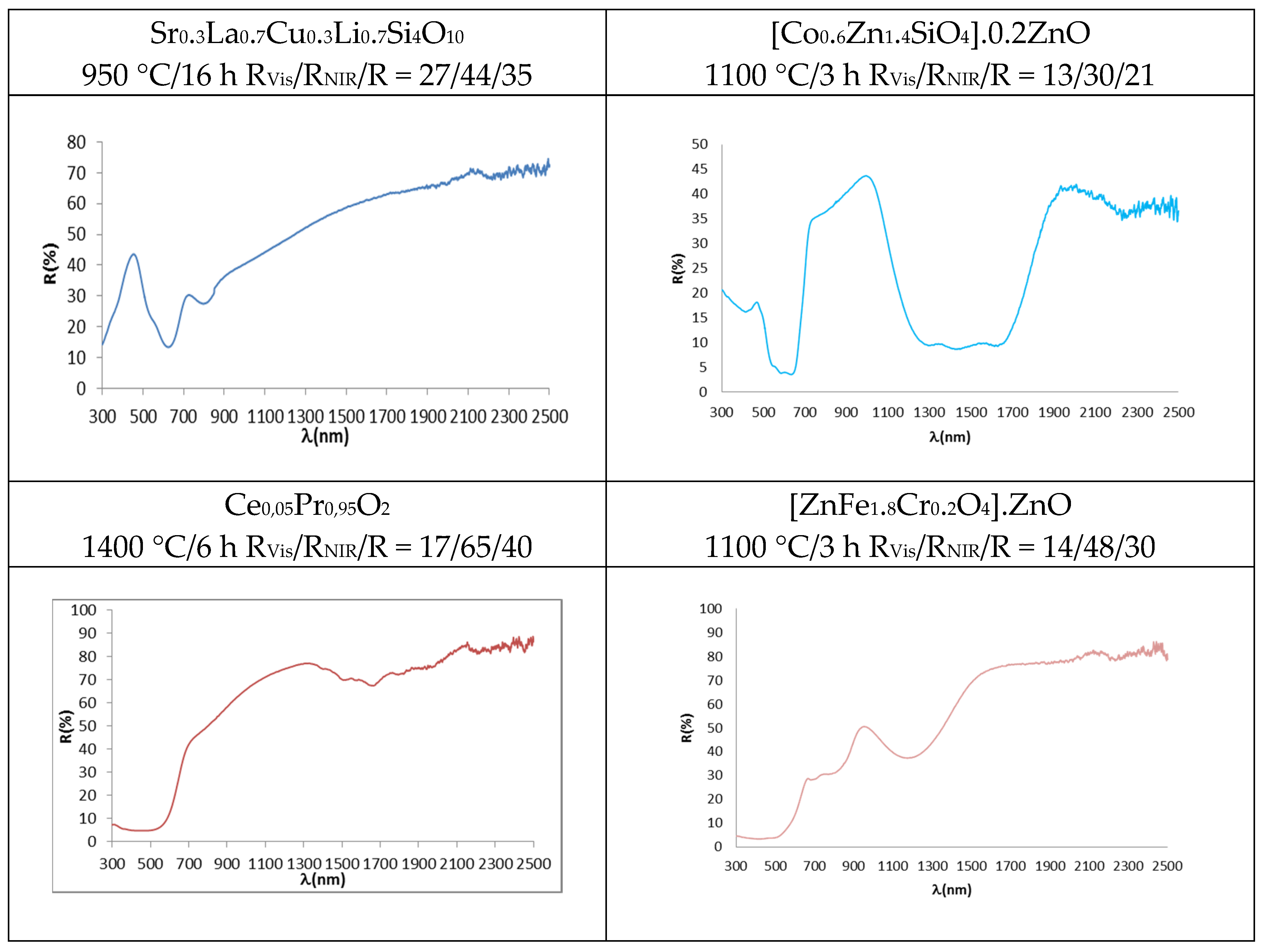
- In the case of blue pigments, the structure of La,Li-SrCuSi4O10 wesselsite is composed of rings of four SiO4 tetrahedra which are linked together by square-planar coordinated copper, thus forming double copper silicate layers which are joined by alkali-earth cations in a distorted cubic geometry. In the corresponding optical spectrum (Figure 5), three broad transitions are observed in the visible region originated from weakly-allowed d-d transitions of copper (despite a symmetry close to D4h at the copper centre) due to vibronic coupling. The three transitions, observed as minimums of reflectance, occur at 800 nm (2B1g → 2B2g), 630 nm (2B1g → 2Eg) and 540 nm (2B1g → 2A1g) [32,33]. As for the classical Co-Zn2SiO4 willemite, its structure is an orthosilicate with all atoms in general position and composed of a framework of tetrahedra accommodating zinc and silicon in three different fourfold crystallographic sites; a deep blue colour is obtained by doping the willemite structure with cobalt, which replaces zinc ions in tetrahedral positions. The advantage of willemite pigments comes from the lower amount of Co needed to obtain suitably saturated blue hues with respect to spinel CoAl2O4 or olivine Co2SiO4; moreover, the use of an excess of ZnO protects the pigment from the attack of ZnO-rich glazes [44]. The blue colour is associated with Co2+ in tetrahedral coordination environment, which shows a strong absorbance with three bands at 540, 590 and 640 nm (for the colour) associated with the splitting of 4A2(F) → 4T1(P) transition due to the Jahn-Teller distortion. An additional strong and broad absorption appears at 1200–1600 nm (due to the coupling of 4A2(F) → 4T1(F) and 4A2(F) → 4T2(F) d-d transitions bands at 1400 nm and 1600 nm, respectively), which hinders NIR reflectance in this range [10,46].
- (b)
- Regarding the red-brown pigments, it has been discussed above that Pr4+ doping in Pr-CeO2 introduces an additional conduction band, and its associated charge transference band absorbs from 610 nm, showing a semiconductor feature with a band gap of 2.0 eV calculated using the Tauc procedure (direct semiconductor) (Figure 5) [18,19,43]. In the case of Cr-franklinite, Cr3+ enters, replacing Fe3+ in the normal spinel ZnFe2O4, and the following absorption bands are detected in the UV-Vis-NIR reflectance spectra of fired powders: (a) intense bands at 300–520 nm, (b) weak band centred at 680 nm, (c) medium band at 780 nm and (d) intense band at 1200 nm. It is difficult to distinguish between the presence of Cr3+ or Fe3+ ions in octahedral sites in the UV-Vis-NIR spectra of samples containing simultaneously both ions in these positions, because its bands are overlapped to a high extent. However, the main characteristic of Fe3+ ions in octahedral sites is the strong absorption associated with the transitions 6A1g(S) → 4A1g,4E(G), which overlaps with the charge transference band at 400–500 nm that dominates the absorption spectrum of the powders, and the band gap associated to this charge transference band is 1.9 eV (Figure 5). The bands at around 770 and 1200 nm are typical characteristics of the zinc ferrite, which are attributed to 6A1g(S) → 4T2g(G) and 6A1g(S) → 4T1g(S) for d-d electron transition of Fe3+ [47,48]. These Fe3+ bands overlap with those of Cr3+ in octahedral coordination, namely: (a) three main parity-forbidden transitions (4A2(4F) → 4T2(4F) at 570 nm and 4A2(4F) → 4T1(4F) at 445 nm, which are partially overlapped, and 4A2(4F) → 4T2(4P) at 235 nm, which overlaps with Cr3+-O2− charge transfer), (b) two weak Cr3+ spin-forbidden transitions (4A2(4F) → 2T1(2G) and 4A2(4F) → 2E(2G) that overlap [49,50].
- (c)
- As for the yellow pigments, in the case of Mo-Y2Ce2O7 solid solutions, the doping of Mo6+ replacing Ce4+ in Y2Ce2O7 needs the formation of cation vacancies (Y2MoxCe2−xO7+x) in order to satisfy the lattice charge balance [24]; Mo6+ doping introduces an additional conduction band and its associated charge transference band O2p–Mo4d absorbs from 505 nm (band gap 2.5 eV, Figure 5), inducing a yellow colour. All wavelengths above 505 nm are practically reflected. In the case of Ni,Sb-TiO2 decorated rutile yellow pigment, the following spin-allowed bands of Ni2+ in octahedral coordination can be observed in Figure 5: 3A2g(3F) → 3T2g(3F) at 1200 nm, 3A2g(3F) → 3T1g(3F) at 800 nm (which overlaps with other spin-forbidden bands) and 3A2g(3F) → 3T1g(3P) at 450 nm (which overlaps with Ni2+-O2− band transfer with a band gap associated using the Tauc plot of 2.3 eV (direct type) [40,43].
- (d)
- Concerning black pigments, in the case of the Sr4Mn2CuO9 black-brown pigment, the broad optical absorption band at the shorter wavelength (400 nm) is attributed to the partial overlap of charge transfer transition and 4A2g → 4T1g d-d transition of Mn4+ in octahedral coordination (the band gap associated of this band is 1.2 eV, Figure 5). Weak transitions from weakly-allowed d-d transitions of copper, that fill the centre of the square faces of the trigonal prisms as described above, can be observed as minimums of reflectance at a at 800 nm (2B1g → 2B2g, very weak band), 715 nm (2B1g → 2Eg) and 540 nm (2B1g → 2A1g) [32,33], but the band at 715 nm is associated by Bae at al. [37] to the 2T2 → 2E transition of Cu2+ in a trigonally disordered tetrahedral crystal field [51]. In the case of the Co,Cr-decorated rutile black-green pigment, the absorption bands of Co2+ in tetrahedral coordination and Co2+ in octahedral coordination in the spinel are overlapped, and the following absorption bands are detected: a charge transfer band at 350 nm, a band at 450 nm (associated to 4A2(4F) → 4T1(4F) of Cr3+ in octahedral coordination), a strong absorbance with three bands at 540, 590 and 640 nm (associated to the splitting of 4A2(F) → 4T1(P) transition of Co2+ in tetrahedral coordination, due to Jahn-Teller distortion), and a strong absorption at a 1200–1600 nm range (due to the coupling of 4A2(F) → 4T1(F) and 4A2(F) → 4T2(F) d-d transitions bands in tetrahedral Co2+) [46,49].
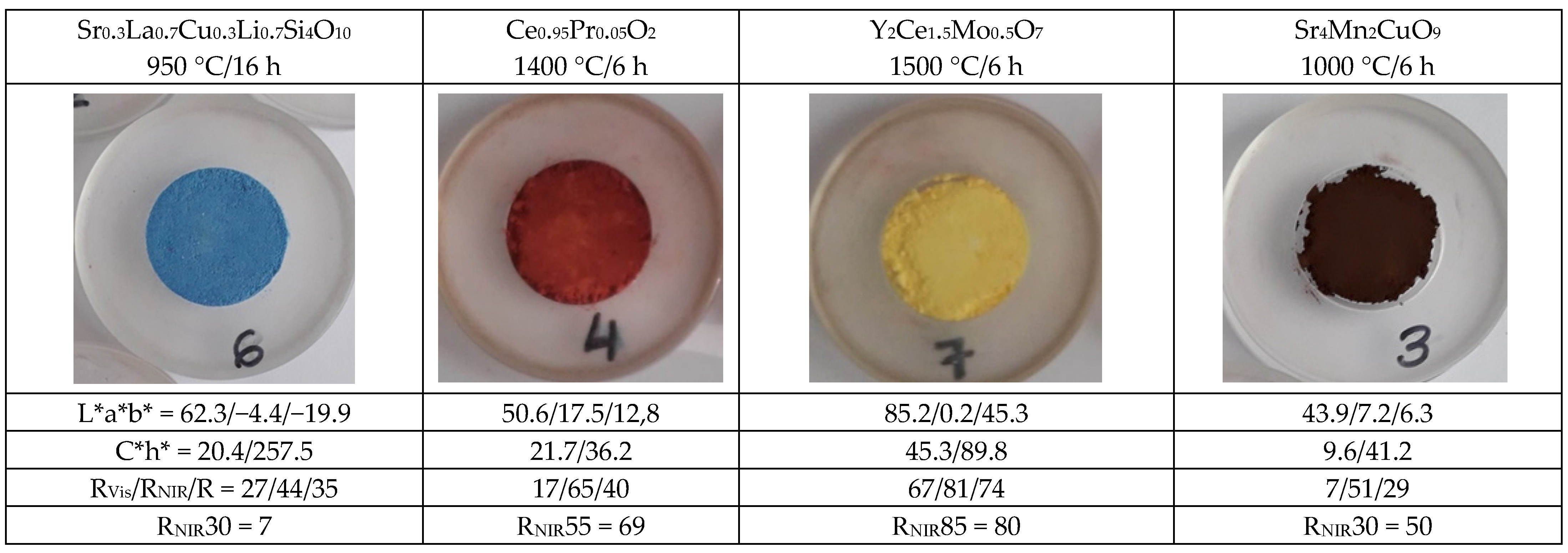
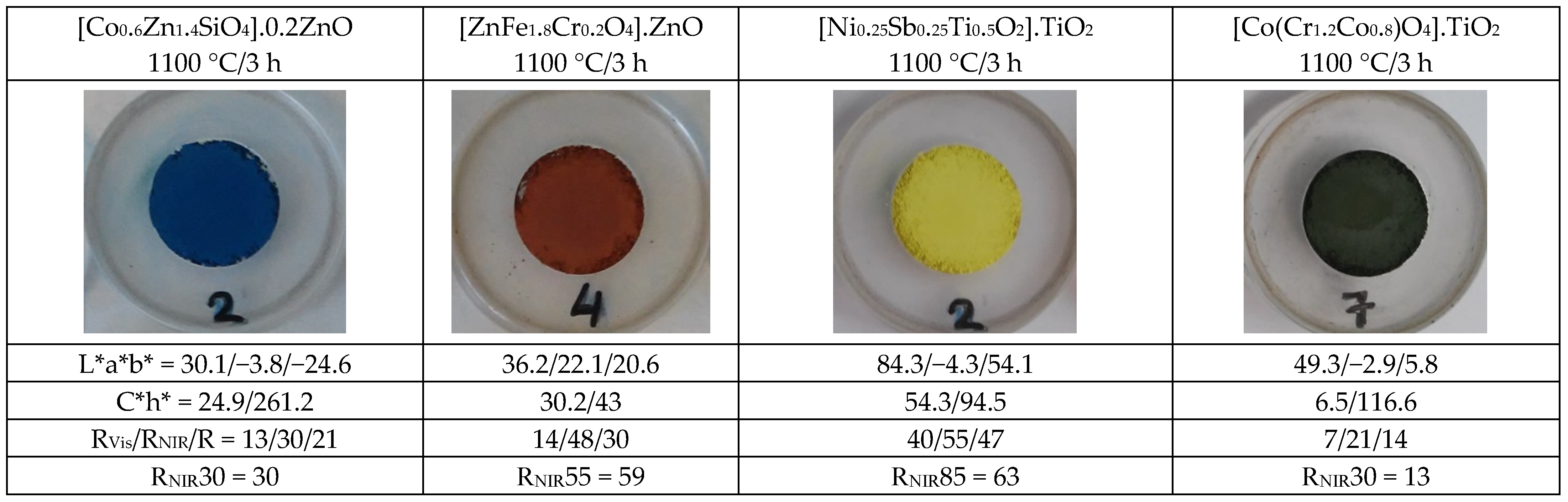
4.3. Colour, Colorimetry (L*a*b* and C*h*) and Reflectance Parameters (RVis/RNIR/R) of the Powders
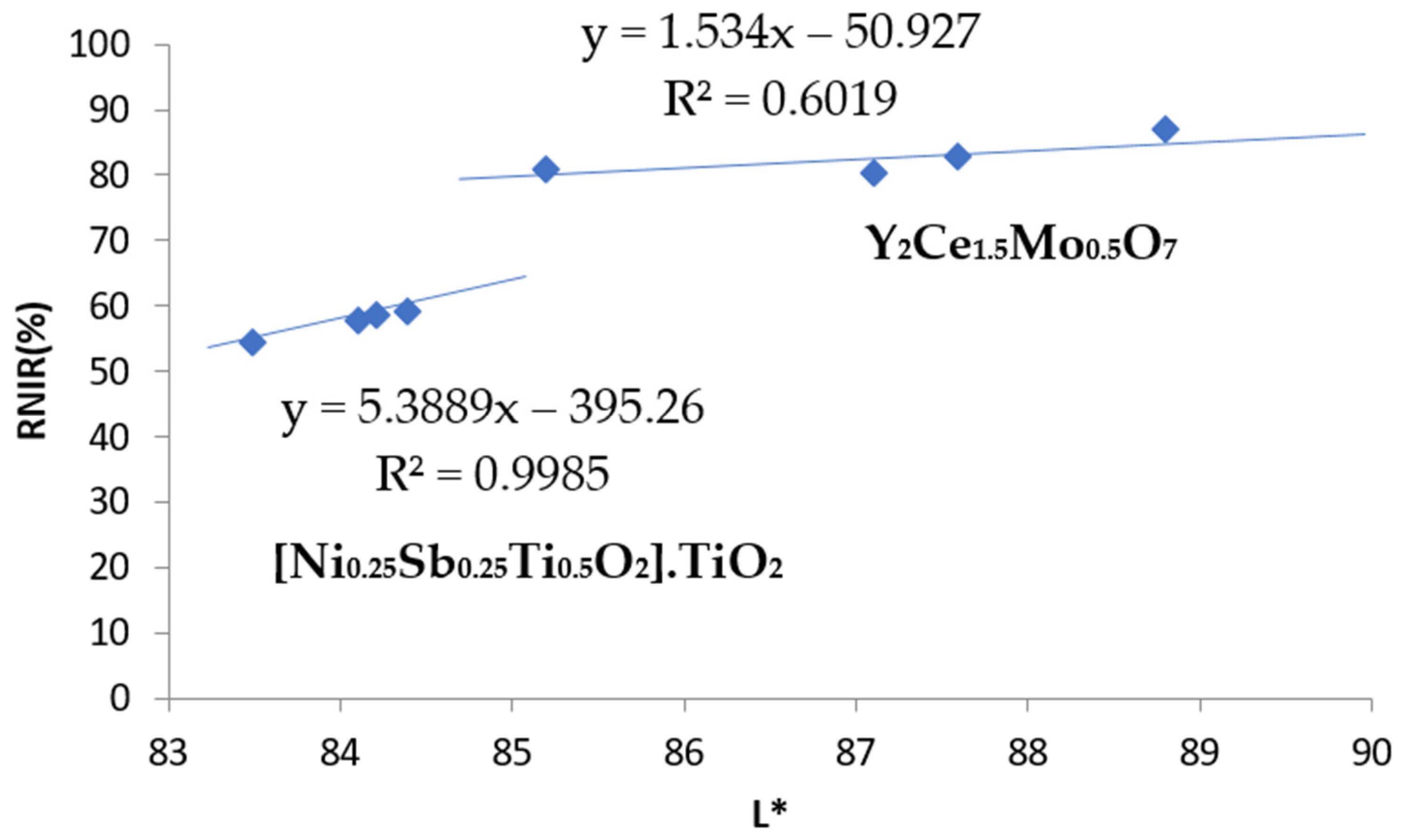
- (a)
- As for blue pigments, the chroma C* is higher for the classical Co-willemite, and in both cases, it is very similar to its b* value. The lightness L* of the rare earth pigment is high and falls out of the Munsell blue range (L* = 25–35), but its RNIR is higher. For the comparison of the performance as cool pigments, CaCO3 was added as binder to the pigments and the evolution of colorimetry and reflectance data are shown in Table 2; likewise, the representation of RNIR versus lightness L* of the blue pigments with gradual addition of the binder CaCO3 is presented in Figure 7. According to Figure 7, it is observed that rare earths pigments always show higher L* and RNIR values, and the values of both pigments (classical and rare earth-based) follow a linear relationship (R2 value > 0.92). Therefore, the normalized RNIR30 value (reflectance associated to a L* = 30), which is proposed for blue hue evaluation, may be estimated as 30% for willemite and 7% for wesselsite blue. Thus, the blue Co-willemite powder shows better performance as a cool pigment.
- (b)
- In the case of red-brown pigments, the chroma C* is higher for Cr-franklinite (C* = 30.2 vs. 21.7 for Pr-cerianite), being this value is closer to its absolute value of a*. The L* value is higher for Pr-cerianite (50.6 vs. 36.2 for Cr-franklinite) and shows higher RNIR (65% vs. 48% for Cr-franklinite). The evolution of colorimetry and reflectance data with the gradual addition of CaCO3 binder to the pigments are shown in Table 3 and the representation of RNIR versus lightness L* follows a linear relationship in Figure 8. The RNIR55 (reflectance associated to a L* = 55, considered for red hues) is 69% for rare-earth Pr-cerianite pigment and 59% for classical Cr-franklinite spinel pigment. Therefore red-brown Pr-cerianite powder shows better performance as a cool pigment.
- (c)
- Regarding yellow pigments, the chroma C* is higher for the Ni,Sb-rutile pigment (C* = 54.3 vs. 45.3 for Mo-cerate pigment), and the lightness L* is similar for both yellow pigments and included in the proposed yellow range (L* = 80–90). The Mo-cerate yellow powder shows higher RNIR (81% versus 55% for Ni,Sb-rutile). The evolution of colorimetry and reflectance data with the gradual addition of CaCO3 binder to the pigments are shown in Table 4 and the representation of RNIR versus lightness L* follows a linear relationship in Figure 9. The RNIR85 (reflectance associated to a L* = 85, proposed for yellow hues) is 80% for the Mo-cerate pigment and 63% for classical Ni,Sb-TiO2 decorated rutile pigment. Therefore Mo-cerate shows better performance as a cool pigment.
- (d)
- Concerning black pigments, from the data in Figure 6, the chroma C* is lower for chromite-rutile black-green (C* = 6.5 vs. 9.6 for Sr4Mn2CuO9), indicating a better black colour, and the hue is on the green range for this pigment (h* = 116.6), whereas the hue of black-brown Sr4Mn2CuO9 falls into the brown range (h* = 41.2). Lightness is low and similar for both dark pigments (44–50), although it is too high to be pure black pigment, since they do not enter into the range proposed above (L* = 30−0). Moreover, the Sr4Mn2CuO9 black-brown shows higher RNIR (51% versus 21% for chromite-rutile black-green). Finally, the evolution of colorimetry and reflectance data with the gradual addition of CaCO3 binder to the pigments are shown in Table 5, and the representation of RNIR versus lightness L* follows a linear relationship in Figure 10. The RNIR30 (reflectance associated to a L* = 30, considered for black hues) is 50% for Sr4Mn2CuO9 black-brown pigment and 13% for chromite-rutile black-green. Therefore, Sr4Mn2CuO9 shows better performance as cool pigment.
4.4. Application of the Pigments: Stability and Colour Yield in Different Media
- (a)
- As for the blue pigments, the application of La,Li-SrCuSi4O10 wesselsite develops pale blue hues in alkyd paint (b* = −3.0) and in double firing frit (b* = −5.6), but it is colourless in porcelain powder application. In agreement with the colour, the UV-Vis-NIR spectra of the applications (Figure 11) present similar features to the powder spectrum in the case of alkyd paint, indicating its stability in the paint. In the double firing frit application, only a strong absorption band centred at 750 nm is observed, which decreases the RNIR dramatically (46%, although its L* is only 80.5). This band is associated to Cu2+ ions dissolved in the alkaline glaze and located in high distorted octahedral sites (due to Jahn Teller distortion) [52], showing that the pigment is not stable in the glaze. Finally, no bands are detected in the application in porcelain powder, also indicating that the pigment is not stable in this medium. In the case of classical Co-willemite decorated ZnO, the blue colour is obtained in the three applications (Table 6) and the features of the spectra are similar in all cases (Figure 12), maintaining the spectrum of the powder, which is associated with tetrahedral Co2+. Nevertheless, in the case of double firing frit, the absorption at 1200–1600 nm range (due to the coupling of 4A2(F) → 4T1(F) and 4A2(F) → 4T2(F)) shows an unusual low intensity and the NIR reflectance increases in this application; this behaviour has been observed in nanopowders of rare earth doped cobalt aluminate [53]. Following DCMA [3] classification, La,Li-SrCuSi4O10 wesselsite pigment belongs to Category B and Co-willemite decorated ZnO to Category A.
- (b)
- In the case of red-brown pigments, the colour of Pr-cerianite is maintained in the three applications, with a* values around 19 in the alkyd paint and double firing frit, although in porcelain powder, a* value decreases to 6.8 (Table 6). The optical spectrum of the powder is maintained in all the applications (Figure 11), and only in alkyd paint the spectrum shows weak absorptions in the NIR range, which can be observed always associated with all the applications with the alkyd resin. Concerning the classical Cr-franklinite spinel pigment, the red-brown colour is maintained in the alkyd paint and in double firing frit (a* between 18 and 20), but it darkens in the porcelain powder application. The reflectance spectra of alkyd and double firing frit applications indicate the stability of the pigment (Figure 12), showing similar features to the powder spectrum; instead, the absorption bands observed at 300 and 500 nm produce the darkening of the sample for the porcelain powder application; moreover, a band at 900 nm and a strong absorption between 1200–1700 diminish the NIR reflectance of this application, probably associated to the decomposition of franklinite and partial reduction of iron (III) to iron (II). Therefore, both Pr-cerianite and Cr-franklinite spinel belongs to Category A but Cr-franklinite is unstable in porcelain powder.
- (c)
- Regarding yellow pigments, the Mo-cerate yellow pigment loses the yellow hue (b* = 45.3 in powder, Figure 6) in the applications, and only in alkyd paint shows an indifferent b* = 18.9 (Table 6). The UV-Vis-NIR reflectance spectra of the applications (Figure 11) only indicate moderate absorption at 505 nm in the alkyd application, whereas in the case of double firing frit and porcelain powder, there is no evidence of absorption at 500 nm, indicating that the pigment is not stable in this medium. The Ni,Sb-rutile pigment, which shows b* = 54.1 in powder (Figure 6), maintains b* = 48 and L* = 81.8 in alkyd paint (into the range of high chromaticity for yellow), and even a higher b* (52.6) value in the double firing frit, but b* decreases to only 17.4 and L* to 79.0 (out of the range) for porcelain powder, indicates that the pigment is not stable and this application is colourless. The UV-Vis-NIR reflectance spectra of the applications (Figure 12) indicate similar features in the case of alkyd paint, whereas the spectrum is different for the double firing application, showing low absorption in the UV range and a broadening of the bands associated with Ni2+ in octahedral coordination. In the case of porcelain powder, no absorption bands are detected. From the proposed classification, Mo-cerate belongs to Category C and, considering the Zn rich glaze used in this study, Ni,Sb-rutile belongs to Category B of the pigments.
- (d)
- Concerning the black pigments, the Sr4Mn2CuO9 black-brown pigment maintains the colour in alkyd paint and double firing frit, whereas only a light darkening is observed in the case of porcelain powder. The corresponding UV-Vis-NIR reflectance spectrum of the applications (Figure 11) shows a similar spectrum to the powder in alkyd paint, whereas in the double firing application it shows a broadening of the absorption bands of Mn4+ in octahedral coordination and lower intensity of reflectance (particularly the band at 715 nm associated with 4A2(4F) → 4T2(4F) transition), which implies a decrease of reflectance in the 700–1000 nm, and thus decreasing its RNIR performance. As for the application in porcelain powder, only a light absorption in the 700–900 nm range is observed, indicating the low stability of the black-brown pigment in this medium. In the case of Co,Cr decorated rutile black-green pigment, the colour is maintained in all applications, with UV-Vis-NIR spectra similar to the powder spectrum, associated with Co2+ in tetrahedral coordination and Cr3+ in octahedral coordination; this confirms the stabilization of the pigment in the three applications, and show a RNIR higher than the powder. Therefore, both Sr4Mn2CuO9 black-brown and Co,Cr decorated rutile black-green belong to Category A of pigments, however, Sr4Mn2CuO9 black-brown is not stable in porcelain stoneware powder.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- CPMA. Classification and Chemical Description of the Complex Inorganic Colour Pigments, 4th ed.; Dry Colour Manufacturers Association: Alexandria, VA, USA, 2010. [Google Scholar]
- Monrós, G. Scheelite and Zircon: Brightness, Color and NIR Reflectance in Ceramics; Nova Science Publishers: New York, NY, USA, 2021; ISBN 978-1-53619-332-9. [Google Scholar]
- CIE Comission International de l´Eclairage. Recommendations on Uniform Color Spaces, Colour Difference Equations, Psychometrics Colour Terms; Bureau Central de la CIE: Paris, France, 1978. [Google Scholar]
- Munsell, A.H. Atlas of the Munsell Color System; Wadsworth, Howland & Co., Inc.: Boston, MA, USA, 1915. [Google Scholar]
- Briggs, D.J.C. The Dimensions of Colour. Available online: http://www.huevaluechroma.com/082.php (accessed on 14 September 2022).
- ISO International Standard 105-J03. Part J03: Calculation of Color Differences. ISO International Standard: London, UK, 2009.
- Hanrahan, P.; Kruger, W. SIGGRAPH’93. In Proceedings of the 20th Annual Conference on Computer Graphics and Interactive Techniques, Anaheim, CA, USA, 2–6 August 1993; pp. 165–174. [Google Scholar]
- Hecht, H.G. The Interpretation of Diffuse Reflectance Spectra. J. Res. Natl. Bur. Stand. Phys. Chem. 1976, 80, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Kortüm, G. Reflectance Spectroscopy, Principles, Methods, Applications; Springer: Berlin/Heidelberg, Germany, 1969; ISBN 978-3-642-88071. [Google Scholar]
- Llusar, M.; Bermejo, T.; Primo, J.E.; Gargori, C.; Esteve, V.; Monrós, G. Karrooite green pigments doped with Co and Zn: Synthesis, color properties and stability in ceramic glazes. Ceram. Int. 2017, 12, 9133–9144. [Google Scholar] [CrossRef]
- ASTM C1371. Standard Test Method for Determination of Emittance of Materials Near Room Temperature Using Portable Emisometers. ASTM International: West Conshohocken, PA, USA, 2004.
- Cerro, S.; Gargori, C.; Llusar, M.; Monrós, G. Orthorhombic (Fe2TiO5)-monoclinic (Cr2TiO5) solid solution series: Synthesis by gel routes, coloring and NIR reflectivity evaluation. Ceram. Int. 2018, 44, 13349–13359. [Google Scholar] [CrossRef]
- Gargori, C.; Cerro, S.; Fas, N.; Llusar, M.; Monrós, G. Red-brown ceramic pigments based on chromium doped ferrian armalcolite, effect of mineralizers. Ceram. Int. 2017, 43, 5490–5497. [Google Scholar] [CrossRef]
- Gargori, C.; Cerro, S.; Fas, N.; Llusar, M.; Monrós, G. Study of the photocatalytic activity and cool characteristics of a novel palette of pigments. Bol. Soc. Esp. Ceram. Vidr. 2017, 56, 166–176. [Google Scholar] [CrossRef]
- Levinson, R.; Berdahl, P.; Akbari, H. Solar spectral optical properties of pigments–Part II: Survey of common colorants. Sol. Energy Mater. Sol. Cells 2005, 89, 351–389. [Google Scholar] [CrossRef]
- Dieke, G.H. Spectra and Energy Levels of Rare Earth Ions in Crystals; Wiley–Interscience: New York, NY, USA, 1968. [Google Scholar]
- Calbo, J.; Sorlí, S.; Llusar, M.; Tena, M.A.; Monrós, G. Minimisation of toxicity in nickel ferrite black pigment. Br. Ceram. Trans. 2003, 103, 3–9. [Google Scholar] [CrossRef]
- Garcia, A.; Llusar, M.; Calbo, J.; Tena, M.A.; Monrós, G. Low-toxicity red ceramic pigments for porcelainised stoneware from lanthanide-cerianite solid solutions. Green Chem. 2001, 3, 238. [Google Scholar] [CrossRef]
- LLusar, M.; Vitaskova, L.; Sucova, P.; Tena, M.A.; Badenes, J.A.; Monrós, G. Red ceramic pigments of terbium-doped ceria prepared through classical and non-conventional coprecipitation routes. J. Eur. Ceram. Soc. 2010, 30, 37–52. [Google Scholar] [CrossRef]
- Smith, A.E.; Mizoguchi, H.; Delaney, K.; Spaldin, N.A.; Sleight, A.W.; Subramanian, M.A. Mn3+ in trigonal bipyramidal coordination: A new blue chromophore. J. Am. Chem. Soc. 2009, 131, 17084–17086. [Google Scholar] [CrossRef]
- Oregon State University Materials with Trigonal Bipyramidal Coordination and Methods of Making the Same. US Patent 8.282.728 B2, 11 June 2009.
- Sheethu, J.; Mundlapudi, L.R. Lanthanum strontium copper silicates as intense blue inorganic pigments with high near-infrared reflectance. Dyes Pigm. 2013, 98, 540–546. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Zhao, X.; Zhang, Y. Sol–gel synthesis and properties of europium–strontium copper silicates blue pigments with high near-infrared reflectance. Dyes Pigm. 2016, 131, 154–159. [Google Scholar] [CrossRef]
- Vishnu, V.S.; Reddy, M.L. Near-infrared reflecting inorganic pigments based on molybdenum and praseodymium doped yttrium cerate: Synthesis, characterization and optical properties. Sol. Energy Mater Sol. Cells 2011, 95, 2685–2692. [Google Scholar] [CrossRef]
- Raj, A.K.; Rao, P.P.; Sameera, S.; Divya, S. Pigments based on terbium-doped yttrium cerate with high NIR reflectance for cool roof and surface coating applications. Dyes Pigm. 2015, 22, 116–125. [Google Scholar] [CrossRef]
- Jovani, M.; Sanz, A.; Beltrán-Mir, H.; Cordoncillo, E. New red-shade environmental-friendly multifunctional pigment based on Tb and Fe doped Y2Zr2O7 for ceramic applications and cool roof coatings. Dyes Pigm. 2016, 133, 33–40. [Google Scholar] [CrossRef]
- Gargori, C.; Galindo, R.; Cerro, S.; García, A.; Llusar, M.; Monrós, G. Synthesis of a new CaxY2−xVxSn2−xO7 yellow pigment. Phys. Procedia 2010, 8, 84–87. [Google Scholar] [CrossRef]
- Lili, L.; Aijun, H.; Mingquan, Y.; Zhao, Z. Synthesis and characterization of Al3+ doped LaFeO3 compounds: A novel inorgànic pigments with high near-infrared reflectance. Sol. Energy Mater Sol. Cells 2015, 132, 377–384. [Google Scholar] [CrossRef]
- Bae, B.; Wendusu; Tamura, S.; Imanaka, N. Novel environmentally friendly inorganic yellow pigments based on gehlenite-type structure. Ceram. Int. 2016, 42, 15104–15106. [Google Scholar] [CrossRef]
- Schildhammer, D.; Fuhrmann, G.; Petschnig, L.; Weinberger, N.; Schottenberger, H.; Huppertz, H. Synthesis and characterization of a new high NIR reflective ytterbium molybdenum oxide and related doped pigments. Dyes Pigm. 2017, 138, 90–99. [Google Scholar] [CrossRef]
- Kim, T.; Kim, S.; Lin, C.; Liu, R.; Chan, T.; Im, S. Melilite-type blue chromophores based on Mn3+ in a trigonal-bipyramidal coordination induced by interstitial oxygen. J. Mater Chem. 2013, 37, 5843–5848. [Google Scholar] [CrossRef]
- Pozza, G.; Ajò, D.; Chiari, G.; De Zuane, F.; Favaro, M. Photoluminescence of the inorganic pigments Egyptian blue, Han blue and Han purple. J. Cult. Heritage 2000, 1, 393–398. [Google Scholar] [CrossRef]
- Kendrick, E.; Kirk, C.J.; Dann, C.J.S.E. Structure and color properties in the Egyptian Blue Family, M1-xM’xCuSi4O10, as a function of M, M’ where M, M’ = Ca, Sr and Ba. Dyes Pigm. 2007, 73, 13–18. [Google Scholar] [CrossRef]
- Seitz, G.; Penin, N.; Decoux, L.; Wattiaux, A.; Duttine, M.; Gaudon, M. Near the Ferric Pseudobrookite Composition (Fe2TiO5). Inorg. Chem. 2016, 55, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, R.; Dave, R.N.; Dongguang, W.; Ramlakhan, M. Synthesis of engineered particulates with tailored properties using dry particle coating. Powder Technol. 2001, 117, 40–67. [Google Scholar] [CrossRef]
- Ouabbas, Y.; Chamayou, A.; Galet, L.; Baron, M.; Thomas, G.; Grosseau, P.; Guilhot, B. Surface modification of silica particles by dry coating: Characterization and powder ageing. Powder Technol. 2009, 190, 200–209. [Google Scholar] [CrossRef]
- Bae, B.; Takeuchi, N.; Tamura, S.; Imanaka, N. Environmentally friendly orange pigments based on hexagonal perovskite-type compounds and their high NIR reflectivity. Dyes Pigm. 2017, 147, 523–528. [Google Scholar] [CrossRef]
- Ozel, E.; Yurdakula, H.; Turana, S.; Ardit, M.; Cruciani, G.; Dondi, M. Co-doped willemite ceramic pigments: Technological behaviour, crystal structure and optical properties. J. Eur. Ceram. Soc. 2010, 30, 3319–3329. [Google Scholar] [CrossRef]
- Benda, P.; Kalendová, A. Anticorrosion properties of pigments based on ferrite coated zinc particles. Phys. Procedia 2013, 44, 185–194. [Google Scholar] [CrossRef]
- Sorlí, S.; Tena, M.A.; Badenes, J.A.; Llusar, M.; Monrós, G. Study of nickel precursors in (Ni,M,Ti)O2 (M = Sb, Nb) yellow ceramic pigments. Br. Ceram. Trans. 2004, 103, 10–14. [Google Scholar] [CrossRef]
- Eliziárioa, S.A.; De Andrade, J.M.; Limac, S.J.G.; Paskocimas, C.A.; Soledade, L.E.B.; Hammer, P.; Longo, E.; Souza, A.G.; Santos, I.M.G. Black and green pigments based on chromium–cobalt spinels. Mater. Chem. Phys. 2011, 129, 619–624. [Google Scholar] [CrossRef]
- Pu, Y.; Wang, G.; Chang, K.; Ling, Y.; Lin, Y.; Fitzmorris, B.C.; Liu, C.; Lu, X.; Tong, Y.; Zhang, J.Z.; et al. Au Nanostructure-Decorated TiO2 Nanowires Exhibiting Photoactivity Across Entire UV-visible Region for Photoelectrochemical Water Splitting. Nano Lett. 2013, 13, 3817–3823. [Google Scholar] [CrossRef] [PubMed]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Tatus Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Schabbacha, L.M.; Marinoski, D.L.; Güthsb, S.; Bernardinc, A.M.; Fredel, M.C. Pigmented glazed ceramic roof tiles in Brazil: Thermal and optical properties related to solar reflectance index. Solar Energy 2018, 159, 113–124. [Google Scholar] [CrossRef]
- Gaffey, S.J. Spectral reflectance of-carbonate minerals in the visible and near infrared (0.35-2.55 microns): Calcite, aragonite, and dolomite. Am. Mineral. 1986, 71, 151–162. [Google Scholar] [CrossRef]
- Forés, A.; Llusar, M.; Badenes, J.A.; Calbo, J.; Tena, M.A.; Monrós, G. Cobalt minimisation in willemite (CoxZn2−xSiO4) ceramic pigments. Green Chem. 2000, 2, 93–100. [Google Scholar] [CrossRef]
- Suwan, M.; Sangwong, N.; Supothina, S. Effect of Co and Pr doping on the properties of solar reflective ZnFe2O4 dark pigment. IOP Conf. Ser. Mater. Sci. Eng. 2017, 182, 012003. [Google Scholar] [CrossRef]
- Pailhé, N.; Wattiaux, A.; Gaudon, M.; Demourges, A. Correlation between structural features and vis-NIR spectra of -Fe2O3 hematite an AFe2O4 spinel oxides (A = Mg, Zn). J. Solid State Chem. 2018, 181, 1040–1047. [Google Scholar] [CrossRef]
- Dondi, M.; Matteucci, F.; Cruciani, G.; Gasparoto, G.; Tobaldi, D.M. Pseudobrookite ceramic pigments: Crystal structural, optical and technological properties. Solid State Sci. 2007, 9, 362–369. [Google Scholar] [CrossRef]
- Prim, S.R.; García, A.; Galindo, R.; Cerro, S.; Llusar, M.; Folgueras, M.V.; Monrós, G. Pink ceramic pigment based on chromium doped M(Al2-xCrx)O4, M=Mg, Zn, normal spinel. Ceram. Int. 2013, 39, 6981–6989. [Google Scholar] [CrossRef]
- Katiyar, R.S.; Kousik, S. Structural and optical properties of Zn1-xCuxO thin films. In Handbook of Zinc Oxide and Related Materials; Feng, Z.C., Ed.; CRC Press: Boca Raton, FL, USA, 2013; Volume 1. [Google Scholar]
- Monrós, G. Sol-Gel Materials for Pigments and Ceramics. In The Sol-Gel Handbook—Synthesis, Characterization and Applications; Levy, D., Zayat, M., Eds.; Wiley: Hoboken, NJ, USA, 2015; Volume 3, pp. 1145–1173. ISBN 9783527334865. [Google Scholar]
- Tong, Y.; Zhang, H.; Wang, S.; Chen, Z.; Bian, B. Highly Dispersed Re-Doped CoAl2O4 Nanopigments: Synthesis and Chromatic Properties. J. Nanomater. 2016, 2016, 4169673. [Google Scholar] [CrossRef]




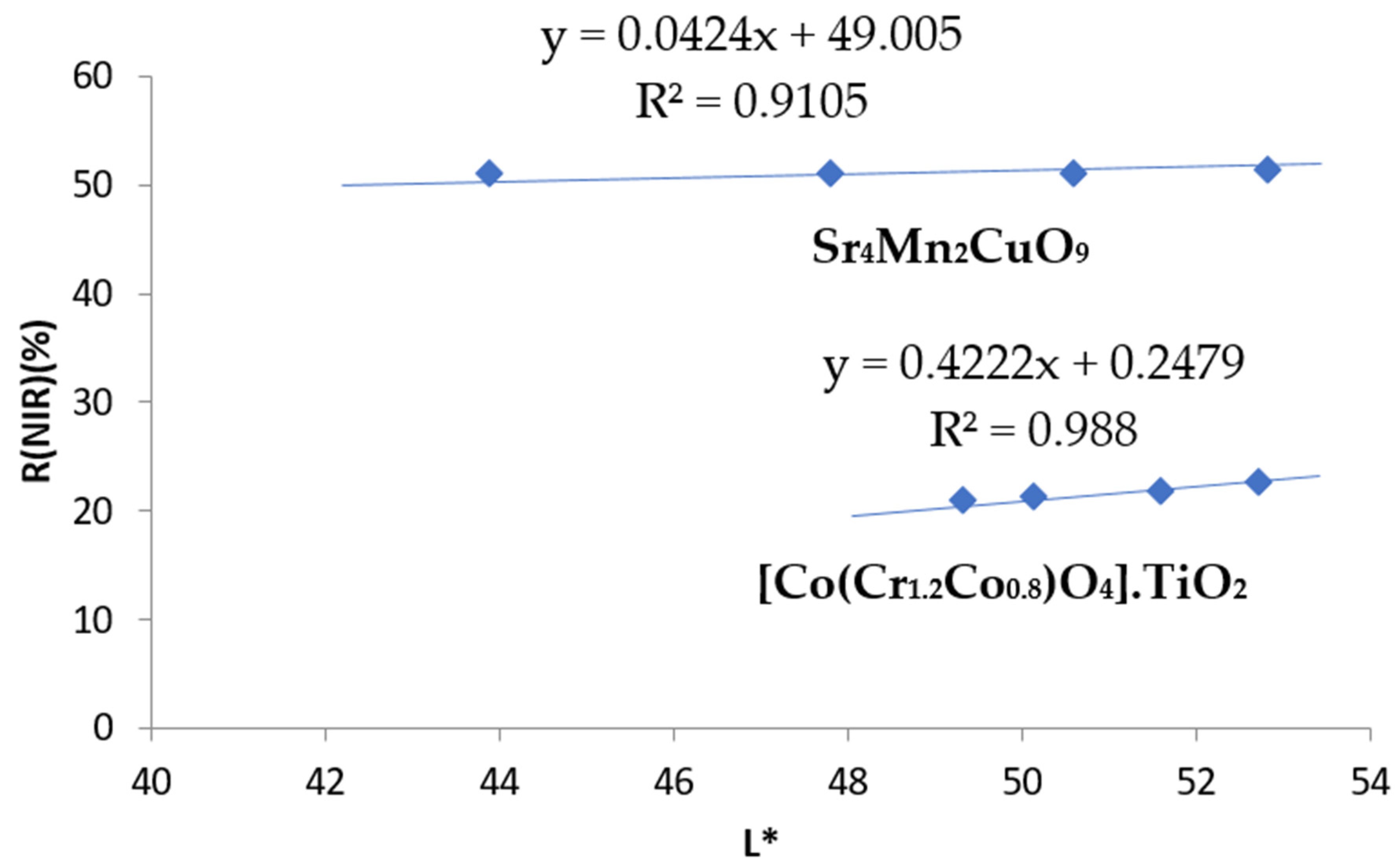

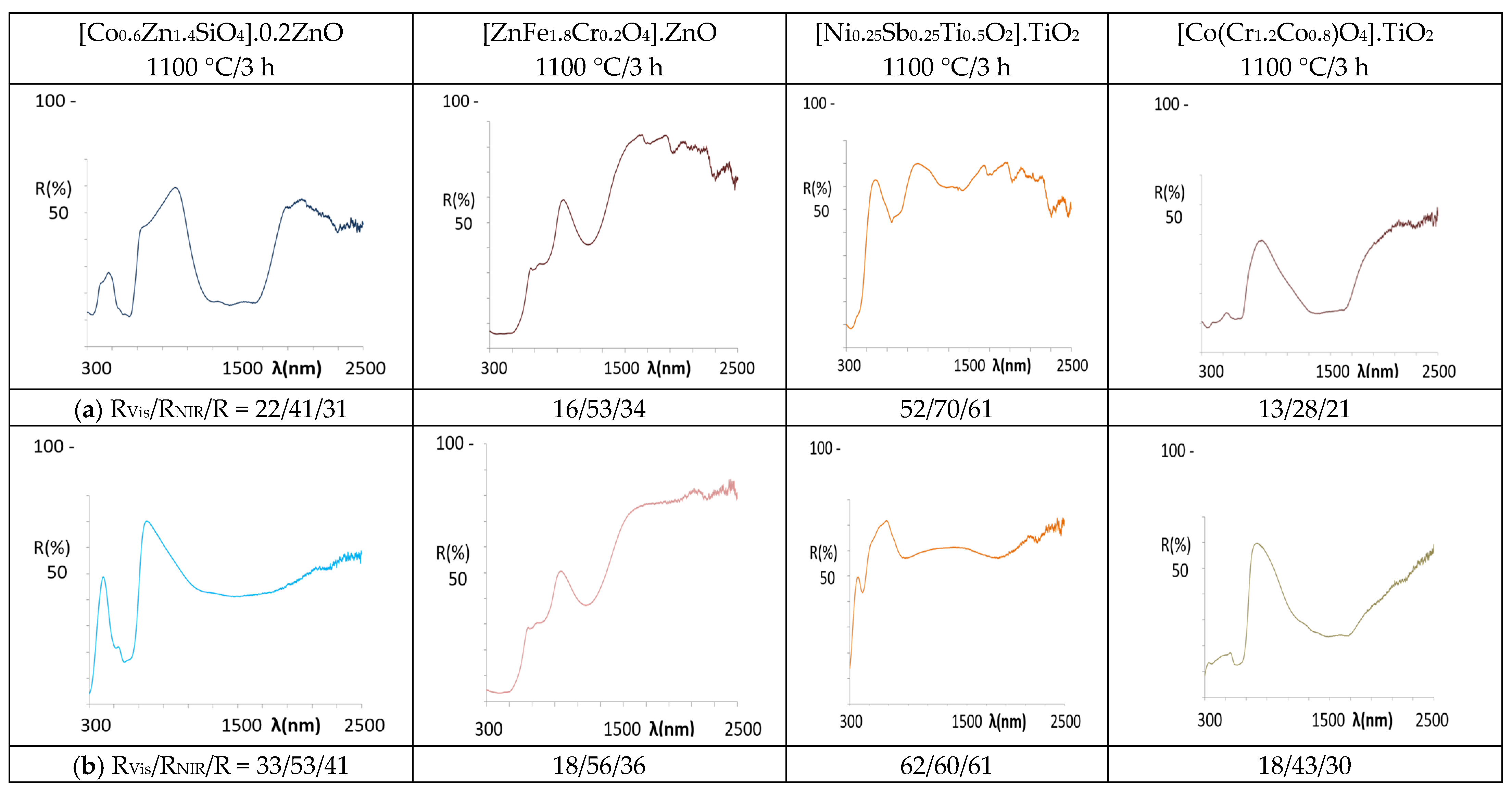
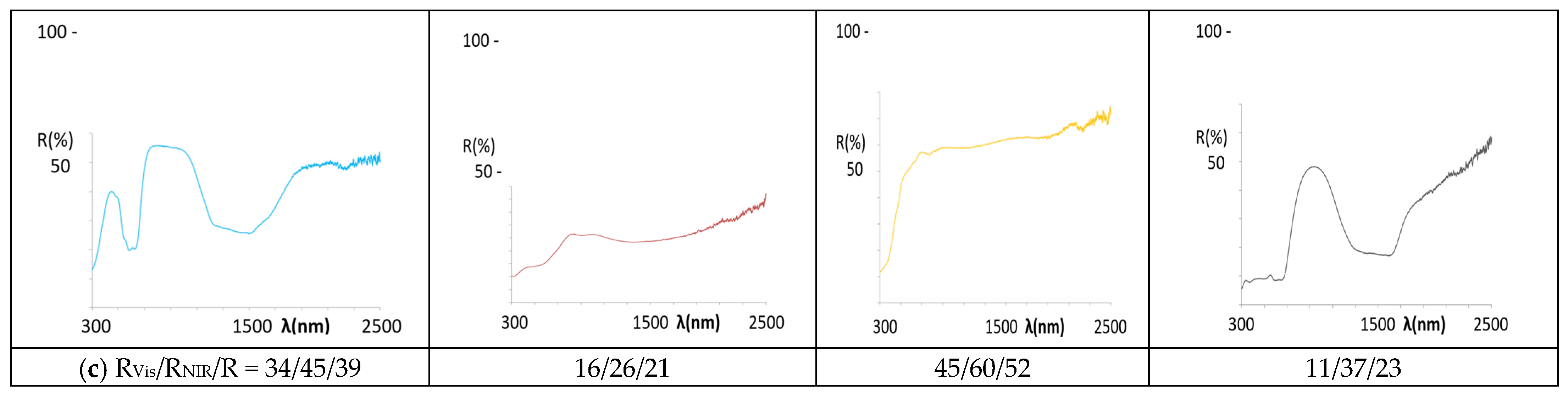
| Stoichiometry | Powder | 10 wt.% In Alkyd Paint | 3 wt.% in Double Firing Frit |
|---|---|---|---|
| Co0.6Zn1.4SiO4 | L*a*b* = 28.0/−4.1/−26.0 RNIR = 27 | L*a*b* = 45.2/−6.3/−16.2 RNIR = 38 | L*a*b* = 54.2/−3.7/−23.0 RNIR = 52 |
| [Co0.6Zn1.4SiO4]·0.2ZnO | L*a*b* = 30.1/−3.8/−24.6 RNIR = 30 | L*a*b* = 47.5/−6.2/−15.0 RNIR = 41 | L*a*b* = 56.2/5.5/−21.1 RNIR = 53 |
| ZnFe1.8Cr0.2O4 | L*a*b* = 33.1/22.6/19.0 RNIR = 46 | L*a*b* = 37.1/20.0/18.0 RNIR = 50 | L*a*b* = 46.1/17.9/19.0 RNIR = 55 |
| [ZnFe1.8Cr0.2O4]·ZnO | L*a*b* = 36.2/22.1/20.6 RNIR = 48 | L*a*b* = 39.4/20.2/18.9 RNIR = 53 | L*a*b* = 48.5/18.1/17.9 RNIR = 56 |
| Ni0.25Sb0.25Ti0.5O2 | L*a*b* = 85.0/−4.0/56.0 RNIR = 53 | L*a*b* = 80.0/−4.0/50.4 RNIR = 64 | L*a*b* = 87.9/−2/53.0 RNIR = 59 |
| [Ni0.25Sb0.25Ti0.5O2]·TiO2 | L*a*b* = 84.3/−4.3/54.1 RNIR = 55 | L*a*b* = 81.8/−4.4/48.0 RNIR = 70 | L*a*b* = 78.7/−0.7/52.6 RNIR = 60 |
| Co(Cr1.2Co0.8)O4 | L*a*b* = 47.6/−2.0/4.6 RNIR = 18 | L*a*b* = 37.6/−2.3/3.6 RNIR = 26 | L*a*b* = 50.6/−4.1/0.6 RNIR = 41 |
| [Co(Cr1.2Co0.8)O4]·TiO2 | L*a*b* = 49.3/−2.9/5.8 RNIR = 21 | L*a*b* = 39.6/−3.4/4.3 RNIR = 28 | L*a*b* = 51.4/−5.2/1.0 RNIR = 43 |
| Sr0.3La0.7Cu0.3Li0.7Si4O10 950 °C/10 h | UV-Vis (%) | NIR (%) | TOTAL (%) | L a*b* |
| 0% CaCO3 | 26.6 | 44.3 | 35.2 | 62.3/−4.4/−19.9 |
| 20% CaCO3 | 45.1 | 60.6 | 52.0 | 74.1/−5.1/−13.2 |
| 38.5% CaCO3 | 53.9 | 66.0 | 59.3 | 79.6/−4.5/−8.4 |
| 50% CaCO3 | 56.1 | 66.5 | 60.7 | 81.1/−4.1/−6.4 |
| [Co0.6Zn1.4SiO4]·0.2ZnO 1100 °C/3 h | UV-Vis (%) | NIR (%) | TOTAL (%) | L a*b* |
| 0% CaCO3 | 13.1 | 29.9 | 21.3 | 30.1/−3.8/−24.6 |
| 20% CaCO3 | 17.9 | 33.8 | 24.6 | 49.7/−3.9/−14.7 |
| 38.5% CaCO3 | 21.3 | 35.2 | 27.2 | 53.2/−4.3/−13.9 |
| 50% CaCO3 | 24.2 | 37.5 | 29.8 | 56.4/−4.1/−13.1 |
| Ce0.95Pr0.05O2 1400 °C/6 h | UV-Vis (%) | NIR (%) | TOTAL (%) | L *a*b* |
| 0% CaCO3 | 17 | 65 | 40 | 50.6/17.5/12.8 |
| 20% CaCO3 | 25.1 | 71.1 | 45.1 | 54.6/14.1/9.3 |
| 38.5% CaCO3 | 30.1 | 70.0 | 47.4 | 60/13.3/9.0 |
| 50% CaCO3 | 32.6 | 70.3 | 49.0 | 62.0/13.0/9.0 |
| 60% CaCO3 | 37.9 | 73.1 | 53.1 | 61.7/11.7/8.1 |
| 80% CaCO3 | 48.3 | 75.3 | 60.0 | 70.8/8.1/6.4 |
| 95% CaCO3 | 63.8 | 80.0 | 70.7 | 81.1/4.4/5.6 |
| 100% CaCO3 | 79.3 | 83.0 | 80.8 | 88.2/0.8/7.4 |
| [ZnFe1.8Cr0.2O4]·ZnO 1100 °C/3 h | UV-Vis (%) | NIR (%) | TOTAL (%) | L*a*b* |
| 0% CaCO3 | 14.0 | 48.1 | 30.2 | 36.2/22.1/20.6 |
| 20% CaCO3 | 18.2 | 46.0 | 29.7 | 41.5/19.0/12.6 |
| 38.5% CaCO3 | 19.1 | 46.6 | 30.7 | 42.1/19.2/13.0 |
| 50% CaCO3 | 19.8 | 47.7 | 31.9 | 45.2/18.7/10.1 |
| 60% CaCO3 | 23.1 | 52.0 | 36.1 | 43.2/19.0/11.1 |
| 80% CaCO3 | 29.2 | 56.1 | 43.0 | 48.1/17.2/12.5 |
| 100% CaCO3 | 79.3 | 83.0 | 80.8 | 88.2/0.8/7.4 |
| Y2Ce1.5Mo0.5O7 1500 °C/6 h | UV-Vis (%) | NIR (%) | TOTAL (%) | L a*b* |
| 0% CaCO3 | 54.0 | 81.1 | 67.3 | 85.2/0.2/45.3 |
| 20% CaCO3 | 66.7 | 80.4 | 72.20 | 87.13/−2.60/30.71 |
| 38.5% CaCO3 | 68.11 | 82.7 | 74.40 | 87.56/−3.12/24.73 |
| 50% CaCO3 | 67.26 | 87.0 | 75.76 | 88.8/−3.02/19.66 |
| 100% CaCO3 | 79.26 | 82.92 | 80.83 | 88.23/0.83/7.37 |
| [Ni0.25Sb0.25Ti0.5O2]·TiO2 1100 °C/3 h | UV-Vis (%) | NIR (%) | TOTAL (%) | L a*b* |
| 0% CaCO3 | 39.6 | 54.7 | 47 | 84.28/−4.29/54.05 |
| 20% CaCO3 | 44.11 | 57.9 | 50.23 | 84.1/−4.05/44.50 |
| 38.5% CaCO3 | 45.59 | 58.6 | 51.36 | 84.2/−3.97/43.83 |
| 50% CaCO3 | 46.90 | 59.5 | 52.48 | 84.4/−3.98/41.78 |
| 100% CaCO3 | 79.3 | 83.0 | 80.8 | 88.2/0.8/7.4 |
| Sr4Mn2CuO9 1000 °C/6 h | UV-Vis (%) | NIR (%) | TOTAL (%) | L*a*b* |
| 0% CaCO3 | 7 | 50.9 | 28.5 | 43.91/7.15/6.29 |
| 20% CaCO3 | 10.16 | 51.0 | 27.63 | 47.79/6.32/5.45 |
| 38.5% CaCO3 | 12.38 | 51.1 | 28.83 | 50.57/5.98/5.08 |
| 50% CaCO3 | 14.63 | 51.3 | 30.76 | 52.77/5.85/5.21 |
| [Co(Cr1.2Co0.8)O4]·TiO2 1100 °C/3 h | UV-Vis (%) | NIR (%) | TOTAL (%) | L*a*b* |
| 0% CaCO3 | 7 | 21 | 14 | 49.3/−2.9/5.8 |
| 20% CaCO3 | 9 | 20 | 14.5 | 50.1/−2.5/5.0 |
| 38.5% CaCO3 | 10 | 21 | 15.5 | 51.6/−2.3/4.9 |
| 50% CaCO3 | 11 | 22 | 16.5 | 52.7/−2.2/4.1 |
| Sr0.3La0.7Cu0.3Li0.7Si4O10 950 °C/10 h | Ce0.05Pr0.95O2 1400 °C/6 h | Y2Ce1.5Mo0.5O7 1500 °C/6 h | Sr4Mn2CuO9 1000 °C/6 h | |
| (a) | 72.4/−3.8/−3.0 | 51.6/19.9/15.5 | 81.3/0.4/18.9 | 46.6/7.8/7.5 |
| (b) | 80.5/−7.0/−5.6 | 65.6/18.2/20.0 | 87.6/−1.8/11.7 | 57.9/2.8/−0.2 |
| (c) | 76.8/1.2/9.8 | 70.3/6.8/11.4 | 79.2/1.5/11.5 | 73.4/1.7/11.6 |
| [Co0.6Zn1.4SiO4]·0.2ZnO 1100 °C/3 h | [ZnFe1.8Cr0.2O4]·ZnO 1100 °C/3 h | [Ni0.25Sb0.25Ti0.5O2]·TiO2 1100 °C/3 h | [Co(Cr1.2Co0.8)O4]·TiO2 1100 °C/3 h | |
| (a) | 47.5/−6.2/−15.0 | 39.4/20.2/18.9 | 81.8/−4.4/48.0 | 39.6/−3.4/4.3 |
| (b) | 56.2/5.5/−21.1 | 48.5/18.1/17.9 | 78.7/−0.7/52.6 | 51.4/−5.2/1.0 |
| (c) | 64.8/−3.4/−13.1 | 46.3/3.4/4.7 | 79.0/−0.6/17.4 | 64.6/0.8/15.2 |
| (A) Powder | (B) Alkyd Paint | (C) Double Firing Frit (1050 °C) | (D) Porcelain Stoneware (1190 °C) | Category | |
|---|---|---|---|---|---|
| Blue | |||||
| Sr0.3La0.7Cu0.3Li0.7Si4O10 950 °C/10 h | 44 | 86 | 46 | ns | B |
| [Co0.6Zn1.4SiO4]·0.2ZnO 1100 °C/3 h | 30 | 41 | 53 | 45 | A |
| Red-brown | |||||
| Ce0.05Pr0.95O2 1400 °C/6 h | 65 | 83 | 82 | 56 | A |
| [ZnFe1.8Cr0.2O4]·ZnO 1100 °C/3 h | 48 | 53 | 56 | ns | A |
| Yellow | |||||
| Y2Ce1.5Mo0.5O7 1500 °C/6 h | 81 | 89 | ns | ns | C |
| [Ni0.25Sb0.25Ti0.5O2]·TiO2 1100 °C/3 h | 55 | 70 | 60 | ns | B |
| Black | |||||
| Sr4Mn2CuO9 1000 °C/6 h | 51 | 66 | 41 | ns | A |
| [Co(Cr1.2Co0.8)O4]·TiO2 1100 °C/3 h | 21 | 28 | 43 | 37 | A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monrós, G.; Badenes, J.A.; Llusar, M. Ecofriendly High NIR Reflectance Ceramic Pigments Based on Rare Earths Compared with Classical Chromophores Prepared by DPC Method. Ceramics 2022, 5, 614-641. https://doi.org/10.3390/ceramics5040046
Monrós G, Badenes JA, Llusar M. Ecofriendly High NIR Reflectance Ceramic Pigments Based on Rare Earths Compared with Classical Chromophores Prepared by DPC Method. Ceramics. 2022; 5(4):614-641. https://doi.org/10.3390/ceramics5040046
Chicago/Turabian StyleMonrós, Guillermo, José A. Badenes, and Mario Llusar. 2022. "Ecofriendly High NIR Reflectance Ceramic Pigments Based on Rare Earths Compared with Classical Chromophores Prepared by DPC Method" Ceramics 5, no. 4: 614-641. https://doi.org/10.3390/ceramics5040046
APA StyleMonrós, G., Badenes, J. A., & Llusar, M. (2022). Ecofriendly High NIR Reflectance Ceramic Pigments Based on Rare Earths Compared with Classical Chromophores Prepared by DPC Method. Ceramics, 5(4), 614-641. https://doi.org/10.3390/ceramics5040046







