Melatonin Attenuates Spinal Cord Injury in Mice by Activating the Nrf2/ARE Signaling Pathway to Inhibit the NLRP3 Inflammasome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Surgical Preparations
2.3. Behavioral Assessments
2.4. Tissue Preparation
2.5. Nissl Staining
2.6. TUNEL Staining
2.7. Transmission Electron Microscope Analysis
2.8. Cell Culture and Treatment
2.9. Cell Viability
2.10. Determination of Intracellular ROS Accumulation
2.11. JC-1 Staining
2.12. Determination of Oxidative Stress
2.13. Western Blot Analysis
2.14. Immunofluorescence Analysis
2.15. Enzyme-Linked Immunosorbent Assay (ELISA)
2.16. Statistical Analysis
3. Results
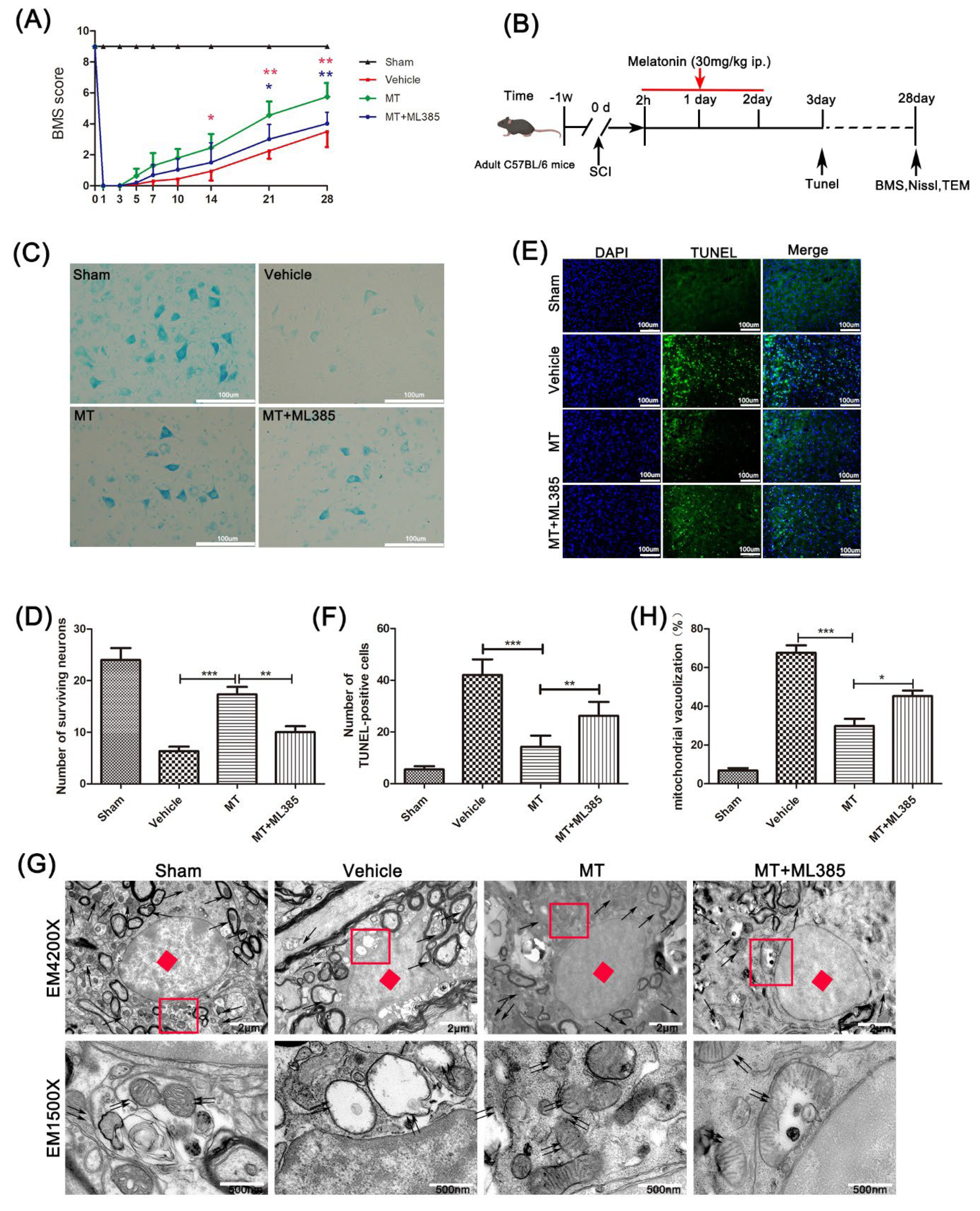
3.1. Melatonin Promotes the Recovery of Motor Neurons after Spinal Cord Injury
3.2. Melatonin Reduces Oxidative Damage to PC12 Cells Induced by H2O2
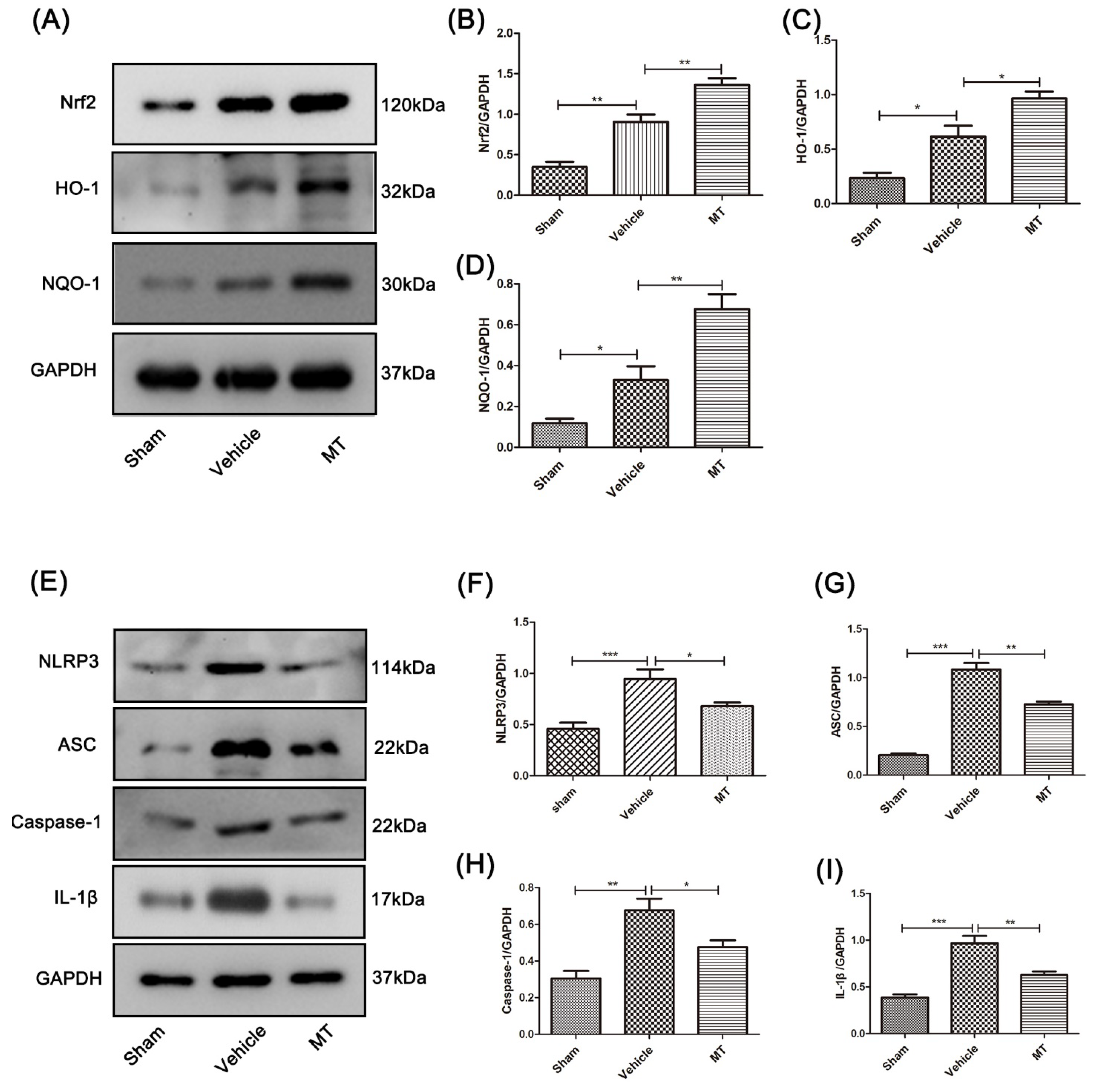
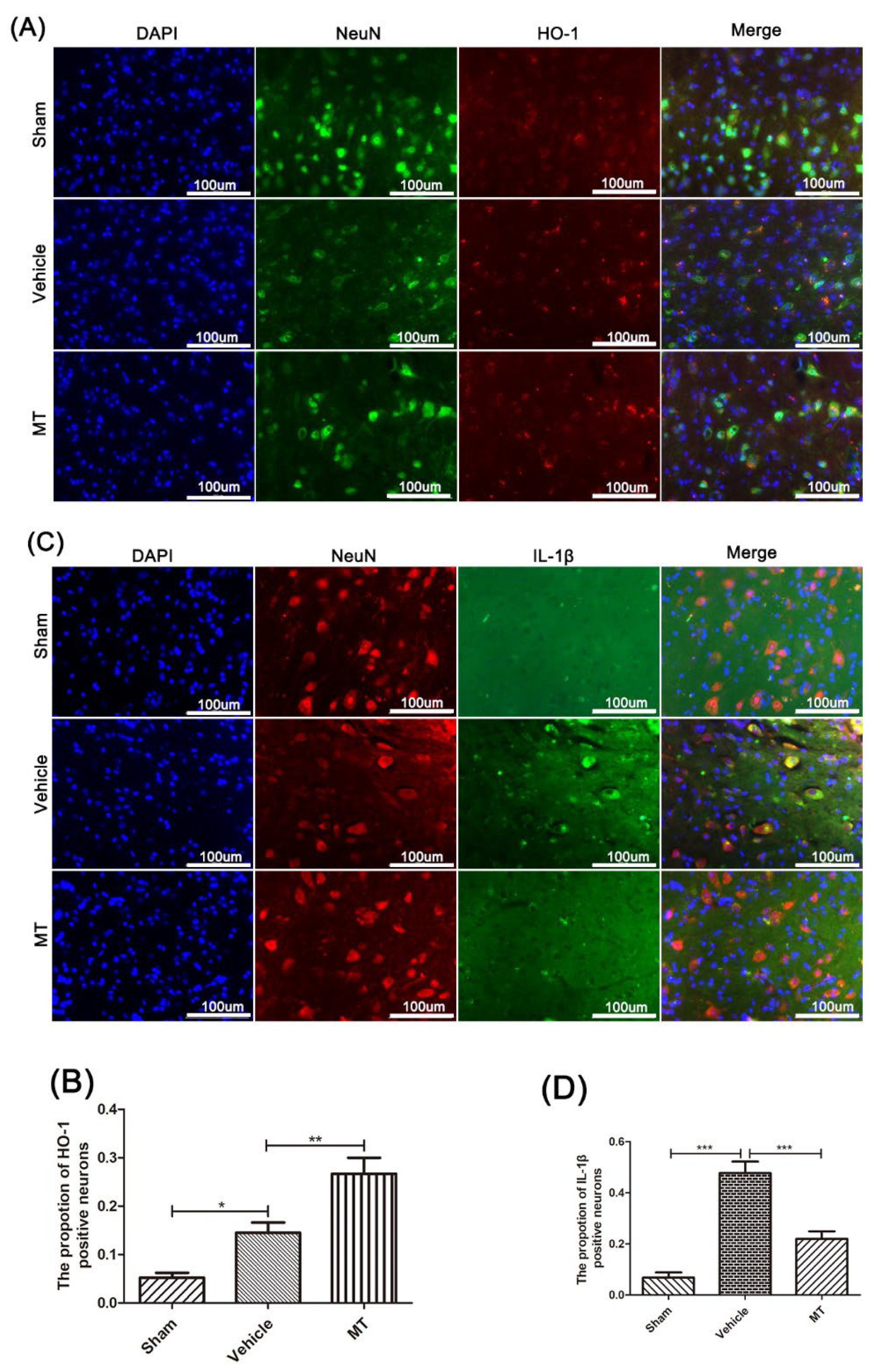
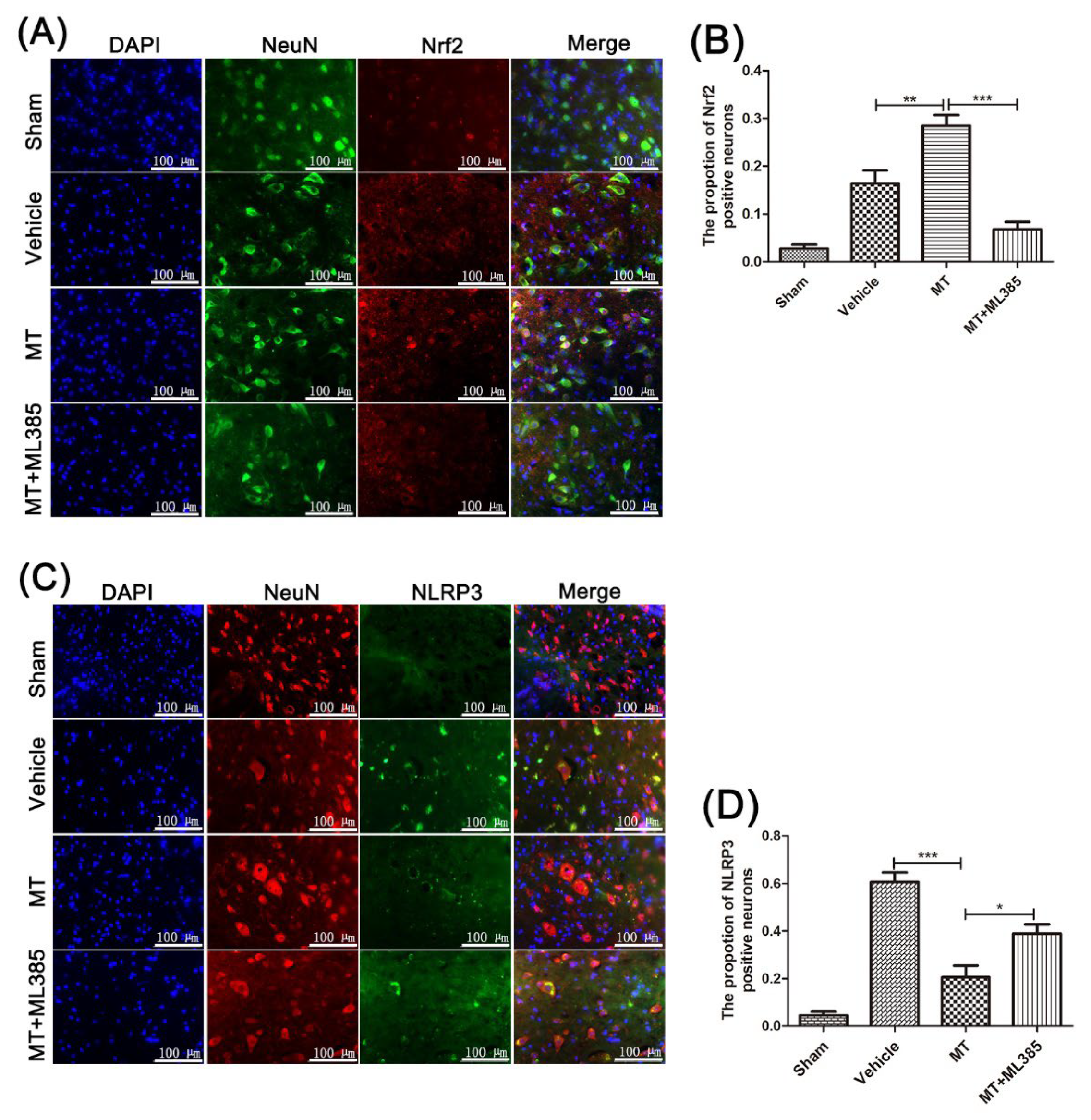
3.3. Melatonin Elevates the Level of Nrf2/ARE-Related Antioxidant Proteins and Inhibits the NLRP3 Inflammasome after SCIin Mice
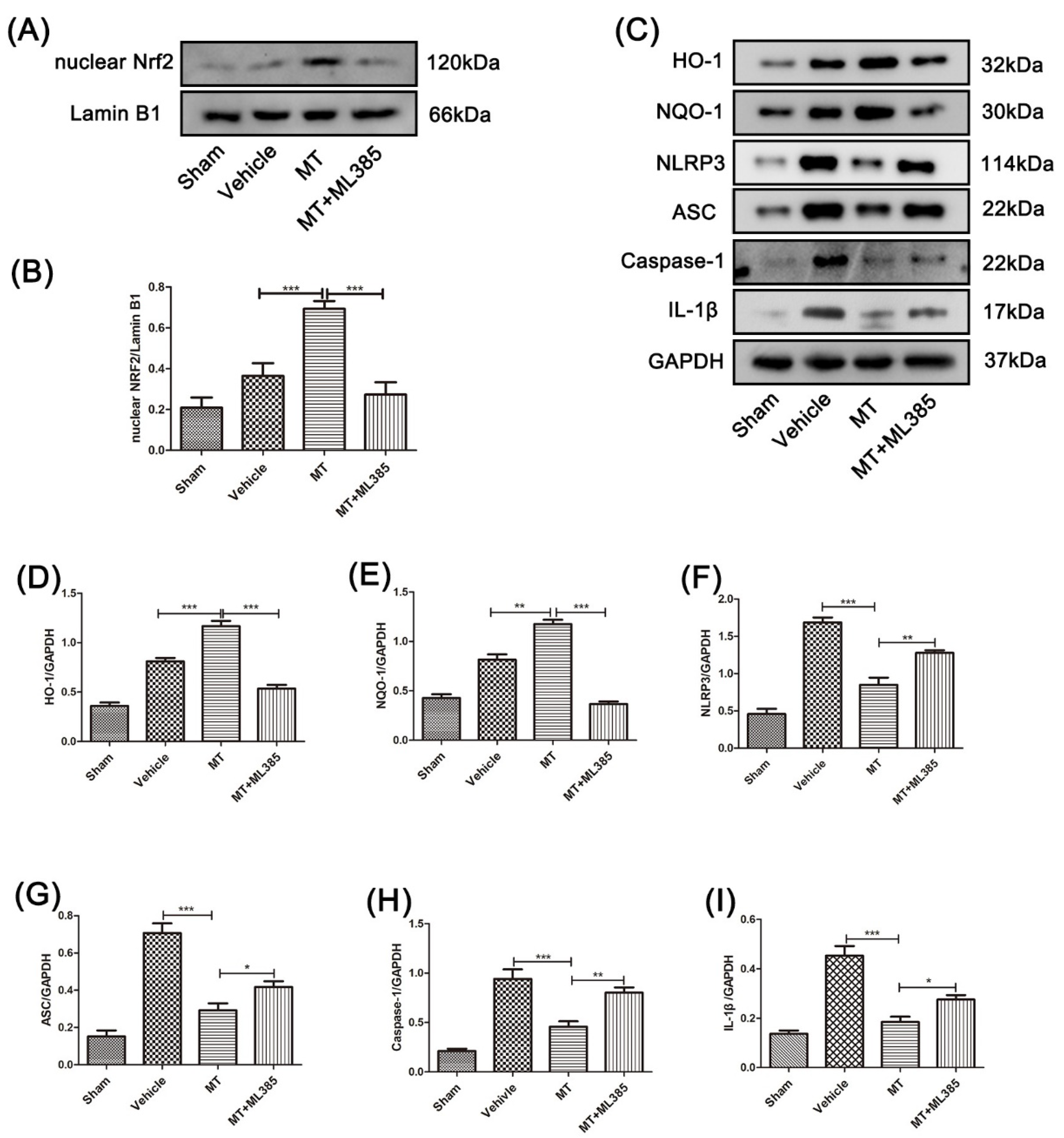
3.4. Melatonin Suppresses the NLRP3 Inflammasome through Activating the Nrf2/ARE Signalingy after SCI in Mice
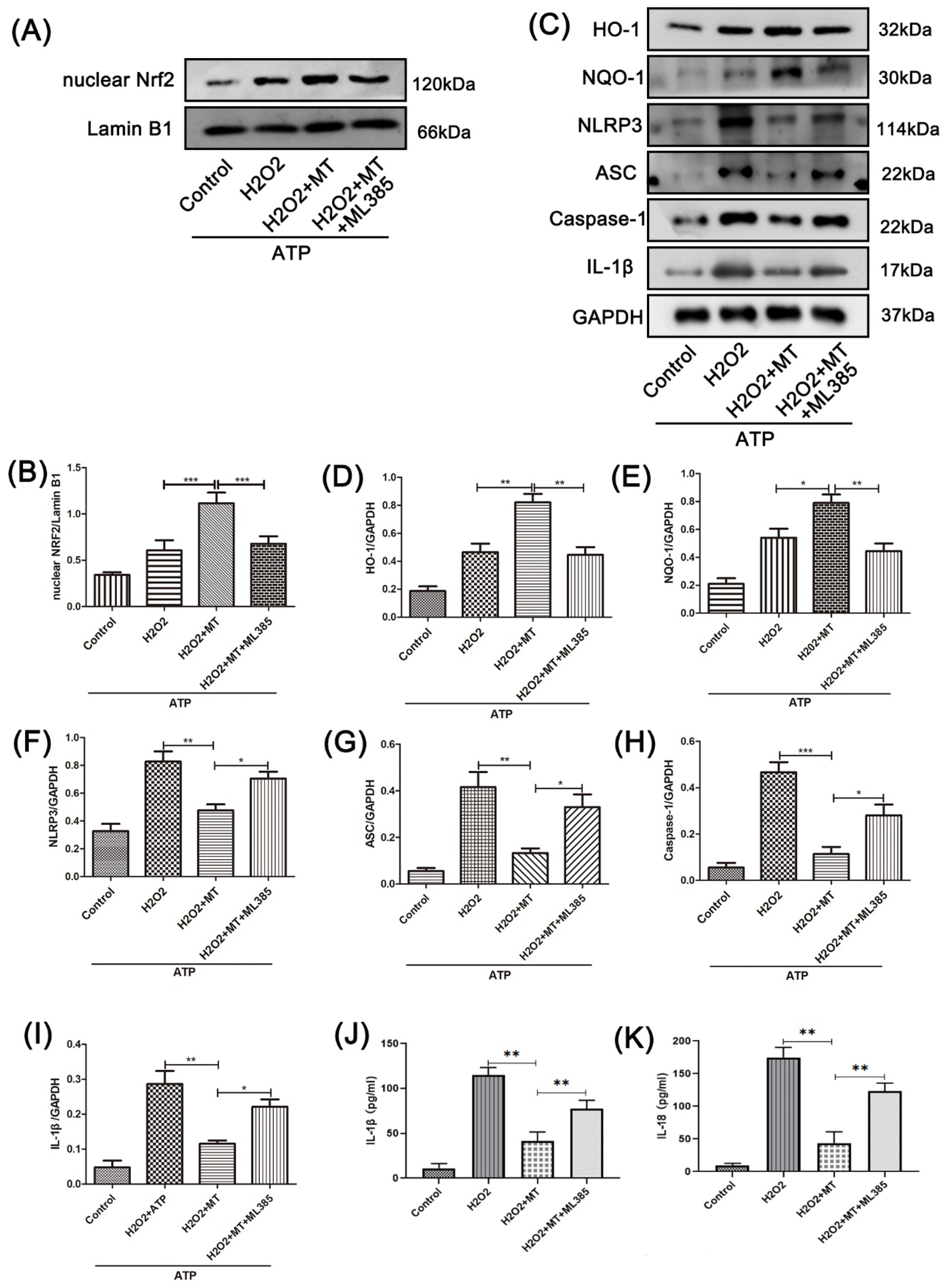
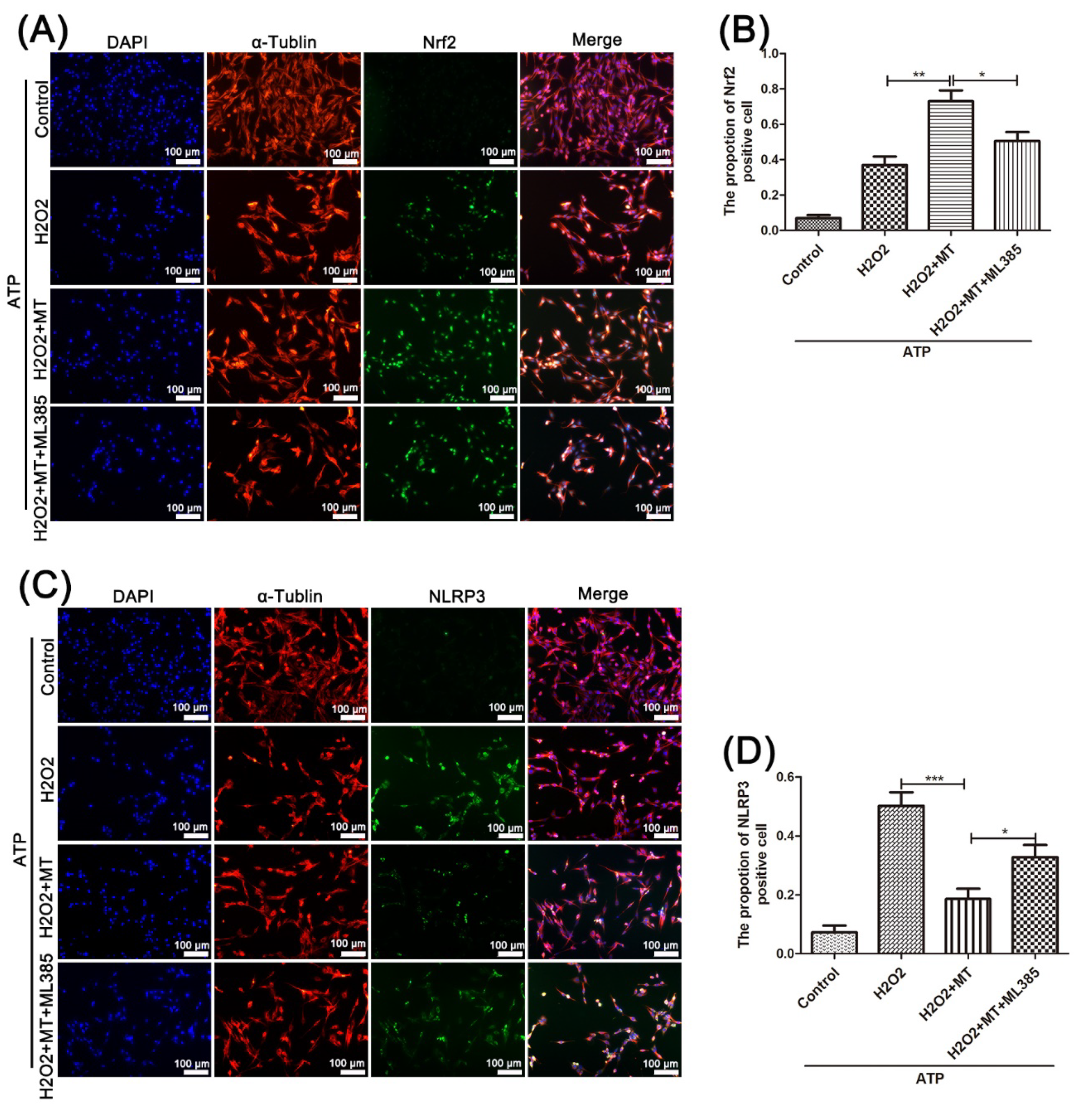
3.5. Melatonin Suppresses the NLRP3 Inflammasome in PC12 Cells Treated with H2O2 via the Nrf2/ARE Defense Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCI | spinal cord injury |
| MT | melatonin |
| ARE | antioxidant response element |
| Nrf2 | nuclear red blood cell 2 related factor 2 |
| SOD | superoxide dismutase |
| MDA | malondialdehyde |
| CNS | central nervous system |
| ROS | reactive oxygen species |
| GSH-PX | glutathione peroxidase |
| TEM | Transmission electron microscopy |
References
- Squair, J.W.; Gautier, M.; Mahe, L.; Soriano, J.E.; Rowald, A.; Bichat, A.; Cho, N.; Anderson, M.A.; James, N.D.; Gandar, J.; et al. Neuroprosthetic Baroreflex Controls Haemodynamics after Spinal Cord Injury. Nature 2021, 590, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.Y.; Kwon, M.J.; Lee, E.M.; Kim, K.; Oh, Y.J.; Kim, H.S.; Hwang, D.H.; Kim, B.G. Role of Proto-Oncogene as a Transcriptional Hub to Regulate the Expression of Regeneration-Associated Genes Following Preconditioning Peripheral Nerve Injury. J. Neurosci. 2021, 41, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Quintá, H.R. Intraspinal Administration of Netrin-1 Promotes Locomotor Recovery after Complete Spinal Cord Transection. J. Neurotrauma 2021, 38, 2084–2102. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive Astrocyte Nomenclature, Definitions, and Future Directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Jiang, W.; Li, M.; He, F.; Zhou, S.; Zhu, L. Targeting the NLRP3 Inflammasome to Attenuate Spinal Cord Injury in Mice. J. Neuroinflam. 2017, 14, 207. [Google Scholar] [CrossRef]
- Iqubal, A.; Ahmed, M.; Iqubal, M.K.; Pottoo, F.H.; Haque, S.E. Polyphenols as Potential Therapeutics for Pain and Inflammation in Spinal Cord Injury. Curr. Mol. Pharmacol. 2021, 14, 714–730. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Y.-Q.; Liu, Y.-K.; Zhang, H.-Q.; Hou, G.-G.; Meng, Q.-G.; Hou, Y. Potential Anti-Neuroinflammatory NF-KB Inhibitors Based on 3,4-Dihydronaphthalen-1(2)-One Derivatives. J. Enzym. Inhib. Med. Chem. 2020, 35, 1631–1640. [Google Scholar] [CrossRef]
- Zhou, L.-Y.; Yao, M.; Tian, Z.-R.; Liu, S.-F.; Song, Y.-J.; Ye, J.; Li, G.; Sun, Y.-L.; Cui, X.-J.; Wang, Y.-J. Muscone Suppresses Inflammatory Responses and Neuronal Damage in a Rat Model of Cervical Spondylotic Myelopathy by Regulating Drp1-Dependent Mitochondrial Fission. J. Neurochem. 2020, 155, 154–176. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, X.; Jiang, W.; Li, W.; Zhu, S.; Liao, C.; Zou, L.; Ding, R.; Chen, J. Advanced Oxidation Protein Products Induce Microglia-Mediated Neuroinflammation via MAPKs-NF-ΚB Signaling Pathway and Pyroptosis after Secondary Spinal Cord Injury. J. Neuroinflam. 2020, 17, 90. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, C.; Xie, K.; Meng, X.; Wang, Y.; Yu, Y. Hydrogen-Rich Saline Alleviated the Hyperpathia and Microglia Activation via Autophagy Mediated Inflammasome Inactivation in Neuropathic Pain Rats. Neuroscience 2019, 421, 17–30. [Google Scholar] [CrossRef]
- Jiang, W.; Li, M.; He, F.; Zhu, L. Inhibition of NLRP3 Inflammasome Attenuates Spinal Cord Injury-Induced Lung Injury in Mice. J. Cell. Physiol. 2019, 234, 6012–6022. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ortiz, M.; Sayed, R.K.A.; Fernández-Martínez, J.; Cionfrini, A.; Aranda-Martínez, P.; Escames, G.; de Haro, T.; Acuña-Castroviejo, D. Melatonin/Nrf2/NLRP3 Connection in Mouse Heart Mitochondria during Aging. Antioxidants 2020, 9, 1187. [Google Scholar] [CrossRef]
- Fan, C.; Feng, J.; Tang, C.; Zhang, Z.; Feng, Y.; Duan, W.; Zhai, M.; Yan, Z.; Zhu, L.; Feng, L.; et al. Melatonin Suppresses ER Stress-Dependent Proapoptotic Effects via AMPK in Bone Mesenchymal Stem Cells during Mitochondrial Oxidative Damage. Stem Cell Res. Ther. 2020, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Arioz, B.I.; Tastan, B.; Tarakcioglu, E.; Tufekci, K.U.; Olcum, M.; Ersoy, N.; Bagriyanik, A.; Genc, K.; Genc, S. Melatonin Attenuates LPS-Induced Acute Depressive-Like Behaviors and Microglial NLRP3 Inflammasome Activation Through the SIRT1/Nrf2 Pathway. Front. Immunol. 2019, 10, 1511. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Su, L.-Y.; Sun, C.; Jiao, L.; Miao, Y.; Xu, M.; Luo, R.; Zuo, X.; Zhou, R.; Zheng, P.; et al. Melatonin Alleviates Morphine Analgesic Tolerance in Mice by Decreasing NLRP3 Inflammasome Activation. Redox Biol. 2020, 34, 101560. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and Inflammation-Story of a Double-Edged Blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Shi, D.; Zhi, Z.; Ao, R.; Yu, B. Melatonin Ameliorates Spinal Cord Injury by Suppressing the Activation of Inflammasomes in Rats. J. Cell. Biochem. 2019, 120, 5183–5192. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a Mitochondria-Targeted Antioxidant: One of Evolution’s Best Ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef]
- Watson, N.; Diamandis, T.; Gonzales-Portillo, C.; Reyes, S.; Borlongan, C.V. Melatonin as an Antioxidant for Stroke Neuroprotection. Cell Transplant. 2016, 25, 883–891. [Google Scholar] [CrossRef]
- Wilkinson, D.; Shepherd, E.; Wallace, E.M. Melatonin for Women in Pregnancy for Neuroprotection of the Fetus. Cochrane Database Syst. Rev. 2016, 3, CD010527. [Google Scholar] [CrossRef]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Buendia, I.; Michalska, P.; Navarro, E.; Gameiro, I.; Egea, J.; León, R. Nrf2-ARE Pathway: An Emerging Target against Oxidative Stress and Neuroinflammation in Neurodegenerative Diseases. Pharmacol. Ther. 2016, 157, 84–104. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, Z.; Ashrafizadeh, M. Melatonin as a Potential Modulator of Nrf2. Fundam. Clin. Pharmacol. 2020, 34, 11–19. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 Signaling Pathway: Pivotal Roles in Inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Shimizu, S.; Tatara, Y.; Mimura, J.; Itoh, K. Regulation of Nrf2 by Mitochondrial Reactive Oxygen Species in Physiology and Pathology. Biomolecules 2020, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tian, H.; Li, X.; Mao, L.; Zhao, X.; Lin, J.; Lin, S.; Xu, C.; Liu, Y.; Guo, Y.; et al. Zinc Promotes Functional Recovery after Spinal Cord Injury by Activating Nrf2/HO-1 Defense Pathway and Inhibiting Inflammation of NLRP3 in Nerve Cells. Life Sci. 2020, 245, 117351. [Google Scholar] [CrossRef]
- Baumann, M.D.; Kang, C.E.; Tator, C.H.; Shoichet, M.S. Intrathecal Delivery of a Polymeric Nanocomposite Hydrogel after Spinal Cord Injury. Biomaterials 2010, 31, 7631–7639. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Huang, S.; Ma, X.-X.; Zhang, W.-Y.; Wang, D.; Jin, S.-Y.; Zhang, Y.-P.; Li, Y.; Li, X. Angiotensin(1-7) Attenuated Angiotensin II-Induced Hepatocyte EMT by Inhibiting NOX-Derived H2O2-Activated NLRP3 Inflammasome/IL-1β/Smad Circuit. Free Radic. Biol. Med. 2016, 97, 531–543. [Google Scholar] [CrossRef]
- Fei, Q.; Ma, H.; Zou, J.; Wang, W.; Zhu, L.; Deng, H.; Meng, M.; Tan, S.; Zhang, H.; Xiao, X.; et al. Metformin Protects against Ischaemic Myocardial Injury by Alleviating Autophagy-ROS-NLRP3-Mediated Inflammatory Response in Macrophages. J. Mol. Cell. Cardiol. 2020, 145. [Google Scholar] [CrossRef]
- Han, Y.; Xu, X.; Tang, C.; Gao, P.; Chen, X.; Xiong, X.; Yang, M.; Yang, S.; Zhu, X.; Yuan, S.; et al. Reactive Oxygen Species Promote Tubular Injury in Diabetic Nephropathy: The Role of the Mitochondrial Ros-Txnip-Nlrp3 Biological Axis. Redox Biol. 2018, 16, 32–46. [Google Scholar] [CrossRef]
- Minutoli, L.; Puzzolo, D.; Rinaldi, M.; Irrera, N.; Marini, H.; Arcoraci, V.; Bitto, A.; Crea, G.; Pisani, A.; Squadrito, F.; et al. ROS-Mediated NLRP3 Inflammasome Activation in Brain, Heart, Kidney, and Testis Ischemia/Reperfusion Injury. Oxidative Med. Cell. Longev. 2016, 2016, 2183026. [Google Scholar] [CrossRef] [PubMed]
- Roosen, K.; Scheld, M.; Mandzhalova, M.; Clarner, T.; Beyer, C.; Zendedel, A. CXCL12 Inhibits Inflammasome Activation in LPS-Stimulated BV2 Cells. Brain Res. 2021, 1763, 147446. [Google Scholar] [CrossRef] [PubMed]
- Chio, J.C.T.; Xu, K.J.; Popovich, P.; David, S.; Fehlings, M.G. Neuroimmunological Therapies for Treating Spinal Cord Injury: Evidence and Future Perspectives. Exp. Neurol. 2021, 341, 113704. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, T.; Lachance, B.B.; Zhong, X.; Shen, G.; Xu, T.; Tang, C.; Jia, X. Critical Roles of Sphingosine Kinase 1 in the Regulation of Neuroinflammation and Neuronal Injury after Spinal Cord Injury. J. Neuroinflam. 2021, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Chou, C.J.; Scheiner, M.; Poeta, E.; Chen, N.Y.; Gunesch, S.; Hoffmann, M.; Sotriffer, C.; Monti, B.; Maurice, T.; et al. Melatonin- and Ferulic Acid-Based HDAC6 Selective Inhibitors Exhibit Pronounced Immunomodulatory Effects and Neuroprotective Effects in a Pharmacological Alzheimer’s Disease Mouse Model. J. Med. Chem. 2021, 64, 3794–3812. [Google Scholar] [CrossRef]
- Bi, J.; Shen, J.; Chen, C.; Li, Z.; Tan, H.; Sun, P.; Lin, Y. Role of Melatonin in the Dynamics of Acute Spinal Cord Injury in Rats. J. Cell. Mol. Med. 2021, 25, 2909–2917. [Google Scholar] [CrossRef]
- Wang, H.; Huo, X.; Han, C.; Ning, J.; Chen, H.; Li, B.; Liu, J.; Ma, W.; Li, Q.; Yu, Y.; et al. Ferroptosis Is Involved in the Development of Neuropathic Pain and Allodynia. Mol. Cell. Biochem. 2021, 476, 3149–3161. [Google Scholar] [CrossRef]
- McIntyre, W.B.; Pieczonka, K.; Khazaei, M.; Fehlings, M.G. Regenerative Replacement of Neural Cells for Treatment of Spinal Cord Injury. Expert Opin. Biol. Ther. 2021, 21, 1411–1427. [Google Scholar] [CrossRef]
- Yao, R.; Ren, L.; Wang, S.; Zhang, M.; Yang, K. Euxanthone Inhibits Traumatic Spinal Cord Injury via Anti-Oxidative Stress and Suppression of P38 and PI3K/Akt Signaling Pathway in a Rat Model. Transl. Neurosci. 2021, 12, 114–126. [Google Scholar] [CrossRef]
- Gao, S.; Xu, T.; Guo, H.; Deng, Q.; Xun, C.; Liang, W.; Sheng, W. Ameliorative Effects of Echinacoside against Spinal Cord Injury via Inhibiting NLRP3 Inflammasome Signaling Pathway. Life Sci. 2019, 237, 116978. [Google Scholar] [CrossRef]
- Al Mamun, A.; Wu, Y.; Monalisa, I.; Jia, C.; Zhou, K.; Munir, F.; Xiao, J. Role of Pyroptosis in Spinal Cord Injury and Its Therapeutic Implications. J. Adv. Res. 2021, 28, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, Z.; Zong, W.; Chen, P.; Li, J.; Wang, M.; Ding, F.; Xie, M.; Wang, W.; Luo, X. Deficiency of the Microglial Hv1 Proton Channel Attenuates Neuronal Pyroptosis and Inhibits Inflammatory Reaction after Spinal Cord Injury. J. Neuroinflam. 2020, 17, 263. [Google Scholar] [CrossRef]
- Shao, A.; Gao, S.; Wu, H.; Xu, W.; Pan, Y.; Fang, Y.; Wang, X.; Zhang, J. Melatonin Ameliorates Hemorrhagic Transformation via Suppression of ROS-Induced NLRP3 Activation after Cerebral Ischemia in Hyperglycemic Rats. Oxid. Med. Cell. Longev. 2021, 2021, 6659282. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.N.; McFarlane, K.E.; Vekaria, H.J.; Bailey, W.M.; Slone, S.A.; Tranthem, L.A.; Zhang, B.; Patel, S.P.; Sullivan, P.G.; Gensel, J.C. Mitochondria Exert Age-Divergent Effects on Recovery from Spinal Cord Injury. Exp. Neurol. 2021, 337, 113597. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Liu, S.; Li, J.; Tian, Y.; Xue, Y.; Liu, X. The Mitochondria-Targeted Anti-Oxidant MitoQ Protects against Intervertebral Disc Degeneration by Ameliorating Mitochondrial Dysfunction and Redox Imbalance. Cell Prolif. 2020, 53, e12779. [Google Scholar] [CrossRef]
- Liu, X.; Liang, F.; Song, W.; Diao, X.; Zhu, W.; Yang, J. Effect of Nrf2 Signaling Pathway on the Improvement of Intestinal Epithelial Barrier Dysfunction by Hyperbaric Oxygen Treatment after Spinal Cord Injury. Cell Stress Chaperones 2021, 26, 433–441. [Google Scholar] [CrossRef]
- Yardım, A.; Kandemir, F.M.; Çomaklı, S.; Özdemir, S.; Caglayan, C.; Kucukler, S.; Çelik, H. Protective Effects of Curcumin Against Paclitaxel-Induced Spinal Cord and Sciatic Nerve Injuries in Rats. Neurochem. Res. 2021, 46, 379–395. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, X.; Zhang, R.; Ma, B.; Niu, S.; Di, X.; Ni, L.; Liu, C. Melatonin Attenuates Smoking-Induced Atherosclerosis by Activating the Nrf2 Pathway via NLRP3 Inflammasomes in Endothelial Cells. Aging 2021, 13, 11363–11380. [Google Scholar] [CrossRef]
- Kryl’skii, E.D.; Popova, T.N.; Safonova, O.A.; Stolyarova, A.O.; Razuvaev, G.A.; de Carvalho, M.A.P. Transcriptional Regulation of Antioxidant Enzymes Activity and Modulation of Oxidative Stress by Melatonin in Rats Under Cerebral Ischemia/Reperfusion Conditions. Neuroscience 2019, 406, 653–666. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Z.; Han, W.; Yuan, Y.; Li, Y.; Zhou, K.; Wang, Q.; Xie, L.; Xu, K.; Zhang, H.; et al. Metformin Promotes Axon Regeneration after Spinal Cord Injury through Inhibiting Oxidative Stress and Stabilizing Microtubule. Oxid. Med. Cell. Longev. 2020, 2020, 9741369. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, H.; Huang, H.; Qu, Z.; Ma, D.; Dang, X.; Dong, Q. Melatonin Attenuates Spinal Cord Injury in Mice by Activating the Nrf2/ARE Signaling Pathway to Inhibit the NLRP3 Inflammasome. Cells 2022, 11, 2809. https://doi.org/10.3390/cells11182809
Wang H, Wang H, Huang H, Qu Z, Ma D, Dang X, Dong Q. Melatonin Attenuates Spinal Cord Injury in Mice by Activating the Nrf2/ARE Signaling Pathway to Inhibit the NLRP3 Inflammasome. Cells. 2022; 11(18):2809. https://doi.org/10.3390/cells11182809
Chicago/Turabian StyleWang, Haoyu, Haifan Wang, Heng Huang, Zhigang Qu, Dong Ma, Xiaoqian Dang, and Quanyu Dong. 2022. "Melatonin Attenuates Spinal Cord Injury in Mice by Activating the Nrf2/ARE Signaling Pathway to Inhibit the NLRP3 Inflammasome" Cells 11, no. 18: 2809. https://doi.org/10.3390/cells11182809
APA StyleWang, H., Wang, H., Huang, H., Qu, Z., Ma, D., Dang, X., & Dong, Q. (2022). Melatonin Attenuates Spinal Cord Injury in Mice by Activating the Nrf2/ARE Signaling Pathway to Inhibit the NLRP3 Inflammasome. Cells, 11(18), 2809. https://doi.org/10.3390/cells11182809





