Transplantation of Human Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Promotes Forelimb Functional Recovery after Cervical Spinal Cord Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture of hiPSCs
2.2. Differentiation of iPSCs into Neural Progenitor Cells
2.3. Purification of Nestin+ Cells by Fluorescence Activated Cell Sorting (FACS) and In Vitro Differentiation
2.4. In Vitro Immunocytochemistry
2.5. Transplantation of Nestin-ZsGreen+ Cells into a Rat Cervical SCI Model
2.6. Behavioral Analyses
2.7. Immunohistochemistry
2.8. Quantification of Gray Matter by MAP2-IR
2.9. Spared White Matter by EC Staining
3. Results
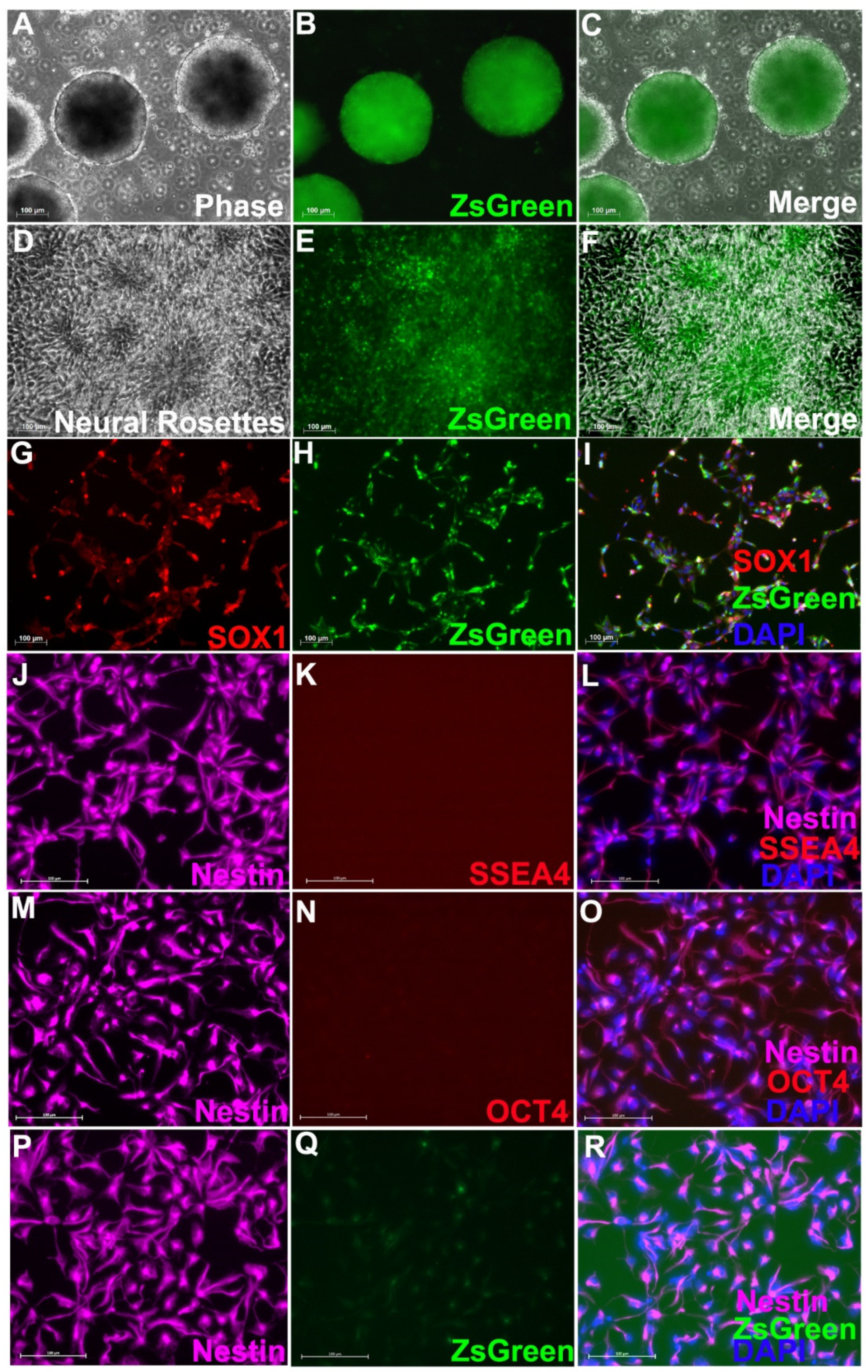
3.1. Neural Progenitor Cells Derived from Nestin-ZsGreen iPSC Knockin Reporter Cell Line
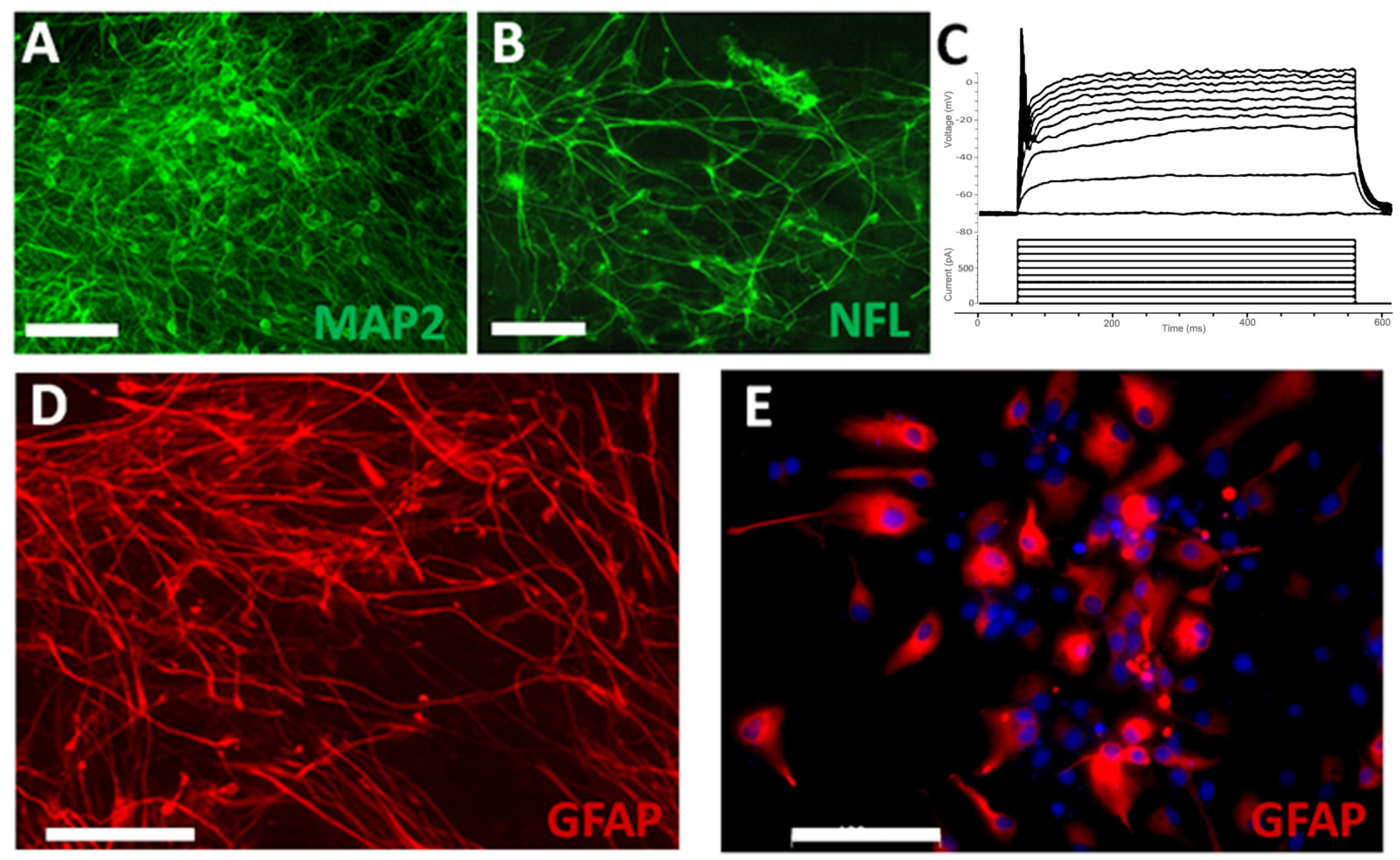
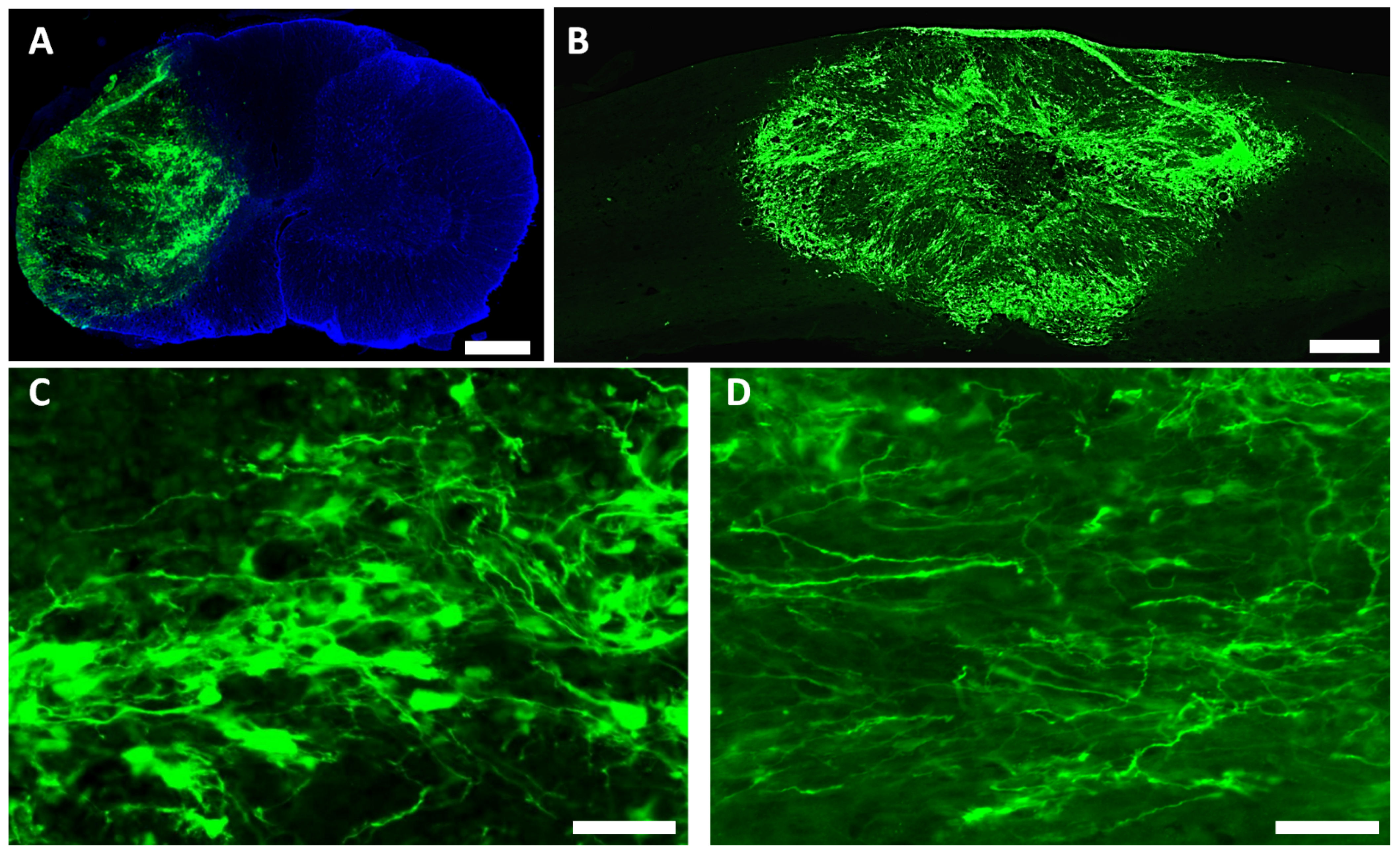
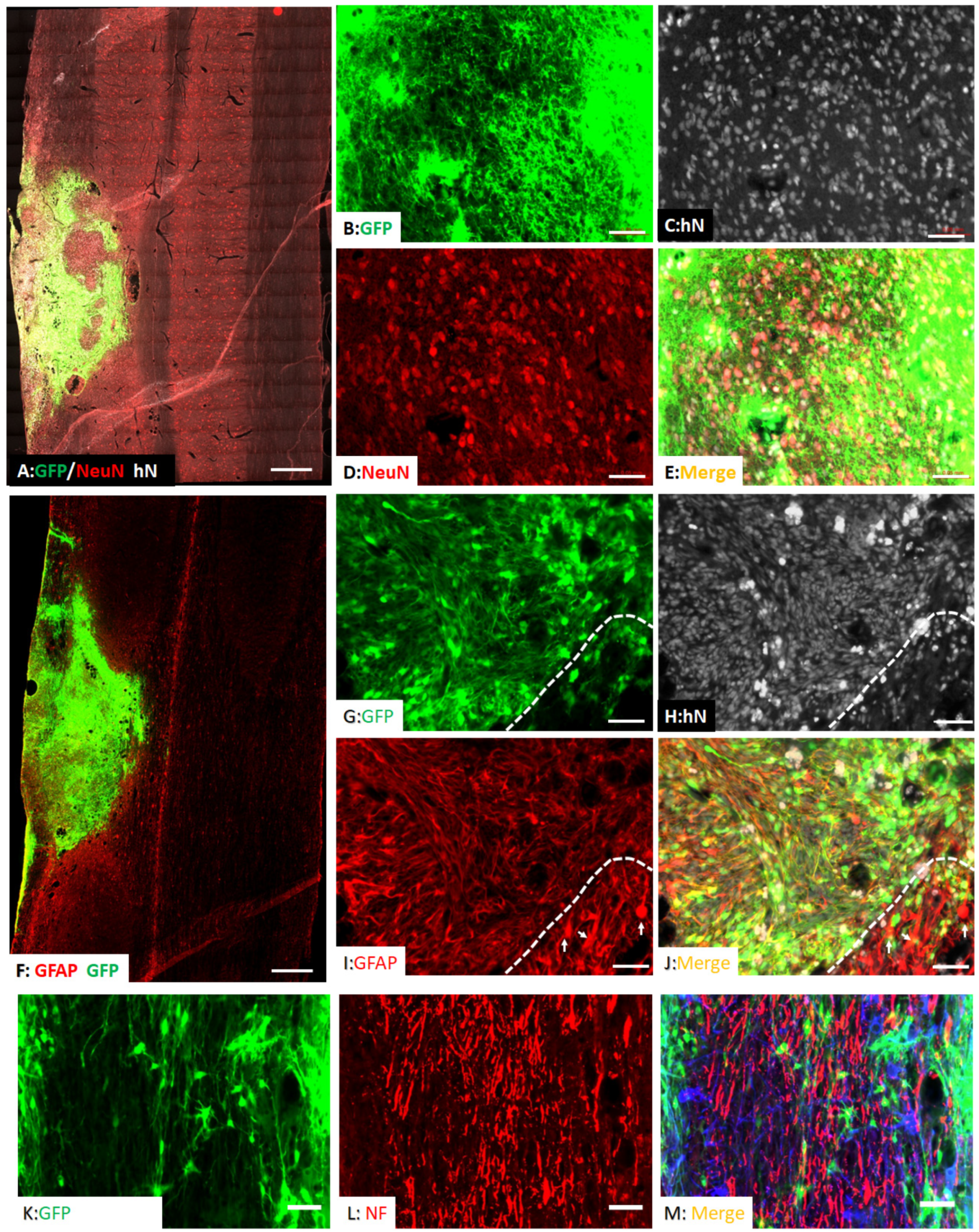
3.2. Survival and Differentiation of Grafted iPSC-Derived Neural Progenitor Cells
3.3. Spared Gray and White Matters after Transplantation of iPSC-NPCs
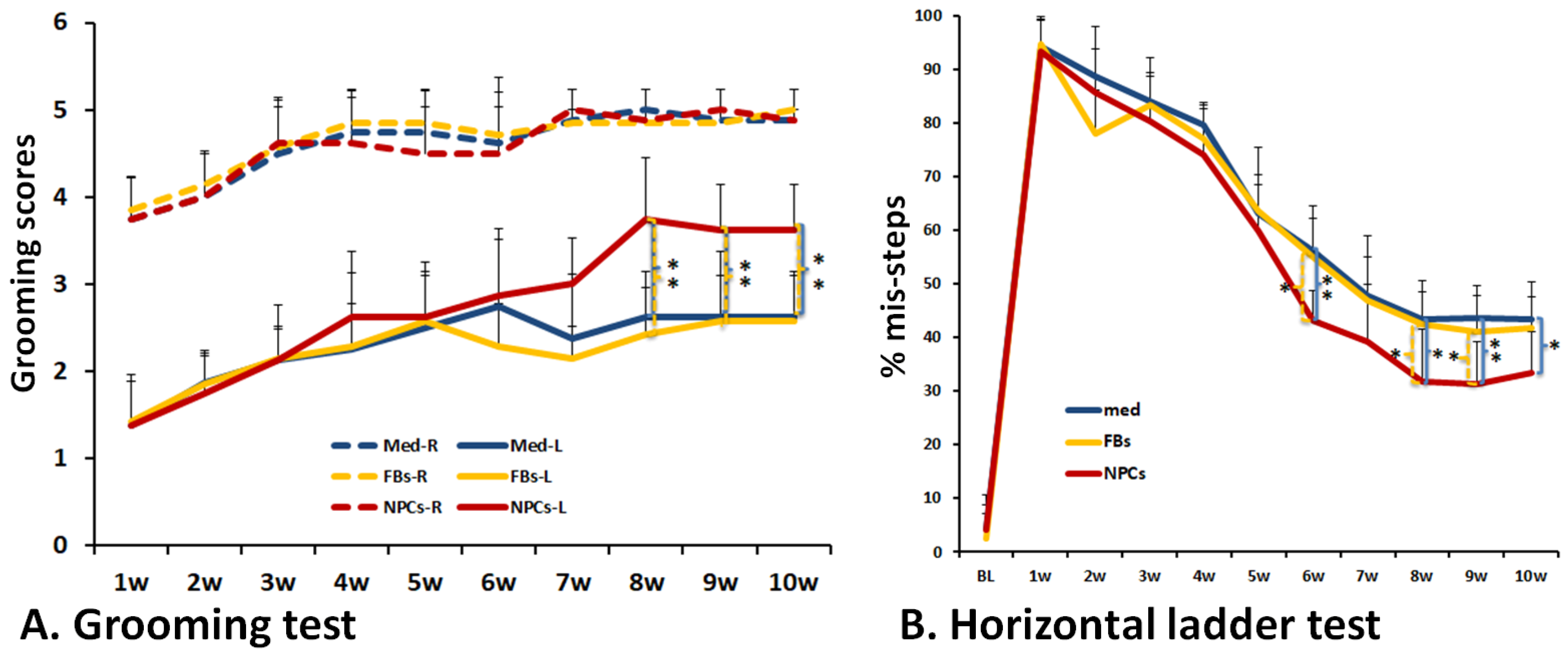
3.4. Improved Forelimb Functional Recovery after Transplantation of Human iPSC-Derived NPCs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Barak, M.; Fedorova, V.; Pospisilova, V.; Raska, J.; Vochyanova, S.; Sedmik, J.; Hribkova, H.; Klimova, H.; Vanova, T.; Bohaciakova, D. Human iPSC-Derived Neural Models for Studying Alzheimer’s Disease: From Neural Stem Cells to Cerebral Organoids. Stem Cell Rev. Rep. 2022, 18, 792–820. [Google Scholar] [CrossRef]
- Giacomelli, E.; Vahsen, B.F.; Calder, E.L.; Xu, Y.; Scaber, J.; Gray, E.; Dafinca, R.; Talbot, K.; Studer, L. Human stem cell models of neurodegeneration: From basic science of amyotrophic lateral sclerosis to clinical translation. Cell Stem Cell 2022, 29, 11–35. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Feng, L.; Liang, L.; Stacey, G.N.; Wang, C.; Wang, Y.; Hu, B. Neuronal cell-based medicines from pluripotent stem cells: Development, production, and preclinical assessment. Stem Cells Transl. Med. 2021, 10, S31–S40. [Google Scholar] [CrossRef]
- Lampert, A.; Bennett, D.L.; McDermott, L.A.; Neureiter, A.; Eberhardt, E.; Winner, B.; Zenke, M. Human sensory neurons derived from pluripotent stem cells for disease modelling and personalized medicine. Neurobiol. Pain 2020, 8, 100055. [Google Scholar] [CrossRef]
- Ford, E.; Pearlman, J.; Ruan, T.; Manion, J.; Waller, M.; Neely, G.G.; Caron, L. Human Pluripotent Stem Cells-Based Therapies for Neurodegenerative Diseases: Current Status and Challenges. Cells 2020, 9, 2517. [Google Scholar] [CrossRef]
- Kim, J.Y.; Nam, Y.; Rim, Y.A.; Ju, J.H. Review of the Current Trends in Clinical Trials Involving Induced Pluripotent Stem Cells. Stem Cell Rev. Rep. 2022, 18, 142–154. [Google Scholar] [CrossRef]
- Sugai, K.; Sumida, M.; Shofuda, T.; Yamaguchi, R.; Tamura, T.; Kohzuki, T.; Abe, T.; Shibata, R.; Kamata, Y.; Ito, S.; et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: Study protocol. Regen. Ther. 2021, 18, 321–333. [Google Scholar] [CrossRef]
- Martin-Lopez, M.; Fernandez-Munoz, B.; Canovas, S. Pluripotent Stem Cells for Spinal Cord Injury Repair. Cells 2021, 10, 3334. [Google Scholar] [CrossRef] [PubMed]
- Nagoshi, N.; Tsuji, O.; Nakamura, M.; Okano, H. Cell therapy for spinal cord injury using induced pluripotent stem cells. Regen. Ther. 2019, 11, 75–80. [Google Scholar] [CrossRef]
- Li, Y.; Shen, P.P.; Wang, B. Induced pluripotent stem cell technology for spinal cord injury: A promising alternative therapy. Neural Regen. Res. 2021, 16, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, N.S.; Truong, V.; Malone, D.; Pengo, T.; Patil, N.; Dutton, J.R.; Parr, A.M. Human induced pluripotent stem cells integrate, create synapses and extend long axons after spinal cord injury. J. Cell. Mol. Med. 2022, 26, 1932–1942. [Google Scholar] [CrossRef] [PubMed]
- Dulin, J.N.; Adler, A.F.; Kumamaru, H.; Poplawski, G.H.D.; Lee-Kubli, C.; Strobl, H.; Gibbs, D.; Kadoya, K.; Fawcett, J.W.; Lu, P.; et al. Injured adult motor and sensory axons regenerate into appropriate organotypic domains of neural progenitor grafts. Nat. Commun. 2018, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Okada, Y.; Itakura, G.; Iwai, H.; Nishimura, S.; Yasuda, A.; Nori, S.; Hikishima, K.; Konomi, T.; Fujiyoshi, K.; et al. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS ONE 2012, 7, e52787. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Wang, Y.; Graham, L.; McHale, K.; Gao, M.; Wu, D.; Brock, J.; Blesch, A.; Rosenzweig, E.S.; Havton, L.A.; et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 2012, 150, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Woodruff, G.; Wang, Y.; Graham, L.; Hunt, M.; Wu, D.; Boehle, E.; Ahmad, R.; Poplawski, G.; Brock, J.; et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron 2014, 83, 789–796. [Google Scholar] [CrossRef]
- Zurita, M.; Vaquero, J.; Zurita, I. Presence and significance of CD-95 (Fas/APO1) expression after spinal cord injury. J. Neurosurg. 2001, 94, 257–264. [Google Scholar] [CrossRef]
- Kong, D.; Feng, B.; Amponsah, A.E.; He, J.; Guo, R.; Liu, B.; Du, X.; Liu, X.; Zhang, S.; Lv, F.; et al. hiPSC-derived NSCs effectively promote the functional recovery of acute spinal cord injury in mice. Stem Cell Res. Ther. 2021, 12, 172. [Google Scholar] [CrossRef]
- Ito, S.; Nagoshi, N.; Kamata, Y.; Kojima, K.; Nori, S.; Matsumoto, M.; Takei, K.; Nakamura, M.; Okano, H. LOTUS overexpression via ex vivo gene transduction further promotes recovery of motor function following human iPSC-NS/PC transplantation for contusive spinal cord injury. Stem Cell Rep. 2021, 16, 2703–2717. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, K.; Imaizumi, K.; Shinozaki, M.; Shibata, S.; Shindo, T.; Kitagawa, T.; Shibata, R.; Kamata, Y.; Kojima, K.; Nagoshi, N.; et al. Cell therapy for spinal cord injury by using human iPSC-derived region-specific neural progenitor cells. Mol. Brain 2020, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- van Den Hauwe, L.; Sundgren, P.C.; Flanders, A.E. Spinal Trauma and Spinal Cord Injury (SCI). In Diseases of the Brain, Head and Neck, Spine 2020–2023: Diagnostic Imaging: IDKD Springer Series; Hodler, J., Kubik-Huch, R.A., von Schulthess, G.K., Eds.; Springer: Cham, Switzerland, 2020; pp. 231–240. [Google Scholar]
- Li, S.; Zhang, A.; Xue, H.; Li, D.; Liu, Y. One-Step piggyBac Transposon-Based CRISPR/Cas9 Activation of Multiple Genes. Mol. Ther. Nucleic Acids 2017, 8, 64–76. [Google Scholar] [CrossRef]
- Macarthur, C.C.; Xue, H.; Van Hoof, D.; Lieu, P.T.; Dudas, M.; Fontes, A.; Swistowski, A.; Touboul, T.; Seerke, R.; Laurent, L.C.; et al. Chromatin insulator elements block transgene silencing in engineered human embryonic stem cell lines at a defined chromosome 13 locus. Stem Cells Dev. 2012, 21, 191–205. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Li, S.; Xue, H.; Schmitt, K.; Hergenroeder, G.W.; Wu, J.; Zhang, Y.; Kim, D.H.; Cao, Q. Human neural progenitors derived from integration-free iPSCs for SCI therapy. Stem Cell Res. 2017, 19, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, S.S.; Wu, Y.; Tuohy, T.M.; Xue, H.; Cai, J.; Back, S.A.; Sherman, L.S.; Fischer, I.; Rao, M.S. CD44 expression identifies astrocyte-restricted precursor cells. Dev. Biol. 2004, 276, 31–46. [Google Scholar] [CrossRef]
- Cao, Q.L.; Howard, R.M.; Dennison, J.B.; Whittemore, S.R. Differentiation of engrafted neuronal-restricted precursor cells is inhibited in the traumatically injured spinal cord. Exp. Neurol. 2002, 177, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Zhang, Y.P.; Iannotti, C.; DeVries, W.H.; Xu, X.M.; Shields, C.B.; Whittemore, S.R. Functional and electrophysiological changes after graded traumatic spinal cord injury in adult rat. Exp. Neurol. 2005, 191, S3–S16. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.L.; Zhang, Y.P.; Howard, R.M.; Walters, W.M.; Tsoulfas, P.; Whittemore, S.R. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp. Neurol. 2001, 167, 48–58. [Google Scholar] [CrossRef]
- Bertelli, J.A.; Mira, J.C. Behavioral evaluating methods in the objective clinical assessment of motor function after experimental brachial plexus reconstruction in the rat. J. Neurosci. Methods 1993, 46, 203–208. [Google Scholar] [CrossRef]
- Soblosky, J.S.; Song, J.H.; Dinh, D.H. Graded unilateral cervical spinal cord injury in the rat: Evaluation of forelimb recovery and histological effects. Behav. Brain Res. 2001, 119, 1–13. [Google Scholar] [CrossRef]
- Gallegos, C.; Carey, M.; Zheng, Y.; He, X.; Cao, Q.L. Reaching and Grasping Training Improves Functional Recovery After Chronic Cervical Spinal Cord Injury. Front. Cell. Neurosci. 2020, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Gensel, J.C.; Tovar, C.A.; Hamers, F.P.; Deibert, R.J.; Beattie, M.S.; Bresnahan, J.C. Behavioral and histological characterization of unilateral cervical spinal cord contusion injury in rats. J. Neurotrauma 2006, 23, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Coggeshall, R.E.; Lekan, H.A. Methods for determining numbers of cells and synapses: A case for more uniform standards of review. J. Comp. Neurol. 1996, 364, 6–15. [Google Scholar] [CrossRef]
- Cao, Q.; He, Q.; Wang, Y.; Cheng, X.; Howard, R.M.; Zhang, Y.; DeVries, W.H.; Shields, C.B.; Magnuson, D.S.K.; Xu, X.M.; et al. Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J. Neurosci. 2010, 30, 2989–3001. [Google Scholar] [CrossRef]
- Cheng, X.; Zheng, Y.; Bu, P.; Qi, X.; Fan, C.; Li, F.; Kim, D.H.; Cao, Q. Apolipoprotein E as a novel therapeutic neuroprotection target after traumatic spinal cord injury. Exp. Neurol. 2018, 299, 97–108. [Google Scholar] [CrossRef]
- Metz, G.A.; Whishaw, I.Q. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: A new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J. Neurosci. Methods 2002, 115, 169–179. [Google Scholar] [CrossRef]
- Kadoya, K.; Lu, P.; Nguyen, K.; Lee-Kubli, C.; Kumamaru, H.; Yao, L.; Knackert, J.; Poplawski, G.; Dulin, J.N.; Strobl, H.; et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat. Med. 2016, 22, 479–487. [Google Scholar] [CrossRef]
- Lien, B.V.; Tuszynski, M.H.; Lu, P. Astrocytes migrate from human neural stem cell grafts and functionally integrate into the injured rat spinal cord. Exp. Neurol. 2019, 314, 46–57. [Google Scholar] [CrossRef]
- Wickersham, I.R.; Lyon, D.C.; Barnard, R.J.; Mori, T.; Finke, S.; Conzelmann, K.K.; Young, J.A.; Callaway, E.M. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 2007, 53, 639–647. [Google Scholar] [CrossRef] [Green Version]
- Weible, A.P.; Schwarcz, L.; Wickersham, I.R.; Deblander, L.; Wu, H.; Callaway, E.M.; Seung, H.S.; Kentros, C.G. Transgenic targeting of recombinant rabies virus reveals monosynaptic connectivity of specific neurons. J. Neurosci. 2010, 30, 16509–16513. [Google Scholar] [CrossRef]
- Kitagawa, T.; Nagoshi, N.; Kamata, Y.; Kawai, M.; Ago, K.; Kajikawa, K.; Shibata, R.; Sato, Y.; Imaizumi, K.; Shindo, T.; et al. Modulation by DREADD reveals the therapeutic effect of human iPSC-derived neuronal activity on functional recovery after spinal cord injury. Stem Cell Rep. 2022, 17, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Imaizumi, K.; Ishikawa, M.; Shibata, S.; Shinozaki, M.; Shibata, T.; Hashimoto, S.; Kitagawa, T.; Ago, K.; Kajikawa, K.; et al. Long-term selective stimulation of transplanted neural stem/progenitor cells for spinal cord injury improves locomotor function. Cell Rep. 2021, 37, 110019. [Google Scholar] [CrossRef]
- Bellak, T.; Fekecs, Z.; Torok, D.; Tancos, Z.; Nemes, C.; Tezsla, Z.; Gal, L.; Polgari, S.; Kobolak, J.; Dinnyes, A.; et al. Grafted human induced pluripotent stem cells improve the outcome of spinal cord injury: Modulation of the lesion microenvironment. Sci. Rep. 2020, 10, 22414. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.I.; Rangappa, N.; Garry, M.G.; Smith, G.M. Functional regeneration of chronically injured sensory afferents into adult spinal cord after neurotrophin gene therapy. J. Neurosci. 2001, 21, 8408–8416. [Google Scholar] [CrossRef] [PubMed]
- Ramer, M.S.; Priestley, J.V.; McMahon, S.B. Functional regeneration of sensory axons into the adult spinal cord. Nature 2000, 403, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Fischer, I.; Tessler, A.; Houle, J.D. Transplants of fibroblasts genetically modified to express BDNF promote axonal regeneration from supraspinal neurons following chronic spinal cord injury. Exp. Neurol. 2002, 177, 265–275. [Google Scholar] [CrossRef]
- Jin, Y.; Tessler, A.; Fischer, I.; Houle, J.D. Fibroblasts genetically modified to produce BDNF support regrowth of chronically injured serotonergic axons. Neurorehabil. Neural Repair 2000, 14, 311–317. [Google Scholar] [CrossRef]
- Fan, C.; Zheng, Y.; Cheng, X.; Qi, X.; Bu, P.; Luo, X.; Kim, D.H.; Cao, Q. Transplantation of D15A-expressing glial-restricted-precursor-derived astrocytes improves anatomical and locomotor recovery after spinal cord injury. Int. J. Biol. Sci. 2013, 9, 78–93. [Google Scholar] [CrossRef]
- Tanimoto, Y.; Yamasaki, T.; Nagoshi, N.; Nishiyama, Y.; Nori, S.; Nishimura, S.; Iida, T.; Ozaki, M.; Tsuji, O.; Ji, B.; et al. In vivo monitoring of remnant undifferentiated neural cells following human induced pluripotent stem cell-derived neural stem/progenitor cells transplantation. Stem Cells Transl. Med. 2020, 9, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Ceto, S.; Wang, Y.; Graham, L.; Wu, D.; Kumamaru, H.; Staufenberg, E.; Tuszynski, M.H. Prolonged human neural stem cell maturation supports recovery in injured rodent CNS. J. Clin. Investig. 2017, 127, 3287–3299. [Google Scholar] [CrossRef] [PubMed]
- Ghobrial, G.M.; Anderson, K.D.; Dididze, M.; Martinez-Barrizonte, J.; Sunn, G.H.; Gant, K.L.; Levi, A.D. Human Neural Stem Cell Transplantation in Chronic Cervical Spinal Cord Injury: Functional Outcomes at 12 Months in a Phase II Clinical Trial. Neurosurgery 2017, 64, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Levi, A.D.; Anderson, K.D.; Okonkwo, D.O.; Park, P.; Bryce, T.N.; Kurpad, S.N.; Aarabi, B.; Hsieh, J.; Gant, K. Clinical Outcomes from a Multi-Center Study of Human Neural Stem Cell Transplantation in Chronic Cervical Spinal Cord Injury. J. Neurotrauma 2019, 36, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Ortuno-Costela, M.D.C.; Cerrada, V.; Garcia-Lopez, M.; Gallardo, M.E. The Challenge of Bringing iPSCs to the Patient. Int. J. Mol. Sci. 2019, 20, 6305. [Google Scholar] [CrossRef]
- Han, X.; Wang, M.; Duan, S.; Franco, P.J.; Kenty, J.H.; Hedrick, P.; Xia, Y.; Allen, A.; Ferreira, L.M.R.; Strominger, J.L.; et al. Generation of hypoimmunogenic human pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2019, 116, 10441–10446. [Google Scholar] [CrossRef]
- Lanza, R.; Russell, D.W.; Nagy, A. Engineering universal cells that evade immune detection. Nat. Rev. Immunol. 2019, 19, 723–733. [Google Scholar] [CrossRef]
- Deuse, T.; Hu, X.; Gravina, A.; Wang, D.; Tediashvili, G.; De, C.; Thayer, W.O.; Wahl, A.; Garcia, J.V.; Reichenspurner, H.; et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 2019, 37, 252–258. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Gallegos, C.M.; Xue, H.; Li, S.; Kim, D.H.; Zhou, H.; Xia, X.; Liu, Y.; Cao, Q. Transplantation of Human Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Promotes Forelimb Functional Recovery after Cervical Spinal Cord Injury. Cells 2022, 11, 2765. https://doi.org/10.3390/cells11172765
Zheng Y, Gallegos CM, Xue H, Li S, Kim DH, Zhou H, Xia X, Liu Y, Cao Q. Transplantation of Human Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Promotes Forelimb Functional Recovery after Cervical Spinal Cord Injury. Cells. 2022; 11(17):2765. https://doi.org/10.3390/cells11172765
Chicago/Turabian StyleZheng, Yiyan, Chrystine M. Gallegos, Haipeng Xue, Shenglan Li, Dong H. Kim, Hongxia Zhou, Xugang Xia, Ying Liu, and Qilin Cao. 2022. "Transplantation of Human Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Promotes Forelimb Functional Recovery after Cervical Spinal Cord Injury" Cells 11, no. 17: 2765. https://doi.org/10.3390/cells11172765
APA StyleZheng, Y., Gallegos, C. M., Xue, H., Li, S., Kim, D. H., Zhou, H., Xia, X., Liu, Y., & Cao, Q. (2022). Transplantation of Human Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Promotes Forelimb Functional Recovery after Cervical Spinal Cord Injury. Cells, 11(17), 2765. https://doi.org/10.3390/cells11172765







