Liver Kinase B1 Functions as a Regulator for Neural Development and a Therapeutic Target for Neural Repair
Abstract
1. Introduction
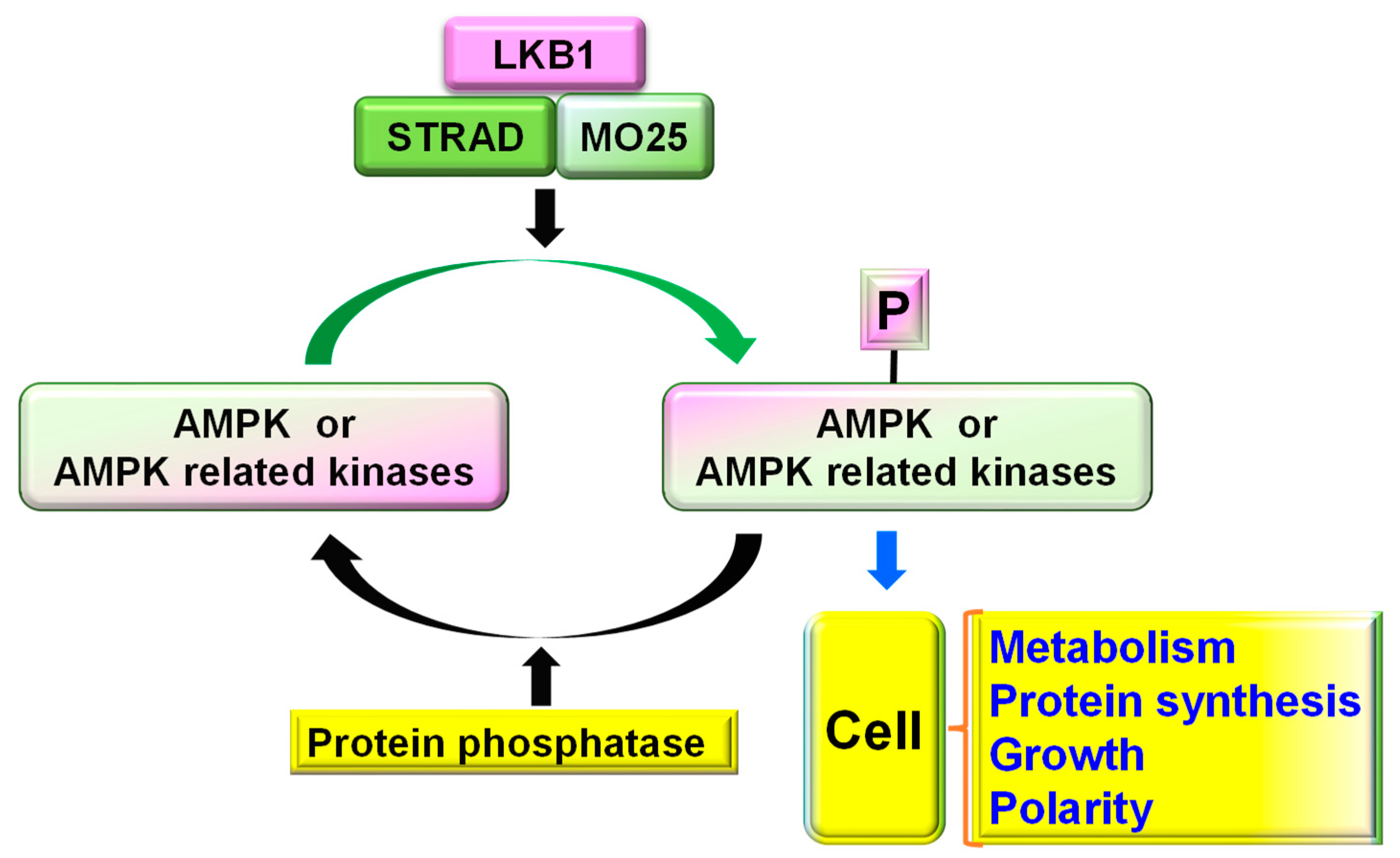
2. LKB1 Gene, Structure, and Overall Cellular Functions
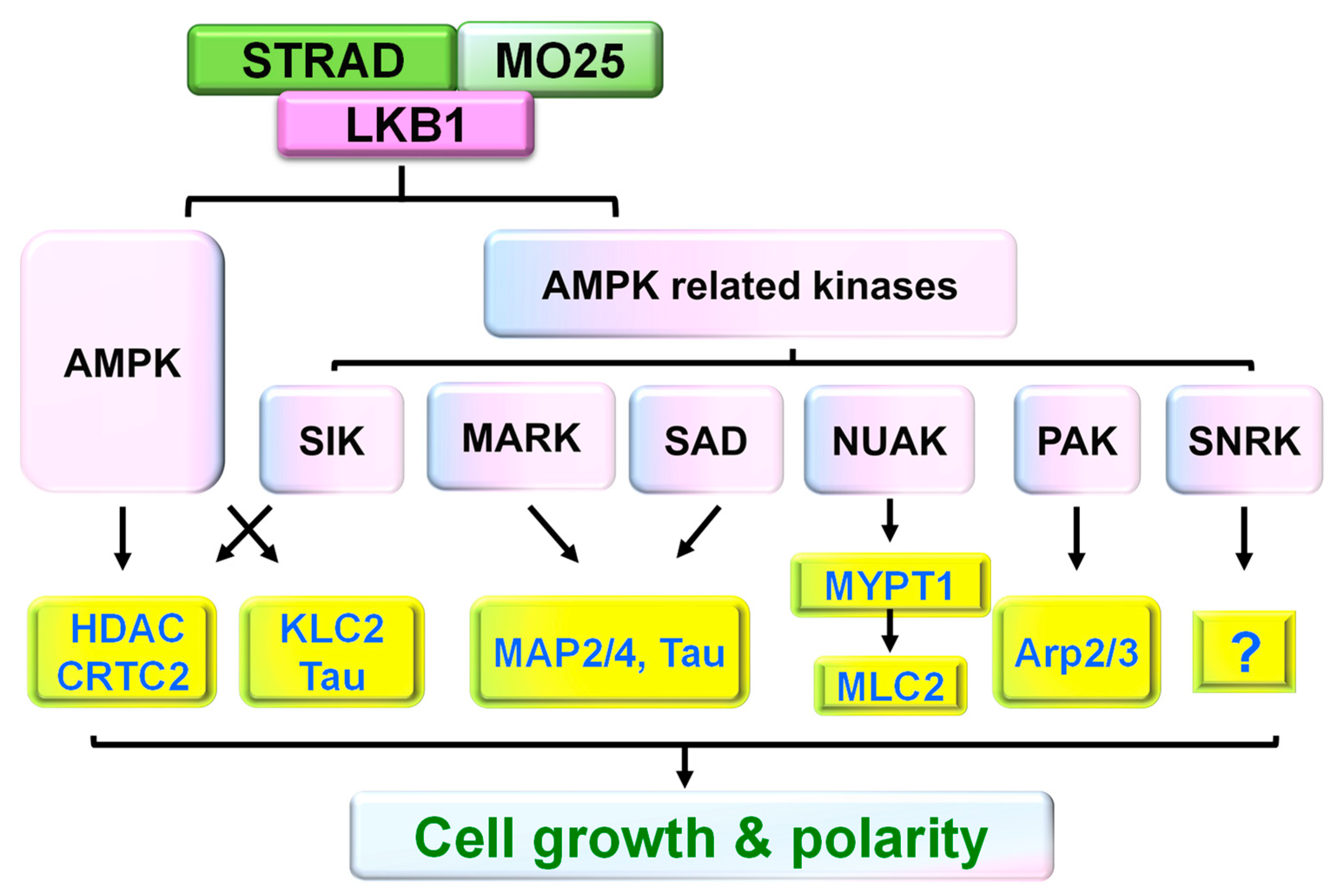
3. LKB1 Forms Protein Complexes for Function in Neural Cells
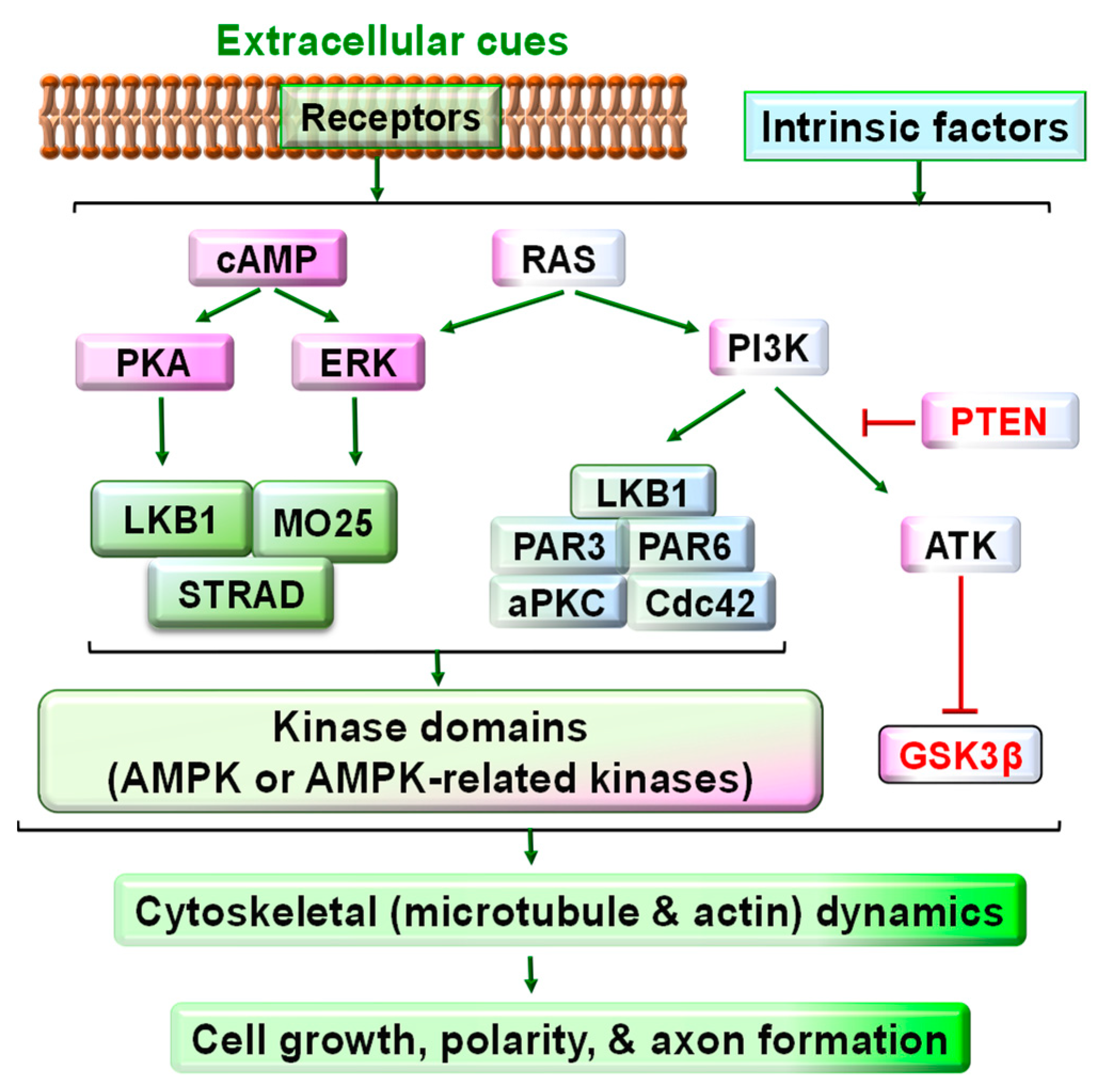
4. Major Signaling Pathways Downstream of LKB1 in Neurons or Other Cell Types
5. Critical Role of LKB1 in Neuronal Polarity and Axon Development
6. LKB1 Regulates Schwann Cell (SC) Function and PNS Myelination during Development
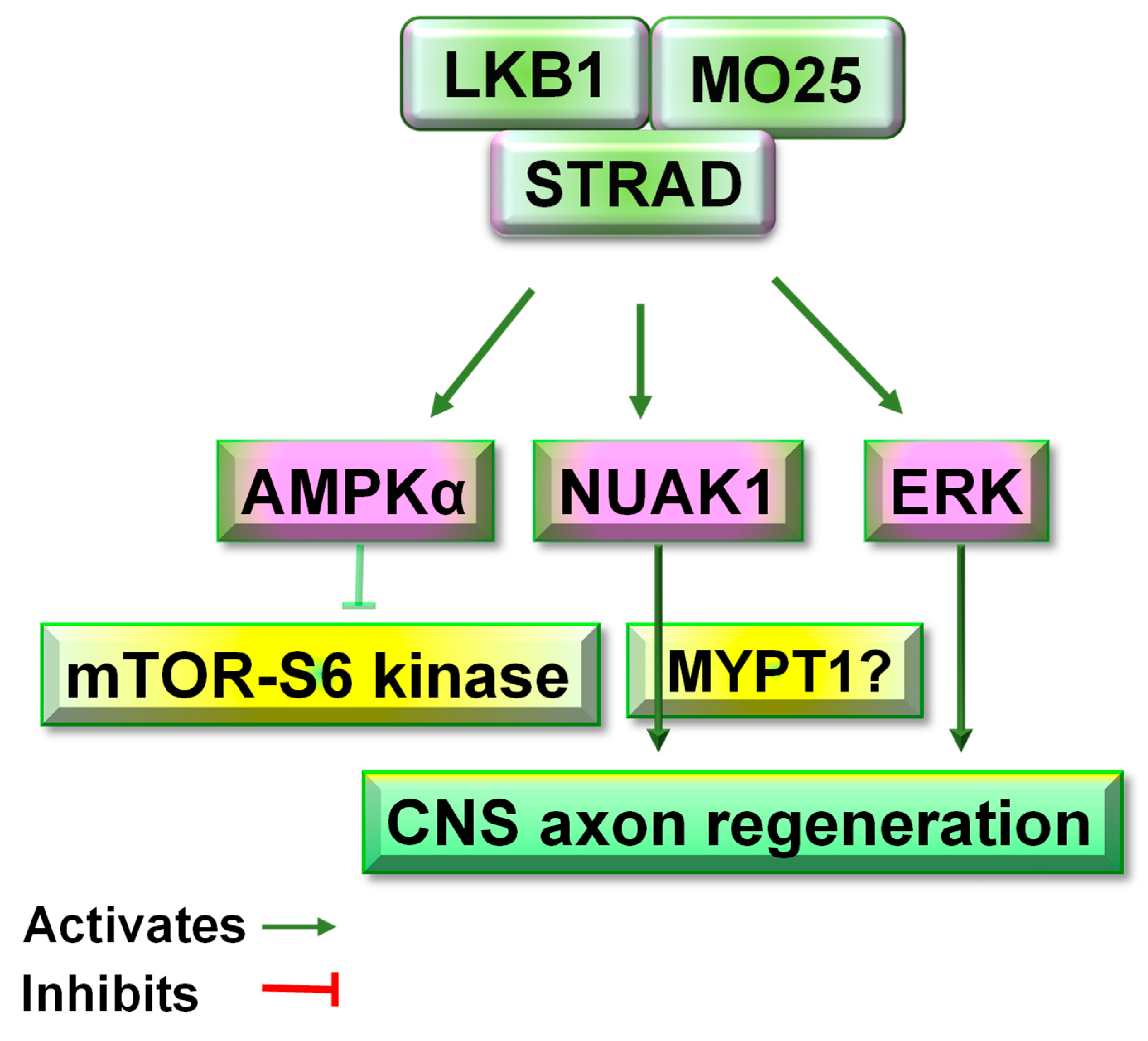
7. LKB1 Acts as a Molecular Target for CNS Axon Regeneration
8. Potential Signal Pathways Downstream of LKB1 in Adult CNS
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hemminki, A.; Markie, D.; Tomlinson, I.; Avizienyte, E.; Roth, S.; Loukola, A.; Bignell, G.; Warren, W.; Aminoff, M.; Hoglund, P.; et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 1998, 391, 184–187. [Google Scholar] [CrossRef]
- Nakau, M.; Miyoshi, H.; Seldin, M.F.; Imamura, M.; Oshima, M.; Taketo, M.M. Hepatocellular carcinoma caused by loss of heterozygosity in Lkb1 gene knockout mice. Cancer Res. 2002, 62, 4549–4553. [Google Scholar]
- Gan, R.Y.; Li, H.B. Recent progress on liver kinase B1 (LKB1): Expression, regulation, downstream signaling and cancer suppressive function. Int. J. Mol. Sci. 2014, 15, 16698–16718. [Google Scholar] [CrossRef]
- Jansen, M.; Ten Klooster, J.P.; Offerhaus, G.J.; Clevers, H. LKB1 and AMPK family signaling: The intimate link between cell polarity and energy metabolism. Physiol. Rev. 2009, 89, 777–798. [Google Scholar] [CrossRef]
- Ohtake, Y.; Sami, A.; Jiang, X.; Horiuchi, M.; Slattery, K.; Ma, L.; Smith, G.M.; Selzer, M.E.; Muramatsu, S.I.; Li, S. Promoting Axon Regeneration in Adult CNS by Targeting Liver Kinase B1. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 102–117. [Google Scholar] [CrossRef]
- Mehenni, H.; Gehrig, C.; Nezu, J.; Oku, A.; Shimane, M.; Rossier, C.; Guex, N.; Blouin, J.L.; Scott, H.S.; Antonarakis, S.E. Loss of LKB1 kinase activity in Peutz-Jeghers syndrome, and evidence for allelic and locus heterogeneity. Am. J. Hum. Genet. 1998, 63, 1641–1650. [Google Scholar] [CrossRef]
- Smith, D.P.; Spicer, J.; Smith, A.; Swift, S.; Ashworth, A. The mouse Peutz-Jeghers syndrome gene Lkb1 encodes a nuclear protein kinase. Hum. Mol. Genet. 1999, 8, 1479–1485. [Google Scholar] [CrossRef]
- Su, J.Y.; Erikson, E.; Maller, J.L. Cloning and characterization of a novel serine/threonine protein kinase expressed in early Xenopus embryos. J. Biol. Chem. 1996, 271, 14430–14437. [Google Scholar] [CrossRef]
- Watts, J.L.; Morton, D.G.; Bestman, J.; Kemphues, K.J. The C. elegans par-4 gene encodes a putative serine-threonine kinase required for establishing embryonic asymmetry. Development 2000, 127, 1467–1475. [Google Scholar] [CrossRef]
- Martin, S.G.; St Johnston, D. A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature 2003, 421, 379–384. [Google Scholar] [CrossRef]
- Abed, A.A.; Gunther, K.; Kraus, C.; Hohenberger, W.; Ballhausen, W.G. Mutation screening at the RNA level of the STK11/LKB1 gene in Peutz-Jeghers syndrome reveals complex splicing abnormalities and a novel mRNA isoform (STK11 c.597(insertion mark)598insIVS4). Hum. Mutat. 2001, 18, 397–410. [Google Scholar] [CrossRef]
- Alessi, D.R.; Sakamoto, K.; Bayascas, J.R. LKB1-dependent signaling pathways. Annu. Rev. Biochem. 2006, 75, 137–163. [Google Scholar] [CrossRef]
- Dorfman, J.; Macara, I.G. STRADalpha regulates LKB1 localization by blocking access to importin-alpha, and by association with Crm1 and exportin-7. Mol. Biol. Cell 2008, 19, 1614–1626. [Google Scholar] [CrossRef]
- Shelly, M.; Poo, M.M. Role of LKB1-SAD/MARK pathway in neuronal polarization. Dev. Neurobiol. 2011, 71, 508–527. [Google Scholar] [CrossRef]
- Barnes, A.P.; Lilley, B.N.; Pan, Y.A.; Plummer, L.J.; Powell, A.W.; Raines, A.N.; Sanes, J.R.; Polleux, F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell 2007, 129, 549–563. [Google Scholar] [CrossRef]
- Jenne, D.E.; Reimann, H.; Nezu, J.; Friedel, W.; Loff, S.; Jeschke, R.; Muller, O.; Back, W.; Zimmer, M. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat. Genet. 1998, 18, 38–43. [Google Scholar] [CrossRef]
- Pooya, S.; Liu, X.; Kumar, V.B.; Anderson, J.; Imai, F.; Zhang, W.; Ciraolo, G.; Ratner, N.; Setchell, K.D.; Yoshida, Y.; et al. The tumour suppressor LKB1 regulates myelination through mitochondrial metabolism. Nat. Commun. 2014, 5, 4993. [Google Scholar] [CrossRef]
- Shen, Y.A.; Chen, Y.; Dao, D.Q.; Mayoral, S.R.; Wu, L.; Meijer, D.; Ullian, E.M.; Chan, J.R.; Lu, Q.R. Phosphorylation of LKB1/Par-4 establishes Schwann cell polarity to initiate and control myelin extent. Nat. Commun. 2014, 5, 4991. [Google Scholar] [CrossRef]
- Beirowski, B.; Babetto, E.; Golden, J.P.; Chen, Y.J.; Yang, K.; Gross, R.W.; Patti, G.J.; Milbrandt, J. Metabolic regulator LKB1 is crucial for Schwann cell-mediated axon maintenance. Nat. Neurosci. 2014, 17, 1351–1361. [Google Scholar] [CrossRef]
- Radu, A.G.; Torch, S.; Fauvelle, F.; Pernet-Gallay, K.; Lucas, A.; Blervaque, R.; Delmas, V.; Schlattner, U.; Lafanechere, L.; Hainaut, P.; et al. LKB1 specifies neural crest cell fates through pyruvate-alanine cycling. Sci. Adv. 2019, 5, eaau5106. [Google Scholar] [CrossRef]
- Baas, A.F.; Kuipers, J.; van der Wel, N.N.; Batlle, E.; Koerten, H.K.; Peters, P.J.; Clevers, H.C. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 2004, 116, 457–466. [Google Scholar] [CrossRef]
- Forcet, C.; Etienne-Manneville, S.; Gaude, H.; Fournier, L.; Debilly, S.; Salmi, M.; Baas, A.; Olschwang, S.; Clevers, H.; Billaud, M. Functional analysis of Peutz-Jeghers mutations reveals that the LKB1 C-terminal region exerts a crucial role in regulating both the AMPK pathway and the cell polarity. Hum. Mol. Genet. 2005, 14, 1283–1292. [Google Scholar] [CrossRef][Green Version]
- Winckler, B. BDNF instructs the kinase LKB1 to grow an axon. Cell 2007, 129, 459–460. [Google Scholar] [CrossRef]
- Zhou, F.Q.; Snider, W.D. Intracellular control of developmental and regenerative axon growth. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1575–1592. [Google Scholar] [CrossRef]
- Liu, K.; Tedeschi, A.; Park, K.K.; He, Z. Neuronal intrinsic mechanisms of axon regeneration. Annu. Rev. Neurosci. 2011, 34, 131–152. [Google Scholar] [CrossRef]
- Esteve-Puig, R.; Canals, F.; Colome, N.; Merlino, G.; Recio, J.A. Uncoupling of the LKB1-AMPKalpha energy sensor pathway by growth factors and oncogenic BRAF. PLoS ONE 2009, 4, e4771. [Google Scholar] [CrossRef]
- Frodin, M.; Gammeltoft, S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol. Cell Endocrinol. 1999, 151, 65–77. [Google Scholar] [CrossRef]
- Hawley, S.A.; Boudeau, J.; Reid, J.L.; Mustard, K.J.; Udd, L.; Makela, T.P.; Alessi, D.R.; Hardie, D.G. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2003, 2, 28. [Google Scholar] [CrossRef]
- Baas, A.F.; Boudeau, J.; Sapkota, G.P.; Smit, L.; Medema, R.; Morrice, N.A.; Alessi, D.R.; Clevers, H.C. Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J. 2003, 22, 3062–3072. [Google Scholar] [CrossRef]
- Veleva-Rotse, B.O.; Smart, J.L.; Baas, A.F.; Edmonds, B.; Zhao, Z.M.; Brown, A.; Klug, L.R.; Hansen, K.; Reilly, G.; Gardner, A.P.; et al. STRAD pseudokinases regulate axogenesis and LKB1 stability. Neural Dev. 2014, 9, 5. [Google Scholar] [CrossRef]
- Boudeau, J.; Baas, A.F.; Deak, M.; Morrice, N.A.; Kieloch, A.; Schutkowski, M.; Prescott, A.R.; Clevers, H.C.; Alessi, D.R. MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 2003, 22, 5102–5114. [Google Scholar] [CrossRef]
- Lee, S.I.; Zhang, W.; Ravi, M.; Weschenfelder, M.; Bastmeyer, M.; Levine, J.M. Atypical protein kinase C and Par3 are required for proteoglycan-induced axon growth inhibition. J. Neurosci. 2013, 33, 2541–2554. [Google Scholar] [CrossRef]
- Xie, Z.; Dong, Y.; Scholz, R.; Neumann, D.; Zou, M.H. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation 2008, 117, 952–962. [Google Scholar] [CrossRef]
- Zhu, H.; Moriasi, C.M.; Zhang, M.; Zhao, Y.; Zou, M.H. Phosphorylation of serine 399 in LKB1 protein short form by protein kinase Czeta is required for its nucleocytoplasmic transport and consequent AMP-activated protein kinase (AMPK) activation. J. Biol. Chem. 2013, 288, 16495–16505. [Google Scholar] [CrossRef]
- Ohtake, Y.; Wong, D.; Abdul-Muneer, P.M.; Selzer, M.E.; Li, S. Two PTP receptors mediate CSPG inhibition by convergent and divergent signaling pathways in neurons. Sci. Rep. 2016, 6, 37152. [Google Scholar] [CrossRef]
- Mehenni, H.; Lin-Marq, N.; Buchet-Poyau, K.; Reymond, A.; Collart, M.A.; Picard, D.; Antonarakis, S.E. LKB1 interacts with and phosphorylates PTEN: A functional link between two proteins involved in cancer predisposing syndromes. Hum. Mol. Genet. 2005, 14, 2209–2219. [Google Scholar] [CrossRef]
- Lizcano, J.M.; Goransson, O.; Toth, R.; Deak, M.; Morrice, N.A.; Boudeau, J.; Hawley, S.A.; Udd, L.; Makela, T.P.; Hardie, D.G.; et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004, 23, 833–843. [Google Scholar] [CrossRef]
- Shaw, R.J.; Kosmatka, M.; Bardeesy, N.; Hurley, R.L.; Witters, L.A.; DePinho, R.A.; Cantley, L.C. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA 2004, 101, 3329–3335. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Mirouse, V.; Billaud, M. The LKB1/AMPK polarity pathway. FEBS Lett. 2011, 585, 981–985. [Google Scholar] [CrossRef]
- Amato, S.; Liu, X.; Zheng, B.; Cantley, L.; Rakic, P.; Man, H.Y. AMP-activated protein kinase regulates neuronal polarization by interfering with PI 3-kinase localization. Science 2011, 332, 247–251. [Google Scholar] [CrossRef]
- Thornton, C.; Bright, N.J.; Sastre, M.; Muckett, P.J.; Carling, D. AMP-activated protein kinase (AMPK) is a tau kinase, activated in response to amyloid beta-peptide exposure. Biochem. J. 2011, 434, 503–512. [Google Scholar] [CrossRef]
- Screaton, R.A.; Conkright, M.D.; Katoh, Y.; Best, J.L.; Canettieri, G.; Jeffries, S.; Guzman, E.; Niessen, S.; Yates, J.R., 3rd; Takemori, H.; et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell 2004, 119, 61–74. [Google Scholar] [CrossRef]
- Du, W.Q.; Zheng, J.N.; Pei, D.S. The diverse oncogenic and tumor suppressor roles of salt-inducible kinase (SIK) in cancer. Expert Opin. Targets 2016, 20, 477–485. [Google Scholar] [CrossRef]
- Walkinshaw, D.R.; Weist, R.; Kim, G.W.; You, L.; Xiao, L.; Nie, J.; Li, C.S.; Zhao, S.; Xu, M.; Yang, X.J. The tumor suppressor kinase LKB1 activates the downstream kinases SIK2 and SIK3 to stimulate nuclear export of class IIa histone deacetylases. J. Biol. Chem. 2013, 288, 9345–9362. [Google Scholar] [CrossRef]
- Proschel, C.; Hansen, J.N.; Ali, A.; Tuttle, E.; Lacagnina, M.; Buscaglia, G.; Halterman, M.W.; Paciorkowski, A.R. Epilepsy-causing sequence variations in SIK1 disrupt synaptic activity response gene expression and affect neuronal morphology. Eur. J. Hum. Genet. 2017, 25, 216–221. [Google Scholar] [CrossRef]
- Bernard, L.P.; Zhang, H. MARK/Par1 Kinase Is Activated Downstream of NMDA Receptors through a PKA-Dependent Mechanism. PLoS ONE 2015, 10, e0124816. [Google Scholar] [CrossRef]
- Nakano, A.; Takashima, S. LKB1 and AMP-activated protein kinase: Regulators of cell polarity. Genes Cells 2012, 17, 737–747. [Google Scholar] [CrossRef]
- Sapir, T.; Shmueli, A.; Levy, T.; Timm, T.; Elbaum, M.; Mandelkow, E.M.; Reiner, O. Antagonistic effects of doublecortin and MARK2/Par-1 in the developing cerebral cortex. J. Neurosci. 2008, 28, 13008–13013. [Google Scholar] [CrossRef]
- Kishi, M.; Pan, Y.A.; Crump, J.G.; Sanes, J.R. Mammalian SAD kinases are required for neuronal polarization. Science 2005, 307, 929–932. [Google Scholar] [CrossRef]
- Zagorska, A.; Deak, M.; Campbell, D.G.; Banerjee, S.; Hirano, M.; Aizawa, S.; Prescott, A.R.; Alessi, D.R. New roles for the LKB1-NUAK pathway in controlling myosin phosphatase complexes and cell adhesion. Sci. Signal. 2010, 3, ra25. [Google Scholar] [CrossRef]
- Ito, M.; Nakano, T.; Erdodi, F.; Hartshorne, D.J. Myosin phosphatase: Structure, regulation and function. Mol. Cell Biochem. 2004, 259, 197–209. [Google Scholar] [CrossRef]
- Yamamoto, H.; Takashima, S.; Shintani, Y.; Yamazaki, S.; Seguchi, O.; Nakano, A.; Higo, S.; Kato, H.; Liao, Y.; Asano, Y.; et al. Identification of a novel substrate for TNFalpha-induced kinase NUAK2. Biochem. Biophys. Res. Commun. 2008, 365, 541–547. [Google Scholar] [CrossRef]
- Juanes-Garcia, A.; Chapman, J.R.; Aguilar-Cuenca, R.; Delgado-Arevalo, C.; Hodges, J.; Whitmore, L.A.; Shabanowitz, J.; Hunt, D.F.; Horwitz, A.R.; Vicente-Manzanares, M. A regulatory motif in nonmuscle myosin II-B regulates its role in migratory front-back polarity. J. Cell Biol. 2015, 209, 23–32. [Google Scholar] [CrossRef]
- Ohmura, T.; Shioi, G.; Hirano, M.; Aizawa, S. Neural tube defects by NUAK1 and NUAK2 double mutation. Dev. Dyn. 2012, 241, 1350–1364. [Google Scholar] [CrossRef]
- Deguchi, A.; Miyoshi, H.; Kojima, Y.; Okawa, K.; Aoki, M.; Taketo, M.M. LKB1 suppresses p21-activated kinase-1 (PAK1) by phosphorylation of Thr109 in the p21-binding domain. J. Biol. Chem. 2010, 285, 18283–18290. [Google Scholar] [CrossRef]
- Daniels, R.H.; Hall, P.S.; Bokoch, G.M. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J. 1998, 17, 754–764. [Google Scholar] [CrossRef]
- Vadlamudi, R.K.; Li, F.; Barnes, C.J.; Bagheri-Yarmand, R.; Kumar, R. p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate. EMBO Rep. 2004, 5, 154–160. [Google Scholar] [CrossRef]
- Edwards, D.C.; Sanders, L.C.; Bokoch, G.M.; Gill, G.N. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat. Cell Biol. 1999, 1, 253–259. [Google Scholar] [CrossRef]
- Jaleel, M.; McBride, A.; Lizcano, J.M.; Deak, M.; Toth, R.; Morrice, N.A.; Alessi, D.R. Identification of the sucrose non-fermenting related kinase SNRK, as a novel LKB1 substrate. FEBS Lett. 2005, 579, 1417–1423. [Google Scholar] [CrossRef]
- Shelly, M.; Cancedda, L.; Heilshorn, S.; Sumbre, G.; Poo, M.M. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell 2007, 129, 565–577. [Google Scholar] [CrossRef]
- Lewis, T.L., Jr.; Courchet, J.; Polleux, F. Cell biology in neuroscience: Cellular and molecular mechanisms underlying axon formation, growth, and branching. J. Cell Biol. 2013, 202, 837–848. [Google Scholar] [CrossRef]
- Nave, K.A. Myelination and the trophic support of long axons. Nat. Rev. Neurosci. 2010, 11, 275–283. [Google Scholar] [CrossRef]
- Dotti, C.G.; Sullivan, C.A.; Banker, G.A. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 1988, 8, 1454–1468. [Google Scholar] [CrossRef]
- Shelly, M.; Cancedda, L.; Lim, B.K.; Popescu, A.T.; Cheng, P.L.; Gao, H.; Poo, M.M. Semaphorin3A regulates neuronal polarization by suppressing axon formation and promoting dendrite growth. Neuron 2011, 71, 433–446. [Google Scholar] [CrossRef]
- Ylikorkala, A.; Rossi, D.J.; Korsisaari, N.; Luukko, K.; Alitalo, K.; Henkemeyer, M.; Makela, T.P. Vascular abnormalities and deregulation of VEGF in Lkb1-deficient mice. Science 2001, 293, 1323–1326. [Google Scholar] [CrossRef]
- Asada, N.; Sanada, K.; Fukada, Y. LKB1 regulates neuronal migration and neuronal differentiation in the developing neocortex through centrosomal positioning. J. Neurosci. 2007, 27, 11769–11775. [Google Scholar] [CrossRef]
- Courchet, J.; Lewis, T.L., Jr.; Lee, S.; Courchet, V.; Liou, D.Y.; Aizawa, S.; Polleux, F. Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell 2013, 153, 1510–1525. [Google Scholar] [CrossRef]
- Song, P.; Xie, Z.; Wu, Y.; Xu, J.; Dong, Y.; Zou, M.H. Protein kinase Czeta-dependent LKB1 serine 428 phosphorylation increases LKB1 nucleus export and apoptosis in endothelial cells. J. Biol. Chem. 2008, 283, 12446–12455. [Google Scholar] [CrossRef]
- Schwamborn, J.C.; Puschel, A.W. The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nat. Neurosci. 2004, 7, 923–929. [Google Scholar] [CrossRef]
- Shi, S.H.; Jan, L.Y.; Jan, Y.N. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell 2003, 112, 63–75. [Google Scholar] [CrossRef]
- Funahashi, Y.; Namba, T.; Fujisue, S.; Itoh, N.; Nakamuta, S.; Kato, K.; Shimada, A.; Xu, C.; Shan, W.; Nishioka, T.; et al. ERK2-mediated phosphorylation of Par3 regulates neuronal polarization. J. Neurosci. 2013, 33, 13270–13285. [Google Scholar] [CrossRef]
- Nishimura, T.; Yamaguchi, T.; Kato, K.; Yoshizawa, M.; Nabeshima, Y.; Ohno, S.; Hoshino, M.; Kaibuchi, K. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat. Cell Biol. 2005, 7, 270–277. [Google Scholar] [CrossRef]
- Govek, E.E.; Newey, S.E.; Van Aelst, L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005, 19, 1–49. [Google Scholar] [CrossRef]
- Namba, T.; Funahashi, Y.; Nakamuta, S.; Xu, C.; Takano, T.; Kaibuchi, K. Extracellular and Intracellular Signaling for Neuronal Polarity. Physiol. Rev. 2015, 95, 995–1024. [Google Scholar] [CrossRef]
- Lilley, B.N.; Pan, Y.A.; Sanes, J.R. SAD kinases sculpt axonal arbors of sensory neurons through long- and short-term responses to neurotrophin signals. Neuron 2013, 79, 39–53. [Google Scholar] [CrossRef][Green Version]
- Biernat, J.; Gustke, N.; Drewes, G.; Mandelkow, E.M.; Mandelkow, E. Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: Distinction between PHF-like immunoreactivity and microtubule binding. Neuron 1993, 11, 153–163. [Google Scholar] [CrossRef]
- Choi, Y.J.; Di Nardo, A.; Kramvis, I.; Meikle, L.; Kwiatkowski, D.J.; Sahin, M.; He, X. Tuberous sclerosis complex proteins control axon formation. Genes Dev. 2008, 22, 2485–2495. [Google Scholar] [CrossRef]
- Biernat, J.; Wu, Y.Z.; Timm, T.; Zheng-Fischhofer, Q.; Mandelkow, E.; Meijer, L.; Mandelkow, E.M. Protein kinase MARK/PAR-1 is required for neurite outgrowth and establishment of neuronal polarity. Mol. Biol. Cell 2002, 13, 4013–4028. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wang, Q.J.; Hu, H.S.; Yu, P.C.; Zhu, J.; Drewes, G.; Piwnica-Worms, H.; Luo, Z.G. Microtubule affinity-regulating kinase 2 functions downstream of the PAR-3/PAR-6/atypical PKC complex in regulating hippocampal neuronal polarity. Proc. Natl. Acad. Sci. USA 2006, 103, 8534–8539. [Google Scholar] [CrossRef]
- Segu, L.; Pascaud, A.; Costet, P.; Darmon, M.; Buhot, M.C. Impairment of spatial learning and memory in ELKL Motif Kinase1 (EMK1/MARK2) knockout mice. Neurobiol. Aging 2008, 29, 231–240. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Jin, Y. Intrinsic Control of Axon Regeneration. Neuron 2016, 90, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Matsuwaki, T.; Asakura, R.; Suzuki, M.; Yamanouchi, K.; Nishihara, M. Age-dependent changes in progranulin expression in the mouse brain. J. Reprod. Dev. 2011, 57, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Ahn, J.H.; Park, J.H.; Won, M.H.; Lee, C.H. Age-dependent changes in the protein expression levels of Redd1 and mTOR in the gerbil hippocampus during normal aging. Mol. Med. Rep. 2016, 13, 2409–2414. [Google Scholar] [CrossRef] [PubMed]
- Iida, A.; Takino, N.; Miyauchi, H.; Shimazaki, K.; Muramatsu, S. Systemic delivery of tyrosine-mutant AAV vectors results in robust transduction of neurons in adult mice. BioMed Res. Int. 2013, 2013, 974819. [Google Scholar] [CrossRef]
- Hawley, S.A.; Davison, M.; Woods, A.; Davies, S.P.; Beri, R.K.; Carling, D.; Hardie, D.G. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J. Biol. Chem. 1996, 271, 27879–27887. [Google Scholar] [CrossRef]
- Samuel, M.A.; Voinescu, P.E.; Lilley, B.N.; de Cabo, R.; Foretz, M.; Viollet, B.; Pawlyk, B.; Sandberg, M.A.; Vavvas, D.G.; Sanes, J.R. LKB1 and AMPK regulate synaptic remodeling in old age. Nat. Neurosci. 2014, 17, 1190–1197. [Google Scholar] [CrossRef]
- Williams, T.; Courchet, J.; Viollet, B.; Brenman, J.E.; Polleux, F. AMP-activated protein kinase (AMPK) activity is not required for neuronal development but regulates axogenesis during metabolic stress. Proc. Natl. Acad. Sci. USA 2011, 108, 5849–5854. [Google Scholar] [CrossRef]
- Hollis, E.R., 2nd; Jamshidi, P.; Low, K.; Blesch, A.; Tuszynski, M.H. Induction of corticospinal regeneration by lentiviral trkB-induced Erk activation. Proc. Natl. Acad. Sci. USA 2009, 106, 7215–7220. [Google Scholar] [CrossRef]
- Kuwako, K.I.; Okano, H. The LKB1-SIK Pathway Controls Dendrite Self-Avoidance in Purkinje Cells. Cell Rep. 2018, 24, 2808–2818.e4. [Google Scholar] [CrossRef]
- Germain, M.; Nguyen, A.P.; Khacho, M.; Patten, D.A.; Screaton, R.A.; Park, D.S.; Slack, R.S. LKB1-regulated adaptive mechanisms are essential for neuronal survival following mitochondrial dysfunction. Hum. Mol. Genet. 2013, 22, 952–962. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, G.; Reynolds, R.; Leclerc, I.; Rutter, G.A. RIP2-mediated LKB1 deletion causes axon degeneration in the spinal cord and hind-limb paralysis. Dis. Model. Mech. 2011, 4, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.; Zhang, A.; Li, H.; Zhang, T.; Jin, Y.; Li, H.; Zhang, J.; Gao, J. LKB1 Is Required for the Development and Maintenance of Stereocilia in Inner Ear Hair Cells in Mice. PLoS ONE 2015, 10, e0135841. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.; Yuan, H.; Wang, X.; Sha, S.H. Noise-Induced Loss of Hair Cells and Cochlear Synaptopathy are Mediated by the Activation of AMPK. J. Neurosci. 2016, 36, 7497–7510. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, S.; Meares, G.P.; Lin, S.X.; Pietruczyk, E.A.; Saher, G.; Spieth, L.; Nave, K.A.; Boullerne, A.I.; Lutz, S.E.; Benveniste, E.N.; et al. Liver kinase B1 depletion from astrocytes worsens disease in a mouse model of multiple sclerosis. Glia 2020, 68, 600–616. [Google Scholar] [CrossRef]
- Ji, J.; Xue, T.F.; Guo, X.D.; Yang, J.; Guo, R.B.; Wang, J.; Huang, J.Y.; Zhao, X.J.; Sun, X.L. Antagonizing peroxisome proliferator-activated receptor gamma facilitates M1-to-M2 shift of microglia by enhancing autophagy via the LKB1-AMPK signaling pathway. Aging Cell 2018, 17, e12774. [Google Scholar] [CrossRef]

| Proteins | Developmental Neurons | Mature CNS Neurons |
|---|---|---|
| AMPKα | Phosphorylate & increase activity [28,38] | Phosphorylate & increase activity [5] |
| NUAK1 | Phosphorylate & increase activity [37] | Upregulate expression, likely increase its activity [5] |
| NUAK2 | Phosphorylate & increase activity [37] | No change in expression [5] |
| SAD A | Phosphorylate & increase activity [15] | Reduce its expression [5] |
| SAD B | Phosphorylate & increase activity [15] | Reduce its expression [5] |
| Akt | Unknown | No change in phosphorylated Akt [5] |
| S6 kinase | Unknown | Decrease its phosphorylation & activity [5] |
| 4E-BP1 | Unknown | No change in phosphorylated 4E-BP1 [5] |
| ERK | Unknown | Increase its phosphorylation & activity [5] |
| SIK | Phosphorylate & increase activity [90] | Unknown |
| MARK | Phosphorylate & increase activity [47] | Unknown |
| PAK | Phosphorylate & decrease activity [56] | Unknown |
| SNRK | Phosphorylate & increase activity [60] | Unknown |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, E.; Li, S. Liver Kinase B1 Functions as a Regulator for Neural Development and a Therapeutic Target for Neural Repair. Cells 2022, 11, 2861. https://doi.org/10.3390/cells11182861
Huang E, Li S. Liver Kinase B1 Functions as a Regulator for Neural Development and a Therapeutic Target for Neural Repair. Cells. 2022; 11(18):2861. https://doi.org/10.3390/cells11182861
Chicago/Turabian StyleHuang, En, and Shuxin Li. 2022. "Liver Kinase B1 Functions as a Regulator for Neural Development and a Therapeutic Target for Neural Repair" Cells 11, no. 18: 2861. https://doi.org/10.3390/cells11182861
APA StyleHuang, E., & Li, S. (2022). Liver Kinase B1 Functions as a Regulator for Neural Development and a Therapeutic Target for Neural Repair. Cells, 11(18), 2861. https://doi.org/10.3390/cells11182861






