Micropatterned Neurovascular Interface to Mimic the Blood–Brain Barrier’s Neurophysiology and Micromechanical Function: A BBB-on-CHIP Model
Abstract
:1. Introduction
2. Materials and Methods
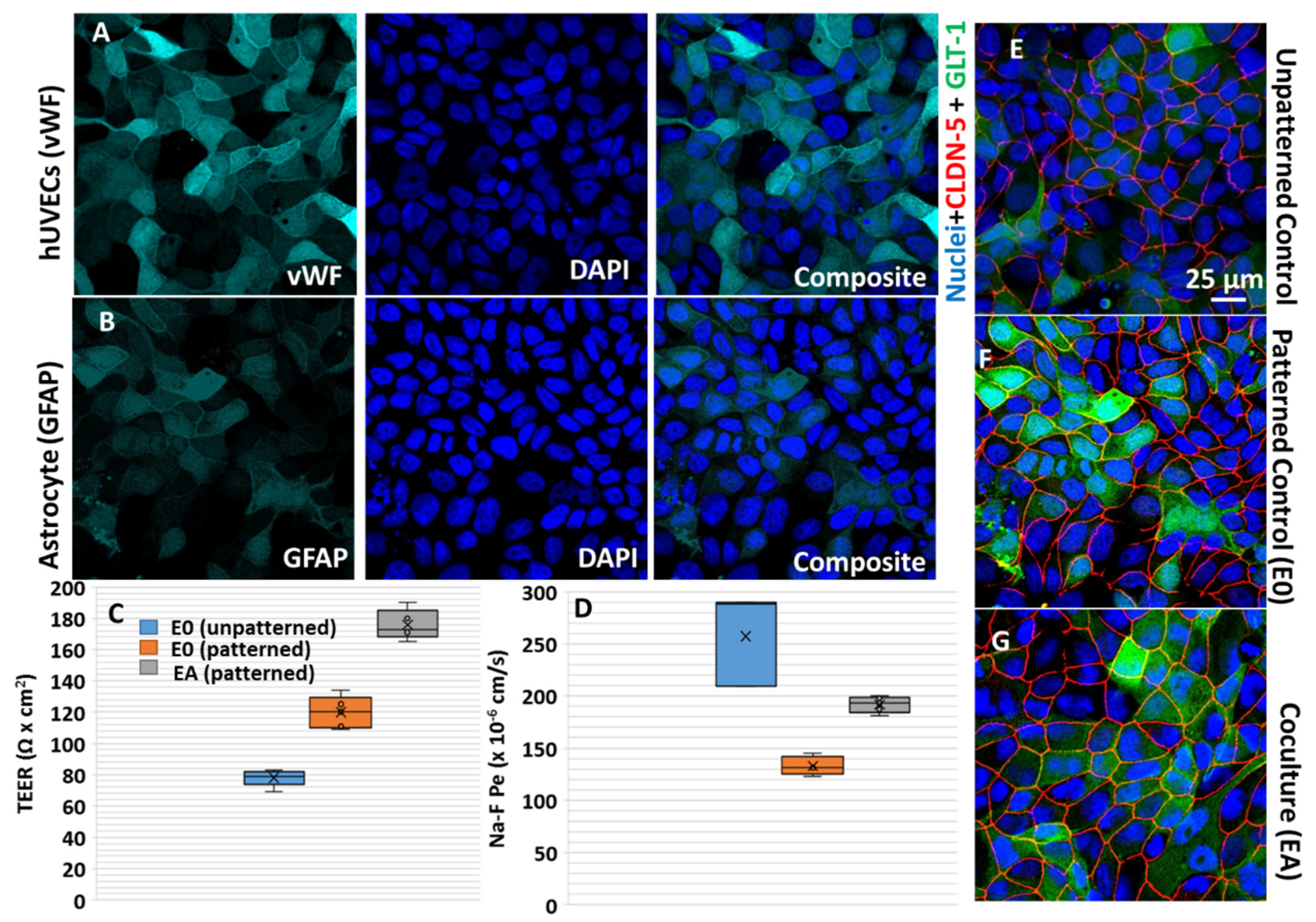
2.1. Cell Culture
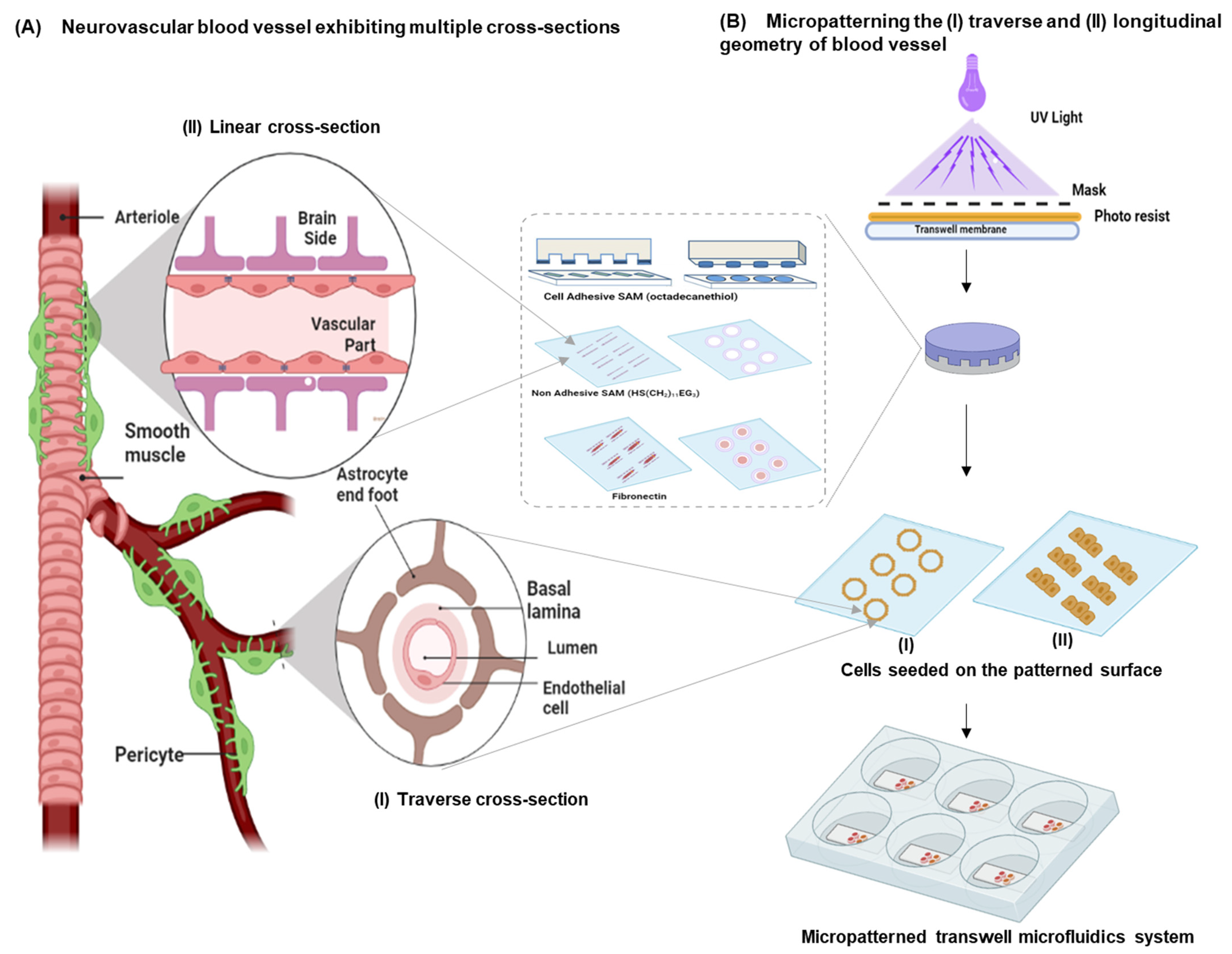
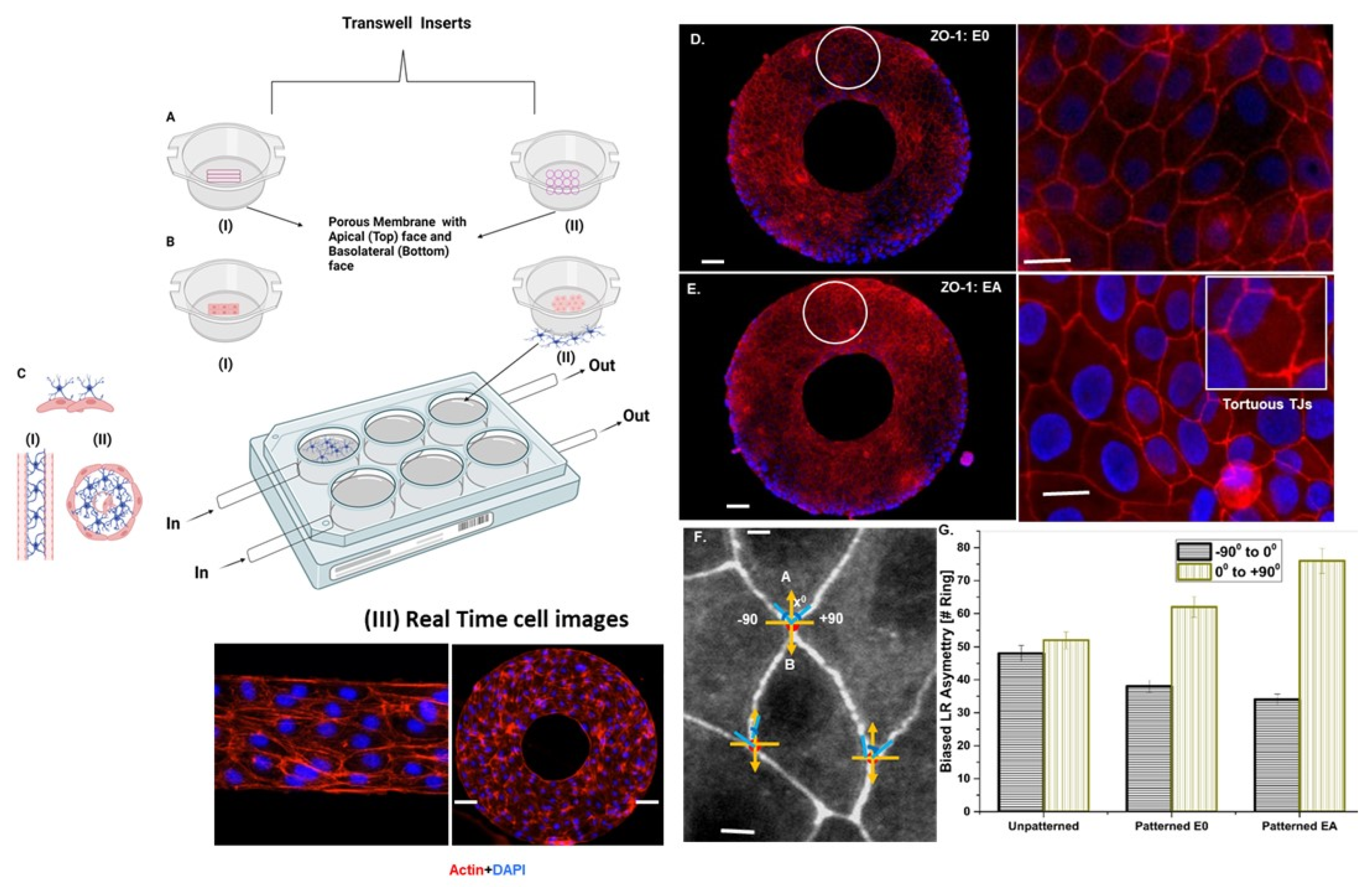
2.2. Micropatterning and Cell Seeding on a Transwell Perfusion System
2.2.1. Analysis of the Transendothelial Electrical Resistance (TEER) and Impedance Spectroscopy
L–R Asymmetric Distribution Analysis
2.2.2. Tight Junction Visualization by Immunofluorescence
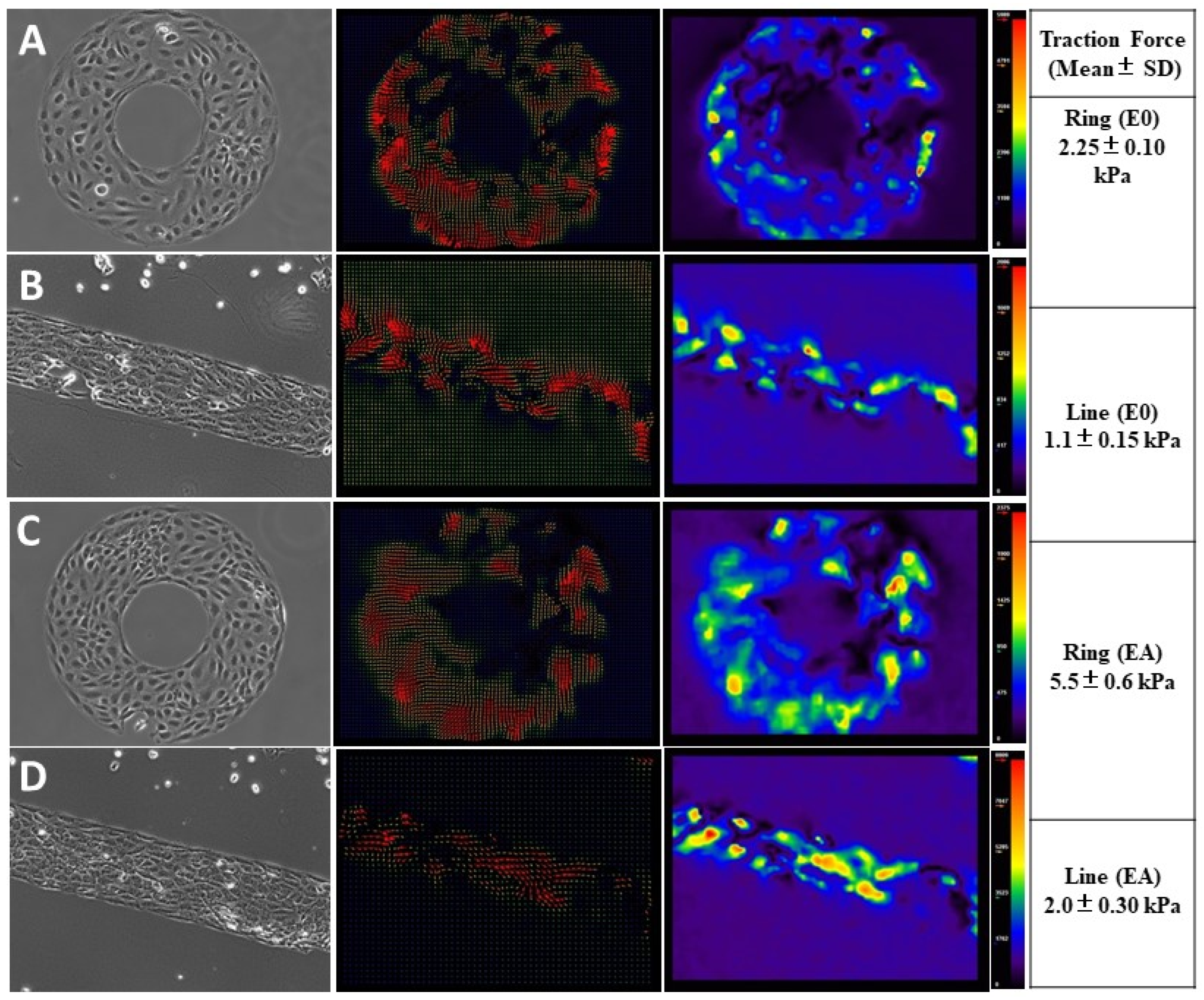
Probing Micromechanical Forces at the Cell–Cell Interface Using Traction Force Microscopy (TFM)
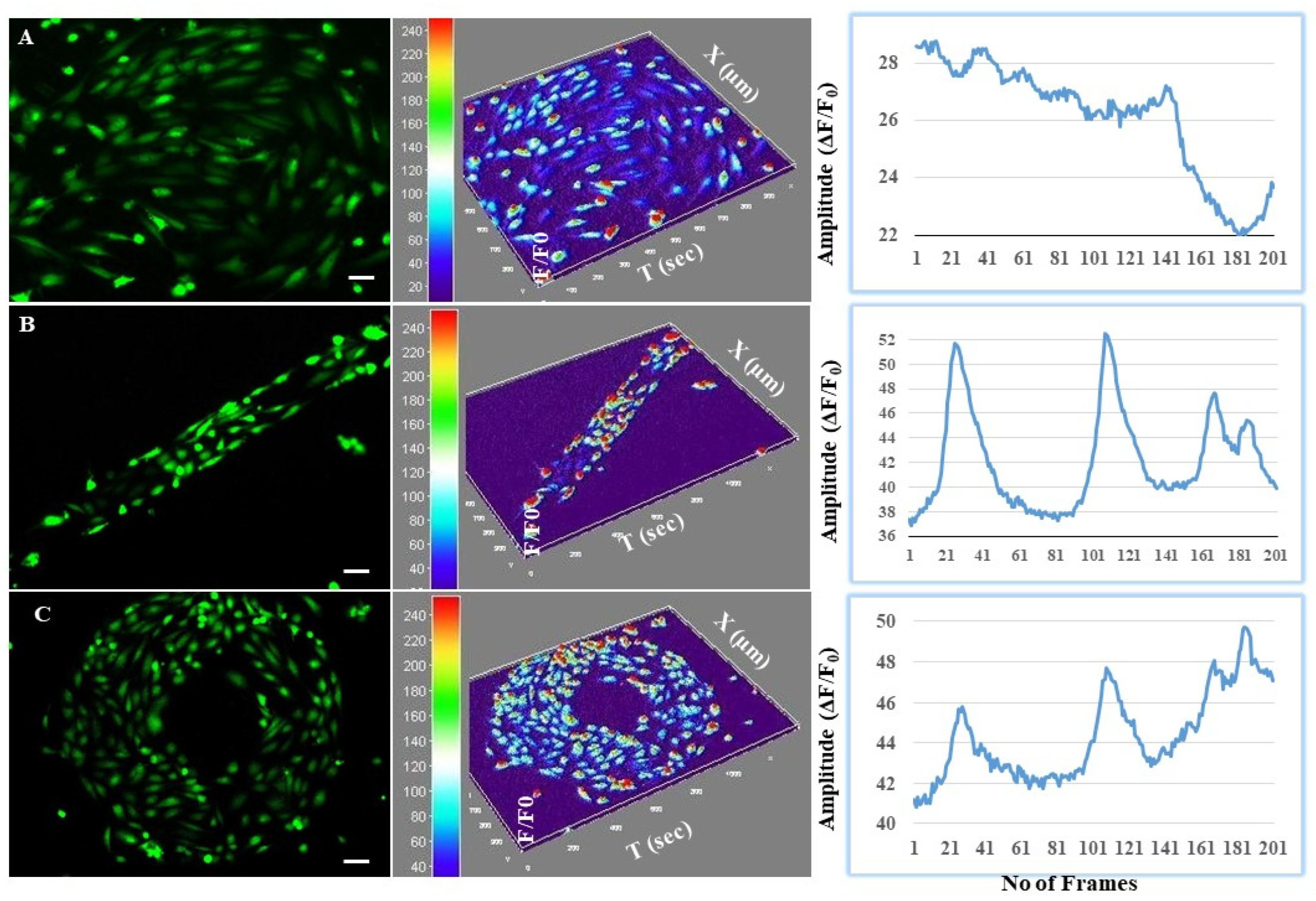
Calcium Imaging to Study the Astrocyte-Mediated Neurovascular Coupling at the BBB Interface
2.2.3. Statistical Analysis
3. Results
3.1. Design Considerations
- An array of endothelial cells exhibiting the multicellular longitudinal and radial architecture of blood vessels. This can be achieved by patterning the endothelial cells as a linear (i.e., longitudinal cross-section) or a ring geometry (i.e., similar to the radial cross-section of a blood vessel). Micropatterning can provide mechanistic information on endothelial cell morphology;
- Constant and continuously perfused transwell chambers that simulate the BBB microvessel physiology. By applying a microfluidics-based perfusion system, information on the disease pathology arising from endothelial functions can be identified;
- Astrocytes that wrap around the endothelial capillaries via end-feet processes at the transluminal surface (i.e., away from the blood vessel lumen) and maintain apicobasal polarity at the tissue–tissue interface of this in vitro neurovascular unit.
3.2. Astrocyte-to-Endothelial Interaction Promoted Tortuosity and Planar Cell Shape via Chiral Distribution of Tight Junctions
3.3. Endothelial Forces Exhibited Directionality and Enhanced Traction Forces with Astrocyte Co-Cultures
3.4. Astrocytes Regulated Intracellular Calcium Oscillations in Endothelial Cells at the BBB Interface
3.5. Perspective of Modeling Proof of Principle Neurodegenerative Disease Pathophysiology on the BBB-on-Chip
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, A.V.; Chandrasekar, V.; Janapareddy, P.; Mathews, D.E.; Laux, P.; Luch, A.; Yang, Y.; Garcia-Canibano, B.; Balakrishnan, S.; Abinahed, J.; et al. Emerging Application of Nanorobotics and Artificial Intelligence to cross the BBB: Advances in Design, Controlled Maneuvering, and Targeting of the Barriers. ACS Chem. Neurosci. 2021, 12, 1835–1853. [Google Scholar] [CrossRef]
- Soares, D.G.; Bordini, E.A.F.; Cassiano, F.B.; Bronze-Uhle, E.S.; Pacheco, L.E.; Zabeo, G.; Hebling, J.; Lisboa-Filho, P.N.; Bottino, M.C.; de Souza Costa, C.A. Characterization of novel calcium hydroxide-mediated highly porous chitosan-calcium scaffolds for potential application in dentin tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2546–2559. [Google Scholar] [CrossRef]
- Théry, M.; Bornens, M. Cell shape and cell division. Curr. Opin. Cell Biol. 2006, 18, 648–657. [Google Scholar] [CrossRef]
- Singh, A.V.; Maharjan, R.S.; Kromer, C.; Laux, P.; Luch, A.; Vats, T.; Chandrasekar, V.; Dakua, S.P.; Park, B.-W. Advances in Smoking Related In Vitro Inhalation Toxicology: A Perspective Case of Challenges and Opportunities from Progresses in Lung-on-Chip Technologies. Chem. Res. Toxicol. 2021, 34, 1984–2002. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Mehta, K.K.; Worley, K.; Dordick, J.S.; Kane, R.S.; Wan, L.Q. Carbon nanotube-induced loss of multicellular chirality on micropatterned substrate is mediated by oxidative stress. ACS Nano 2014, 8, 2196–2205. [Google Scholar] [CrossRef]
- Théry, M.; Racine, V.; Pépin, A.; Piel, M.; Chen, Y.; Sibarita, J.-B.; Bornens, M. The extracellular matrix guides the orientation of the cell division axis. Nat. Cell 2005, 7, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Elbakary, B.; Badhan, R.K.S. A dynamic perfusion based blood-brain barrier model for cytotoxicity testing and drug permeation. Sci. Rep. 2020, 10, 3788. [Google Scholar] [CrossRef]
- Cucullo, L.; Hossain, M.; Puvenna, V.; Marchi, N.; Janigro, D. The role of shear stress in Blood-Brain Barrier endothelial physiology. BMC Neurosci. 2011, 12, 40. [Google Scholar] [CrossRef]
- Porras Hernández, A.M.; Barbe, L.; Pohlit, H.; Tenje, M.; Antfolk, M. Brain microvasculature endothelial cell orientation on micropatterned hydrogels is affected by glucose level variations. Sci. Rep. 2021, 11, 19608. [Google Scholar] [CrossRef]
- Kérourédan, O.; Bourget, J.-M.; Rémy, M.; Crauste-Manciet, S.; Kalisky, J.; Catros, S.; Thébaud, N.B.; Devillard, R. Micropatterning of endothelial cells to create a capillary-like network with defined architecture by laser-assisted bioprinting. J. Mater. Sci. Mater. Med. 2019, 30, 28. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.Q.; Chin, A.S.; Worley, K.E.; Ray, P. Cell chirality: Emergence of asymmetry from cell culture. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150413. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, V.; Singh, A.V.; Maharjan, R.S.; Dakua, S.P.; Balakrishnan, S.; Dash, S.; Laux, P.; Luch, A.; Singh, S.; Pradhan, M. Perspectives on the Technological Aspects and Biomedical Applications of Virus-Like Particles/Nanoparticles in Reproductive Biology: Insights on the Medicinal and Toxicological Outlook. Adv. NanoBiomed Res. 2022, 2, 2200010. [Google Scholar] [CrossRef]
- Sip, C.G.; Bhattacharjee, N.; Folch, A. Microfluidic transwell inserts for generation of tissue culture-friendly gradients in well plates. Lab Chip 2014, 14, 302–314. [Google Scholar] [CrossRef]
- Maharjan, R.S.; Singh, A.V.; Hanif, J.; Rosenkranz, D.; Haidar, R.; Shelar, A.; Singh, S.P.; Dey, A.; Patil, R.; Zamboni, P.; et al. Investigation of the Associations between a Nanomaterial’s Microrheology and Toxicology. ACS Omega 2022, 7, 13985–13997. [Google Scholar] [CrossRef]
- Man, S.; Tucky, B.; Cotleur, A.; Drazba, J.; Takeshita, Y.; Ransohoff, R.M. CXCL12-Induced Monocyte-Endothelial Interactions Promote Lymphocyte Transmigration Across an In Vitro Blood-Brain Barrier. Sci. Transl. Med. 2012, 4, 119ra114. [Google Scholar] [CrossRef]
- Dwivedi, C.; Pandey, H.; Pandey, A.C.; Patil, S.; Ramteke, P.W.; Laux, P.; Luch, A.; Singh, A.V. In Vivo Biocompatibility of Electrospun Biodegradable Dual Carrier (Antibiotic + Growth Factor) in a Mouse Model—Implications for Rapid Wound Healing. Pharmaceutics 2019, 11, 180. [Google Scholar] [CrossRef]
- Raymond, M.J.; Ray, P.; Kaur, G.; Singh, A.V.; Wan, L.Q. Cellular and Nuclear Alignment Analysis for Determining Epithelial Cell Chirality. Ann. Biomed. Eng. 2016, 44, 1475–1486. [Google Scholar] [CrossRef]
- Singh, A.V.; Maharjan, R.S.; Jungnickel, H.; Romanowski, H.; Hachenberger, Y.U.; Reichardt, P.; Bierkandt, F.; Siewert, K.; Gadicherla, A.; Laux, P.; et al. Evaluating Particle Emissions and Toxicity of 3D Pen Printed Filaments with Metal Nanoparticles As Additives: In Vitro and in Silico Discriminant Function Analysis. ACS Sustain. Chem. Eng. 2021, 9, 11724–11737. [Google Scholar] [CrossRef]
- Singh, A.V.; Maharjan, R.-S.; Kanase, A.; Siewert, K.; Rosenkranz, D.; Singh, R.; Laux, P.; Luch, A. Machine-Learning-Based Approach to Decode the Influence of Nanomaterial Properties on Their Interaction with Cells. ACS Appl. Mater. Interfaces 2021, 13, 1943–1955. [Google Scholar] [CrossRef]
- Asmat, T.M.; Tenenbaum, T.; Jonsson, A.-B.; Schwerk, C.; Schroten, H. Impact of Calcium Signaling during Infection of Neisseria meningitidis to Human Brain Microvascular Endothelial Cells. PLoS ONE 2014, 9, e114474. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Patil, R.; Thombre, D.K.; Gade, W.N. Micro-nanopatterning as tool to study the role of physicochemical properties on cell–surface interactions. J. Biomed. Mater. Res. Part A 2013, 101, 3019–3032. [Google Scholar] [CrossRef]
- Singh, A.V. Astrocytes Increase ATP Exocytosis Mediated Calcium Signaling in Response to Microgroove Structures. Sci. Rep. 2015, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Dad Ansari, M.H.; Dayan, C.B.; Giltinan, J.; Wang, S.; Yu, Y.; Kishore, V.; Laux, P.; Luch, A.; Sitti, M. Multifunctional magnetic hairbot for untethered osteogenesis, ultrasound contrast imaging and drug delivery. Biomaterials 2019, 219, 119394. [Google Scholar] [CrossRef] [PubMed]
- Gee, K.R.; Brown, K.A.; Chen, W.N.U.; Bishop-Stewart, J.; Gray, D.; Johnson, I. Chemical and physiological characterization of fluo-4 Ca2+-indicator dyes. Cell Calcium 2000, 27, 97–106. [Google Scholar] [CrossRef]
- Singh, A.V.; Baylan, S.; Park, B.-W.; Richter, G.; Sitti, M. Hydrophobic pinning with copper nanowhiskers leads to bactericidal properties. PLoS ONE 2017, 12, e0175428. [Google Scholar] [CrossRef]
- Gidwani, M.; Singh, A.V. Nanoparticle Enabled Drug Delivery across the Blood Brain Barrier: In vivo and in vitro Models, Opportunities and Challenges. Curr. Pharm. Biotechnol. 2013, 14, 1201–1212. [Google Scholar] [CrossRef]
- Man, S.; Ubogu, E.E.; Williams, K.A.; Tucky, B.; Callahan, M.K.; Ransohoff, R.M. Human Brain Microvascular Endothelial Cells and Umbilical Vein Endothelial Cells Differentially Facilitate Leukocyte Recruitment and Utilize Chemokines for T Cell Migration. Clin. Dev. Immunol. 2008, 2008, 384982. [Google Scholar] [CrossRef]
- Thomsen, L.B.; Burkhart, A.; Moos, T. A Triple Culture Model of the Blood-Brain Barrier Using Porcine Brain Endothelial cells, Astrocytes and Pericytes. PLoS ONE 2015, 10, e0134765. [Google Scholar] [CrossRef]
- Xu, Y.; Pan, S.; Liu, J.; Dong, F.; Cheng, Z.; Zhang, J.; Qi, R.; Zang, Q.; Zhang, C.; Wang, X.; et al. GATA3-induced vWF upregulation in the lung adenocarcinoma vasculature. Oncotarget 2017, 8, 110517–110529. [Google Scholar] [CrossRef]
- Delsing, L.; Herland, A.; Falk, A.; Hicks, R.; Synnergren, J.; Zetterberg, H. Models of the blood-brain barrier using iPSC-derived cells. Mol. Cell. Neurosci. 2020, 107, 103533. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, Y.; Obermeier, B.; Cotleur, A.; Sano, Y.; Kanda, T.; Ransohoff, R.M. An in vitro blood-brain barrier model combining shear stress and endothelial cell/astrocyte co-culture. J. Neurosci. Methods 2014, 232, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood–brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Guatimosim, S.; Guatimosim, C.; Song, L.-S. Imaging Calcium Sparks in Cardiac Myocytes. In Light Microscopy; Chiarini-Garcia, H., Melo, R.C.N., Eds.; Humana Press: Totowa, NJ, USA, 2011; Volume 689, pp. 205–214. [Google Scholar]
- Singh, A.V.; Zamboni, P. Anomalous Venous Blood Flow and Iron Deposition in Multiple Sclerosis. J. Cereb. Blood Flow Metab. 2009, 29, 1867–1878. [Google Scholar] [CrossRef]
- Sheykhansari, S.; Kozielski, K.; Bill, J.; Sitti, M.; Gemmati, D.; Zamboni, P.; Singh, A.V. Redox metals homeostasis in multiple sclerosis and amyotrophic lateral sclerosis: A review. Cell Death Dis. 2018, 9, 348. [Google Scholar] [CrossRef]
- Singh, A.V.; Vyas, V.; Montani, E.; Cartelli, D.; Parazzoli, D.; Oldani, A.; Zeri, G.; Orioli, E.; Gemmati, D.; Zamboni, P. Investigation of in vitro cytotoxicity of the redox state of ionic iron in neuroblastoma cells. J. Neurosci. Rural Pract. 2012, 3, 301. [Google Scholar] [CrossRef]
- Picht, E.; Zima, A.V.; Blatter, L.A.; Bers, D.M. SparkMaster: Automated calcium spark analysis with ImageJ. Am. J. Physiol.-Cell Physiol. 2007, 293, C1073–C1081. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, Z.-L.; Norris, E.H.; Strickland, S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat. Commun. 2014, 5, 3413. [Google Scholar] [CrossRef]
- Adriani, G.; Ma, D.; Pavesi, A.; Kamm, R.D.; Goh, E.L.K. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood–brain barrier. Lab Chip 2017, 17, 448–459. [Google Scholar] [CrossRef]
- Okeyo, K.O.; Kibe, Y.; Adachi, T. Controlling macroscale cell alignment in self-organized cell sheets by tuning the microstructure of adhesion-limiting micromesh scaffolds. Mater. Today Adv. 2021, 12, 100194. [Google Scholar] [CrossRef]
- Wan, L.Q.; Ronaldson, K.; Park, M.; Taylor, G.; Zhang, Y.; Gimble, J.M.; Vunjak-Novakovic, G. Micropatterned mammalian cells exhibit phenotype-specific left-right asymmetry. Proc. Natl. Acad. Sci. USA 2011, 108, 12295–12300. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Le Gac, S.; Verdonschot, N.; van den Berg, A.; Koopman, B.; Rouwkema, J. Endothelial cell alignment as a result of anisotropic strain and flow induced shear stress combinations. Sci. Rep. 2016, 6, 29510. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.T.; Ocheltree, S.M.; Norwood, K.M.; Egleton, R.D. Decreased blood-brain barrier permeability to fluorescein in streptozotocin-treated rats. Neurosci. Lett. 2007, 411, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Haddad-Tóvolli, R.; Dragano, N.R.V.; Ramalho, A.F.S.; Velloso, L.A. Development and Function of the Blood-Brain Barrier in the Context of Metabolic Control. Front. Neurosci. 2017, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J. Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 2002, 200, 629–638. [Google Scholar] [CrossRef]
- Takano, T.; Tian, G.-F.; Peng, W.; Lou, N.; Libionka, W.; Han, X.; Nedergaard, M. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 2006, 9, 260–267. [Google Scholar] [CrossRef]
- Bhalerao, A.; Sivandzade, F.; Archie, S.R.; Chowdhury, E.A.; Noorani, B.; Cucullo, L. In vitro modeling of the neurovascular unit: Advances in the field. Fluids Barriers CNS 2020, 17, 22. [Google Scholar] [CrossRef]
- De Bock, M.; Wang, N.; Decrock, E.; Bol, M.; Gadicherla, A.K.; Culot, M.; Cecchelli, R.; Bultynck, G.; Leybaert, L. Endothelial calcium dynamics, connexin channels and blood-brain barrier function. Prog. Neurobiol. 2013, 108, 1–20. [Google Scholar] [CrossRef]
- Camand, E.; Peglion, F.; Osmani, N.; Sanson, M.; Etienne-Manneville, S. N-cadherin expression level modulates integrin-mediated polarity and strongly impacts on the speed and directionality of glial cell migration. J. Cell Sci. 2012, 125, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Leckband, D.E.; le Duc, Q.; Wang, N.; de Rooij, J. Mechanotransduction at cadherin-mediated adhesions. Curr. Opin. Cell Biol. 2011, 23, 523–530. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.V.; Chandrasekar, V.; Laux, P.; Luch, A.; Dakua, S.P.; Zamboni, P.; Shelar, A.; Yang, Y.; Pandit, V.; Tisato, V.; et al. Micropatterned Neurovascular Interface to Mimic the Blood–Brain Barrier’s Neurophysiology and Micromechanical Function: A BBB-on-CHIP Model. Cells 2022, 11, 2801. https://doi.org/10.3390/cells11182801
Singh AV, Chandrasekar V, Laux P, Luch A, Dakua SP, Zamboni P, Shelar A, Yang Y, Pandit V, Tisato V, et al. Micropatterned Neurovascular Interface to Mimic the Blood–Brain Barrier’s Neurophysiology and Micromechanical Function: A BBB-on-CHIP Model. Cells. 2022; 11(18):2801. https://doi.org/10.3390/cells11182801
Chicago/Turabian StyleSingh, Ajay Vikram, Vaisali Chandrasekar, Peter Laux, Andreas Luch, Sarada Prasad Dakua, Paolo Zamboni, Amruta Shelar, Yin Yang, Vaibhav Pandit, Veronica Tisato, and et al. 2022. "Micropatterned Neurovascular Interface to Mimic the Blood–Brain Barrier’s Neurophysiology and Micromechanical Function: A BBB-on-CHIP Model" Cells 11, no. 18: 2801. https://doi.org/10.3390/cells11182801
APA StyleSingh, A. V., Chandrasekar, V., Laux, P., Luch, A., Dakua, S. P., Zamboni, P., Shelar, A., Yang, Y., Pandit, V., Tisato, V., & Gemmati, D. (2022). Micropatterned Neurovascular Interface to Mimic the Blood–Brain Barrier’s Neurophysiology and Micromechanical Function: A BBB-on-CHIP Model. Cells, 11(18), 2801. https://doi.org/10.3390/cells11182801









