Strategies to Reduce Radiation Stress in Open-Field Ginger and Turmeric Production
Abstract
:1. Introduction
2. Materials and Methods
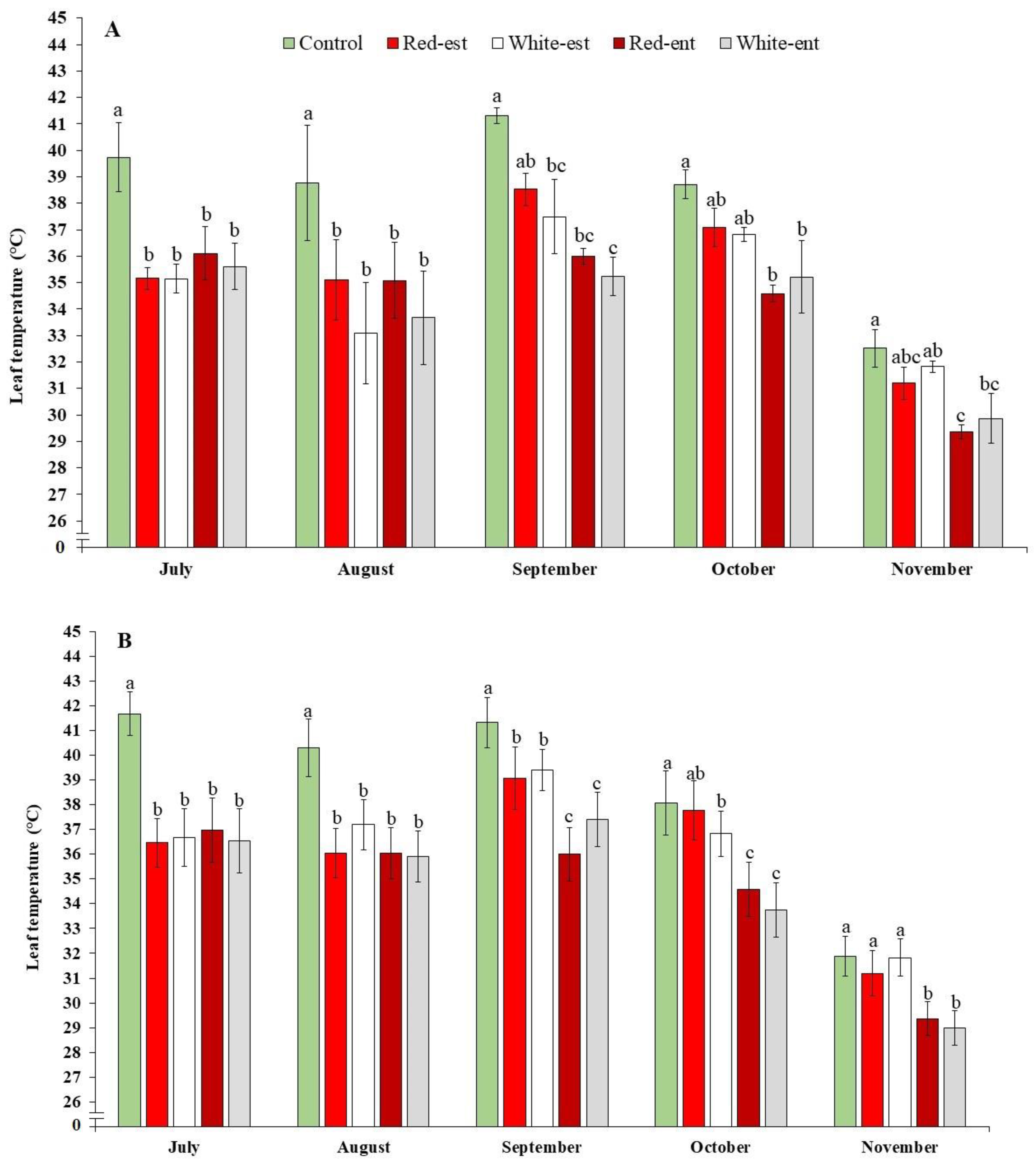
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rao, M.R.; Reddy, I.B.; Gopal, S.V.R.; Bhaskar, D.; Ramana, T. A comparative study of antimicrobial activity of Curcuma amada and Alpinia galanga of Zingiberaceae family. Asian J. Chem. 2008, 20, 5293–5300. [Google Scholar]
- Ruby, A.J.; Kuttan, G.; Babu, K.D.; Rajasekharan, K.N.; Kuttan, R. Anti-tumor and antioxidant activity of natural curcuminoids. Cancer Lett. 1995, 94, 79–83. [Google Scholar] [CrossRef]
- Ma, R.H.; Ni, Z.J.; Zhu, Y.Y.; Thakur, K.; Zhang, F.; Zhang, Y.Y.; Hu, F.; Zhang, J.G.; Wei, Z.J. A recent update on the multifaceted health benefits associated with ginger and its bioactive components. Food Funct. 2021, 12, 519–542. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, J.P.; Behera, L.; Praveena, J.; Sawant, S.; Mishra, A.; Sharma, S.S.; Ghosh, L.; Mishra, A.P.; Sahoo, A.R.; Pradhan, P.; et al. The golden spice turmeric (Curcuma longa) and its feasible benefits in prospering human health—A review. Am. J. Plant Sci. 2021, 12, 455–475. [Google Scholar] [CrossRef]
- Bag, B.B. Ginger processing in India (Zingiber officinale): A review. Int. J. Curr. Microbio. App. Sci. 2018, 7, 1639–1651. [Google Scholar] [CrossRef]
- Nair, K.P. Turmeric (Curcuma longa L.) and Ginger (Zingiber officinale Rosc.)—World’s Invaluable Medicinal Spices. The Agronomy and Economy of Turmeric and Ginger, 1st ed.; Springer Nature: Cham, Switzerland, 2019; pp. 1–243. ISBN 9780123948014. [Google Scholar]
- Li, S.; Yuan, W.; Deng, G.; Wang, P.; Yang, P.; Aggarwal, B.B. Chemical Composition and Product Quality Control of Turmeric (Curcuma longa L.). Pharm. Crops 2011, 2, 28–54. [Google Scholar] [CrossRef]
- Popuri, A.K.; Pagala, B. Extraction of Curcumin from Turmeric Roots. Int. J. Innov. Res. Stud. 2013, 2, 289–299. [Google Scholar]
- OEC. Available online: https://oec.world/en/profile/hs/ginger (accessed on 4 May 2022).
- Statista. Available online: https://www.statista.com/statistics/798301/us-turmeric-imports-by-country/ (accessed on 22 February 2022).
- Babu, P.; Jayachandran, B.K. Mulch requirement of ginger (Zingiber officinale R.) under shade. J. Spices Aromat. Crops 1997, 6, 141–143. [Google Scholar]
- Hossain, M.A.; Akamine, H.; Ishimine, Y.; Teruya, R.; Aniya, Y.; Yamawaki, K. Effects of relative light intensity on the growth, yield and curcumin content of turmeric (Curcuma longa L.) in Okinawa, Japan. Plant Prod. Sci. 2009, 12, 29–36. [Google Scholar] [CrossRef]
- Ravindran, P.N.; Nirmal Babu, K.; Shivaraman, K.N. Botany and Crop Improvement of Ginger. In Ginger: The Genus Zingiber, 1st ed.; Ravindran, P.N., Nirmal Babu, K., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 15–86. [Google Scholar]
- Sharangi, A.B.; Gowda, M.P.; Das, S. Responses of turmeric to light intensities and nutrients in a forest ecosystem: Retrospective insight. Trees For. People 2022, 7, 100208. [Google Scholar] [CrossRef]
- Kratky, B.; Bernabe, C.; Arakaki, E.; White, F.; Miyasaka, S. Shading Reduces Yields of Edible Ginger Rhizomes Grown in Sub-Irrigated Pots; University of Hawaii: Honolulu, HI, USA, 2013. [Google Scholar]
- Ajithkumar, K.; Jayachandran, B.K.; Ravi, V. Influence of shade regimes on photosynthetic rate and stomatal characters of ginger (Zingiber officinale R.). J. Spices Aromat. Crops 2002, 11, 26–29. [Google Scholar]
- Alam, B.; Chaturvedi, M.; Singh, R.; Newaj, R.; Dhyani, S.K. Physiological determinants for adaptive potential of turmeric (Curcuma longa) for its growth and yield under different regimes of shade in semiarid Region of Central India. Indian J. Agrofor. 2014, 16, 25–29. [Google Scholar]
- Srikrishnah, S.; Sutharsan, S. Effect of different shade levels on growth and tuber yield of turmeric (Curcuma longa L.) in the batticaloa district of Sri Lanka. Am. Eurasian J. Agric. Environ. Sci. 2015, 15, 813–816. [Google Scholar]
- Lötze, E.; Daiber, S.H.; Midgley, S.J.E. Evaluating the efficacy of a pre-harvest combination of calcium and boron as foliar application to reduce sunburn on ‘Cripps Pink’ apples. Acta Hortic. 2018, 1217, 61–68. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Moutinho-Pereira, J.; Correia, C. Kaolin, an emerging tool to alleviate the effects of abiotic stresses on crop performance. Sci. Hortic. 2019, 250, 310–316. [Google Scholar] [CrossRef]
- Glenn, D.M. The mechanisms of plant stress mitigation by kaolin-based particle films and applications in horticultural and agricultural crops. HortScience 2012, 47, 710–711. [Google Scholar] [CrossRef]
- Sharma, R.R.; Reddy, S.V.R.; Datta, S.C. Particle films and their applications in horticultural crops. Appl. Clay Sci. 2015, 116, 54–68. [Google Scholar] [CrossRef]
- Conde, A.; Neves, A.; Breia, R.; Pimentel, D.; Dinis, L.T.; Bernardo, S.; Correia, C.M.; Cunha, A.; Gerós, H.; Moutinho-Pereira, J. Kaolin particle film application stimulates photoassimilate synthesis and modifies the primary metabolome of grape leaves. J. Plant Physiol. 2018, 223, 47–56. [Google Scholar] [CrossRef]
- Glenn, D.M.; Puterka, G.J.; Drake, S.R.; Unruh, T.R.; Knight, A.L.; Baherle, P.; Prado, E.; Baugher, T.A. Particle film application influences apple leaf physiology, fruit yield, and fruit quality. J. Am. Soc. Hortic. Sci. 2001, 126, 175–181. [Google Scholar] [CrossRef]
- Glenn, D.M.; Prado, E.; Erez, A.; McFerson, J.; Puterka, G.J. A reflective, processed-kaolin particle film affects fruit temperature, radiation reflection, and solar injury in apple. J. Am. Soc. Hortic. Sci. 2002, 127, 188–193. [Google Scholar] [CrossRef]
- Glenn, D.M.; Puterka, G.J. Particle films: A new technology for agriculture. Hortic. Rev. 2005, 31, 1–44. [Google Scholar]
- Jifon, J.L.; Syvertsen, J.P. Kaolin particle film applications can increase photosynthesis and water use efficiency of “Ruby Red” grapefruit leaves. J. Am. Soc. Hort. Sci. 2003, 128, 107–112. [Google Scholar] [CrossRef]
- Salvatierra, J.P. Physiological and Horticultural Responses of Citrus to Colored Particle Films. Master’s Thesis, University of Florida, Gainesville, FL, USA, 2019. [Google Scholar]
- Sengupta, M.; Xie, Y.; Lopez, A.; Habte, A.; Maclaurin, G.; Shelby, J. The National Solar Radiation Data Base (NSRDB). Renew. Sustain. Energy Rev. 2018, 89, 51–60. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 10 April 2021).
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-3. 2020. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 12 April 2021).
- Cao, B.; Xia, J.; Lv, Y.; Chen, Z.; Xu, K. Effect of a mist culture system on photosynthesis and nitrogen metabolism in ginger. Protoplasma 2020, 257, 1359–1371. [Google Scholar] [CrossRef]
- Chudiwal, A.; Jain, D.P.; Somani, R.S. Alpinia galanga Willd–An overview on phyto-pharmacological properties. Indian J. Nat. Prod. Resour. 2010, 1, 143–149. [Google Scholar]
- Ferreira, M.I.; Lima, G.P.P.; Rodrigues, L.; Silva, M.B.; Jadoski, C.; Gonçalves, G.G.; Ming, L.C. Biomass production and photosynthetic efficiency of turmeric grown in different shade conditions. Acta Hortic. 2014, 1125, 41–46. [Google Scholar] [CrossRef]
- Vastrad, N.V.; Hedge, R.V.; Giritammanavar, V.A. Influence of light and vermicompost on growth and yield of ginger (Zingiber officinale Rosc.). Karnataka J. Agric. 2006, 19, 936–940. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A.; Wahab, P.E.M.; Halim, M.R.A. Effect of different light intensities on total phenolics and flavonoids synthesis and anti-oxidant activities in young ginger varieties (Zingiber officinale Roscoe). Int. J. Mol. Sci. 2010, 11, 3885–3897. [Google Scholar] [CrossRef]
- Aly, M.M.; El Sawy, A.; El Gendy, R.A. Comparative study of different shading types on growth and yield of ginger plants. Middle East J. 2019, 8, 1264–1270. [Google Scholar] [CrossRef]
- Sivaraman, K. Studies on Productivity of Turmeric—Maize and Onion Intercropping Systems under Varied Population and Nitrogen Levels. Ph.D. Dissertation, Tamil Nadu Agricultural University, Coimbatore, India, 1992. [Google Scholar]
- Ruberti, I.; Sessa, G.; Ciolfi, A.; Possenti, M.; Carabelli, M.; Morelli, G. Plant adaptation to dynamically changing environment: The shade avoidance response. Biotechnol. Adv. 2012, 30, 1047–1058. [Google Scholar] [CrossRef]
- Chmura, D.J.; Modrzyński, J.; Chmielarz, P.; Tjoelker, M.G. Plasticity in seedling morphology, biomass allocation and physiology among ten temperate tree species in response to shade is related to shade tolerance and not leaf habit. Plant Biol. 2017, 19, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Miner, B.G.; Sultan, S.E.; Morgan, S.G.; Padilla, D.K.; Relyea, R.A. Ecological consequences of phenotypic plasticity. Trends Ecol. Evol. 2005, 20, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.M.; Wu, T.; Geng, Y.F.; Chai, Y.; Hao, J.B. Phenotypic plasticity of lianas in response to altered light environment. Ecol. Res. 2016, 31, 375–384. [Google Scholar] [CrossRef]
- Ravindran, P.N.; Babu, K.N.; Shivaraman, K.N. Botany and crop improvement of tumeric. In Turmeric: The Genus Curcuma, 1st ed.; Ravindran, P.N., Nirmal Babu, K., Sivaraman, K.N., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 15–70. [Google Scholar]
- Chitra, R.; Hemalatha, P. Effect of intercrops on growth and yield of turmeric (Curcuma longa L.). J. Spices Aromat. Crops 2017, 26, 51–54. [Google Scholar] [CrossRef]
- Xizhen, A.; Jinfeng, S.; Xia, X. Ginger production in South East Asia. In Ginger: The Genus Zingiber, 1st ed.; Ravindran, P.N., Nirmal Babu, K., Eds.; CRC Press: Boca Raton, FL, USA, 2005; Volume 1, pp. 241–278. [Google Scholar]
- Li, H.L.; Huang, M.J.; Tan, D.Q.; Liao, Q.H.; Zou, Y.; Jiang, Y.S. Effects of soil moisture content on the growth and physiological status of ginger (Zingiber officinale Roscoe). Acta Physiol. Plant. 2018, 40, 125. [Google Scholar] [CrossRef]
- Wang, Y.; An, A.Z.; Li, R.K.; Yang, X.; Huang, Y.F.; Shao, R.X.; Ye, Y.L. The nutritional status and fluorescence characteristics of maize cultivars with different chlorophyll content and yields. Photosynthetica 2019, 57, 295–302. [Google Scholar] [CrossRef]
- Babu, M.S.; Kumar, B.P.; Swami, D.V.; Krishna, K.U.; Emmanuel, N. Impact of shade net condition on growth, rhizome and yield characters of ginger. J. Pharmacogn. Phytochem. 2019, 8, 3481–3485. [Google Scholar]
- Abul-Soud, M.; Emam, M.; Abdrabbo, M. Intercropping of some brassica crops with mango trees under different net house color. Res. J. Agric. Biol. Sci. 2014, 10, 70–79. [Google Scholar]
- Tinyane, P.P.; Soundy, P.; Sivakumar, D. Growing ‘Hass’ avocado fruit under different coloured shade netting improves the marketable yield and affects fruit ripening. Sci. Hortic. 2018, 230, 43–49. [Google Scholar] [CrossRef]
- Manja, K.; Aoun, M. The use of nets for tree fruit crops and their impact on the production: A review. Sci. Hortic. 2019, 246, 110–122. [Google Scholar] [CrossRef]
- Wang, Q.L.; Chen, J.H.; He, N.Y.; Guo, F.Q. Metabolic reprogramming in chloroplasts under heat stress in plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef]
- Flores, S.; Retana-Cordero, M.; Fisher, P.R.; Freyre, R.; Gómez, C. Effect of photoperiod, propagative material, and production period on greenhouse-grown ginger and turmeric plants. HortScience 2021, 56, 1476–1485. [Google Scholar] [CrossRef]
- Azizi, A.; Hokmabadi, H.; Piri, S.; Rabie, V. Effect of kaolin application on water stress in pistachio cv. ‘Ohadi’. J. Nuts 2013, 4, 9–14. [Google Scholar] [CrossRef]
- Gharaghani, A.; Javarzari, A.M.; Vahdati, K. Kaolin particle film alleviates adverse effects of light and heat stresses and improves nut and kernel quality in persian walnut. Sci. Hortic. 2018, 239, 35–40. [Google Scholar] [CrossRef]
- Cantore, V.; Pace, B.; Albrizio, R. Kaolin-based particle film technology affects tomato physiology, yield and quality. Env. Exp. Bot. 2009, 66, 279–288. [Google Scholar] [CrossRef]
- Rosati, A. Physiological effects of kaolin particle film technology: A review. Funct. Plant Sci. Biotech. 2007, 1, 100–105. [Google Scholar]
- Liu, S.; Sun, B.; Cao, B.; Lv, Y.; Chen, Z.; Xu, K. Effects of soil waterlogging and high-temperature stress on photosynthesis and photosystem II of ginger (Zingiber officinale). Protoplasma 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ćosić, M.; Stričević, R.; Djurović, N.; Lipovac, A.; Bogdan, I.; Pavlović, M. Effects of irrigation regime and application of kaolin on canopy temperatures of sweet pepper and tomato. Sci. Hortic. 2018, 238, 23–31. [Google Scholar] [CrossRef]
- Boari, F.; Cucci, G.; Donadio, A.; Schiattone, M.I.; Cantore, V. Kaolin influences tomato response to salinity: Physiological aspects. Acta Agric. Scand. Sect. B Soil Plant Sci. 2014, 64, 559–571. [Google Scholar] [CrossRef]
- Sotelo-Cuitiva, Y.M.; Restrepo-Díaz, H.; García-Castro, A.; Ramírez-Godoy, A.; Flórez-Roncancio, V.J. Effect of kaolin film particle applications (Surround WP®) and water deficit on physiological characteristics in rose cut plants (Rosa spp L.). Am. J. Plant Sci. 2011, 2, 354–358. [Google Scholar] [CrossRef]
- Mahmoud, S.H.; El-Tanahy, A.M.M.; El-Sawy, S.M. Amelioration productivity of potato crop grown under high temperature condition spraying with kaolin and α-tocopherol. Plant Arch. 2020, 20, 3568–3575. [Google Scholar]
- Glenn, D.M.; Cooley, N.; Walker, R.; Clingeleffer, P.; Shellie, K. Impact of kaolin particle film and water deficit on wine grape water use efficiency and plant water relations. HortScience 2010, 45, 1178–1187. [Google Scholar] [CrossRef]
- Dash, P.K.; Chase, C.A.; Agehara, S.; Zotarelli, L. Heat stress mitigation effects of kaolin and s-abscisic acid during the establishment of strawberry plug transplants. Sci. Hortic. 2020, 267, 109276. [Google Scholar] [CrossRef]
- Spiers, J.D.; Matta, F.B.; Marshall, D.A. Effects of kaolin clay particle film on southern highbush (Vaccinium corymbosum L.) blueberry plants. Small Fruit Rev. 2008, 2, 29–36. [Google Scholar] [CrossRef]
- Le Grange, M.; Wand, S.J.E.; Theron, K.I. Effect of kaolin applications on apple fruit quality and gas exchange of apple leaves. Acta Hortic. 2004, 636, 545–550. [Google Scholar] [CrossRef]
- Sheikh, A.S.; Mall, I.P. Effect of antitranspirants on transpiration and water use efficiency of chillies. J. Ind. Bot. Soc. 1978, 57, 6–8. [Google Scholar]
- Tworkoski, T.J.; Michael Glenn, D.; Puterka, G.J. Response of bean to applications of hydrophobic mineral particles. Can. J. Plant Sci. 2002, 82, 217–219. [Google Scholar] [CrossRef]
- Glenn, D.M. Effect of highly processed calcined kaolin residues on apple water use efficiency. Sci. Hortic. 2016, 205, 127–132. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Ferreira, H.; Moutinho-Pereira, J.; Correia, C. The role of nighttime water balance on Olea europaea plants subjected to contrasting water regimes. J. Plant Physiol. 2018, 226, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Sunaga, M.; Ito, M.; Yuchen, Q.; Matsushima, Y.; Sakoda, K.; Yamori, W. Minimizing VPD fluctuations maintains higher stomatal conductance and photosynthesis, resulting in improvement of plant growth in lettuce. Front. Plant Sci. 2021, 12, 458. [Google Scholar] [CrossRef]
- Tommaso, F.; Tombesi, S.; Luciani, E.; Sabbatini, P.; Berrios, J.G.; Palliotti, A. Kaolin treatments on Pinot noir grapevines for the control of heat stress damages. BIO Web Conf. 2019, 13, 04004. [Google Scholar] [CrossRef]
- El-Shayeb, N.S.; Hassan, R.H.; Ahmed, M.A.; Abdelkader, M.A. Evaluation of growth, yield and active ingredients in fenugreek plants under different potassium fertilizer rates and kaolin application. Eur. J. Med. Plants 2020, 31, 28–37. [Google Scholar] [CrossRef]
- Faghih, S.; Zamani, Z.; Fatahi, R.; Liaghat, A. Effects of deficit irrigation and kaolin application on vegetative growth and fruit traits of two early ripening apple cultivars. Biol. Res. 2019, 52, 43. [Google Scholar] [CrossRef] [PubMed]
| Month | Max. Temp. (°C) | Min. Temp. (°C) | Mean Temp. (°C) | Max. RH (%) | Min. RH (%) | Mean RH (%) | Mean Solar Irradiance (kWh·m−2·d−1) | DLI (mol·m−2·d−1) | Total Rainfall (mm) |
|---|---|---|---|---|---|---|---|---|---|
| June | 36.6 | 21.4 | 27.4 | 98.0 | 48.0 | 83.4 | 5.1 | 39.4 | 205.2 |
| July | 37.1 | 20.1 | 26.2 | 99.0 | 46.0 | 88.0 | 5.3 | 38.2 | 230.0 |
| August—Full sun | 35.5 | 21.0 | 26.4 | 99.0 | 51.0 | 87.3 | 4.9 | 36.8 | 195.8 |
| August—Shade | 30.3 | 21.7 | 27.1 | 100.0 | 38.5 | 85.9 | 3.2 | 13.7 | 195.8 |
| September—Full sun | 35.6 | 21.4 | 29.6 | 100.0 | 28.5 | 75.7 | 4.6 | 31.3 | 123.7 |
| September—Shade | 31.2 | 21.1 | 28.2 | 100.0 | 23.7 | 81.1 | 3.1 | 11.9 | 123.7 |
| October—Full sun | 33.4 | 19.9 | 26.5 | 100.0 | 19.9 | 76.0 | 3.8 | 28.6 | 13.0 |
| October—Shade | 29.3 | 12.4 | 21.7 | 100.0 | 18.7 | 77.9 | 3.0 | 11.1 | 13.0 |
| November—Full sun | 30.2 | 0.4 | 18.3 | 100.0 | 26.0 | 84.1 | 3.2 | 25.2 | 95.5 |
| November—Shade | 27.5 | 1.8 | 10.6 | 100.0 | 31.4 | 77.4 | 2.7 | 9.6 | 95.5 |
| December—Full sun | 25.2 | −1.0 | 15.5 | 100.0 | 27.6 | 85.3 | 2.9 | 20.1 | 213.6 |
| December—Shade | 20.4 | 0.0 | 14.5 | 100.0 | 32.4 | 83.9 | 2.5 | 7.7 | 213.6 |
| January—Full sun | 26.1 | 4.0 | 15.9 | 100.0 | 27.4 | 80.5 | 3.1 | 22.3 | 125.5 |
| January—Shade | 23.2 | 0.9 | 13.4 | 100.0 | 19.9 | 71.0 | 2.4 | 8.5 | 125.5 |
| Month | Max. Temp. (°C) | Min. Temp. (°C) | Mean Temp. (°C) | Max. RH (%) | Min. RH (%) | Mean RH (%) | Mean Solar Irradiance (kWh·m−2·d−1) | DLI (mol·m−2·d−1) | Total Rainfall (mm) |
|---|---|---|---|---|---|---|---|---|---|
| June | 35.7 | 18.3 | 26.2 | 100.0 | 37.1 | 78.7 | 4.9 | 37.1 | 208.5 |
| July | 36.5 | 21.6 | 27.3 | 100.0 | 38.3 | 79.4 | 5.0 | 38.5 | 132.3 |
| August | 36.8 | 21.2 | 27.1 | 100.0 | 40.4 | 82.1 | 4.8 | 34.4 | 171.8 |
| September | 36.4 | 14.1 | 26.8 | 100.0 | 31.2 | 83.5 | 4.4 | 30.1 | 118.4 |
| October | 31.7 | 11.3 | 26.1 | 100.0 | 27.5 | 74.6 | 4.0 | 26.4 | 69.2 |
| November | 29.4 | 5.5 | 17.7 | 100.0 | 35.3 | 72.2 | 3.4 | 23.9 | 30.3 |
| Treatment | Shoot Fresh Mass (FM) (g) | Root FM (g) | Rhizome FM (g) | Shoot Dry Mass (DM) (g) | Root DM (g) | Rhizome DM (g) | SPAD Index |
|---|---|---|---|---|---|---|---|
| Ginger | |||||||
| Propagative Material (PM) | |||||||
| R z | 335.4 a y | 49.4 a | 424.8 b | 36.9 a | 5.7 a | 86.4 b | 46.6 a |
| 2GR | 480.9 a | 60.9 a | 879.9 a | 47.7 a | 6.8 a | 167.5 a | 40.8 b |
| Treatment (T) | |||||||
| Full sun | 329.9 a | 68.7 a | 667.8 a | 35.4 a | 8.1 a | 127.2 a | 39.6 b |
| Shade | 473.8 a | 41.7 b | 619.3 a | 46.3 a | 5.6 b | 118.4 a | 47.9 a |
| PM × T | |||||||
| R-Full sun | 316.8 a | 57.5 a | 452.5 a | 33.5 a | 6.7 a | 83.4 a | 43.9 a |
| R-Shade | 354.0 a | 41.3 a | 397.2 a | 38.0 a | 5.0 a | 76.7 a | 49.4 a |
| 2GR-Full sun | 345.6 a | 79.8 a | 926.2 a | 35.7 a | 8.6 a | 181.9 a | 35.2 a |
| 2GR-Shade | 593.7 a | 42.0 a | 841.3 a | 54.3 a | 5.8 a | 173.6 a | 46.4 a |
| PM | NS | NS | *** | NS | NS | *** | * |
| T | NS | * | NS | NS | * | NS | ** |
| PM × T | NS | NS | NS | NS | NS | NS | NS |
| Turmeric | |||||||
| Propagative Material (PM) | |||||||
| R | 582.8 a | 68.1 b | 691.5 a | 62.1 a | 8.3 b | 120.3 a | 32.8 a |
| 2GR | 442.8 a | 130.0 a | 692.8 a | 55.2 a | 14.9 a | 116.7 a | 33.7 a |
| Treatment (T) | |||||||
| Full sun | 452.8 a | 118.3 a | 688.2 a | 47.4 a | 14.1 a | 114.4 a | 30.3 b |
| Shade | 572.9 a | 79.8 b | 696.1 a | 53.5 a | 9.3 b | 118.2 a | 36.2 a |
| PM × T | |||||||
| R-Full sun | 529.8 a | 78.0 a | 619.7 a | 55.6 a | 8.6 a | 119.3 a | 30.0 a |
| R-Shade | 635.8 a | 58.2 a | 663.3 a | 59.2 a | 7.1 a | 124.8 a | 35.6 a |
| 2 GR-Full sun | 375.7 a | 158.5 a | 656.7 a | 46.8 a | 16.4 a | 113.5 a | 30.5 a |
| 2 GR-Shade | 510.0 a | 101.5 a | 628.8 a | 54.3 a | 13.7 a | 122.2 a | 36.8 a |
| PM | NS | *** | NS | NS | *** | NS | NS |
| T | NS | ** | NS | NS | ** | NS | *** |
| PM × T | NS | NS | NS | NS | NS | NS | NS |
| Treatment y | Shoot Fresh Mass (FM) (g) | Root FM (g) | Rhizome FM (g) | Shoot Dry Mass (DM) (g) | Root DM (g) | Rhizome DM (g) | SPAD Index | Ax (μmol CO2·m−2·s−1) | E (mmol H2O·m−2·s−1) | gs (mmol H2O·m−2·s−1) | WUEi (μmol CO2·mmol H2O) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ginger | |||||||||||
| Control | 724.1 c w | 78.3 b | 869.9 b | 93.5 c | 9.6 b | 198.3 c | 36.7 b | 9.9 d | 3.3 a | 213.2 c | 2.8 c |
| Redest | 1116.3 b | 82.8 b | 1386.6 a | 123.8 b | 10.2 b | 321.4 b | 50.3 a | 13.2 c | 3.0 ab | 341.3 b | 4.2 b |
| Whiteest | 1098.5 b | 87.3 b | 1495.7 a | 133.4 b | 11.6 b | 303.6 b | 48.9 a | 14.0 bc | 2.9 ab | 315.1 b | 5.0 a |
| Redent | 1345.6 a | 100.4 a | 1600.5 a | 156.7 a | 13.4 a | 375.1 a | 50.7 a | 17.5 a | 2.7 bc | 424.7 a | 6.9 a |
| Whiteent | 1298.7 a | 98.3 a | 1634.9 a | 161.5 a | 12.9 a | 349.5 a | 49.2 a | 14.7 b | 2.4 c | 508.9 a | 5.8 a |
| Treatment (T) | ** | ** | ** | *** | *** | ** | ** | *** | *** | ** | ** |
| Variety (V) | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| T × V | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Turmeric | |||||||||||
| Control | 2012.3 c | 100.3 c | 3064.1 b | 145.6 c | 14.3 b | 278.9 c | 34.2 b | 9.5 c | 4.7 a | 305.4 c | 1.9 c |
| Redest | 2345.2 b | 223.5 b | 4353.8 a | 184.1 b | 18.5 b | 369.0 b | 45.0 a | 12.9 b | 4.5 a | 442.1 b | 2.7 b |
| Whiteest | 2298.7 b | 201.1 b | 4397.2 a | 167.4 b | 22.5 ab | 394.2 b | 44.7 a | 12.1 bc | 4.3 a | 407.6 b | 2.9 b |
| Redent | 3067.3 a | 269.8 a | 4512.9 a | 210.3 a | 25.8 a | 445.3 a | 46.5 a | 15.6 a | 3.2 b | 716.3 a | 5.4 a |
| Whiteent | 3102.7 a | 254.2 a | 4452.5 a | 206.9 a | 33.2 a | 468.2 a | 45.3 a | 16.2 a | 3.4 b | 647.8 a | 4.2 a |
| Treatment (T) | *** | *** | ** | *** | ** | *** | ** | ** | ** | *** | * |
| Variety (V) | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| T × V | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Retana-Cordero, M.; Flores, S.; Freyre, R.; Gómez, C. Strategies to Reduce Radiation Stress in Open-Field Ginger and Turmeric Production. Agronomy 2022, 12, 1910. https://doi.org/10.3390/agronomy12081910
Retana-Cordero M, Flores S, Freyre R, Gómez C. Strategies to Reduce Radiation Stress in Open-Field Ginger and Turmeric Production. Agronomy. 2022; 12(8):1910. https://doi.org/10.3390/agronomy12081910
Chicago/Turabian StyleRetana-Cordero, Marlon, Sofia Flores, Rosanna Freyre, and Celina Gómez. 2022. "Strategies to Reduce Radiation Stress in Open-Field Ginger and Turmeric Production" Agronomy 12, no. 8: 1910. https://doi.org/10.3390/agronomy12081910
APA StyleRetana-Cordero, M., Flores, S., Freyre, R., & Gómez, C. (2022). Strategies to Reduce Radiation Stress in Open-Field Ginger and Turmeric Production. Agronomy, 12(8), 1910. https://doi.org/10.3390/agronomy12081910








