An Investigation into Using Temporary Immersion Bioreactors to Micropropagate Moringa oleifera Lam. Callus, Roots, and Shoots
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Room and In Vitro Conditions
2.2. Seed Preparation
2.3. Experiment 1
2.3.1. Pre-Culturing Phase
2.3.2. Shooting Phase
2.4. Experiment 2
2.4.1. Pre-Culturing Phase
2.4.2. Shooting Phase
3. Results and Discussion
3.1. Experiment 1
3.2. Experiment 2
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: An overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef] [PubMed]
- Mughal, H.M.; Ali, G.; Srivastava, P.S.; Iqbal, M. Improvement of drumstick (Moringa pterygosperma Gaertn.)—A unique source of food and medicine through tissue culture. Hamdard Med. 1999, 42, 37–42. [Google Scholar]
- Stephenson, K.K.; Fahey, J.W. Development of Tissue Culture Methods for the Rescue and Propagation of Endangered Moringa Spp. Germplasm. Econ. Bot. 2004, 58, S116–S124. [Google Scholar] [CrossRef]
- Steinitz, B.; Tabib, Y.; Gaba, V.; Gefen, T.; Vaknin, Y. Vegetative micro-cloning to sustain biodiversity of threatened Moringa species. In Vitro Cell. Dev. Biol. Plant 2009, 45, 65–71. [Google Scholar] [CrossRef]
- Lyam, P.T.; Musa, M.L.; Jamaleddine, Z.O.; Okere, U.A.; Odofin, W.T. The Potential of Temporary Immersion Bioreactors (TIBs) in Meeting Crop Production Demand in Nigeria. J. Biol. Life Sci. 2012, 3, 67–86. [Google Scholar] [CrossRef]
- Debnath, S. Bioreactors and molecular analysis in berry crop micropropagation—A review. Can. J. Plant Sci. 2011, 91, 147–157. [Google Scholar] [CrossRef]
- Businge, E.; Trifonova, A.; Schneider, C.; Rödel, P.; Egertsdotter, U. Evaluation of a new temporary immersion bioreactor system for micropropagation of cultivars of Eucalyptus, Birch and Fir. Forests 2017, 8, 196. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Welander, M.; Persson, J.; Asp, H.; Zhu, L.H. Evaluation of a new vessel system based on temporary immersion system for micropropagation. Sci. Hortic. 2014, 179, 227–232. [Google Scholar] [CrossRef]
- Welander, M.; Sayegh, A.; Hagwall, F.; Kuznetsova, T.; Holefors, A. Technical improvement of a new bioreactor for large scale micropropagation of several Vaccinium cultivars. Acta Hortic. 2017, 1180, 387–392. [Google Scholar] [CrossRef]
- Mordocco, A.M.; Brumbley, J.A.; Lakshmanan, P. Development of a temporary immersion system (RITA®) for mass production of sugarcane (Saccharum spp. interspecific hybrids). In Vitro Cell. Dev. Biol. Plant 2009, 45, 450–457. [Google Scholar] [CrossRef]
- Escalona, M.; Lorenzo, J.C.; Gonzalez, B.; Daquinta, M.; González, J.L.; Desjardins, Y.; Borroto, C.G. Pineapple (Ananas comosus L. Merr) micropropagation in temporary immersion systems. Plant Cell Rep. 1999, 18, 743–748. [Google Scholar] [CrossRef]
- Latawa, J.; Shukla, M.R.; Saxena, P.K. An efficient temporary immersion system for micropropagation of hybrid hazelnut. Botany 2016, 94, 1–8. [Google Scholar] [CrossRef]
- Jeong, C.-S.; Chakrabarty, D.; Hahn, E.-J.; Lee, H.-L.; Paek, K.-Y. Effects of oxygen, carbon dioxide and ethylene on growth and bioactive compound production in bioreactor culture of ginseng adventitious roots. Biochem. Eng. J. 2006, 27, 252–263. [Google Scholar] [CrossRef]
- Coetser, E.; Du Toit, E.; Prinsloo, G. Temporary immersion bioreactors for clonal production of Moringa oleifera tissues. Acta Hortic. 2021, 1306, 13–18. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Lin, M.; Li, S.; Tang, Y.; Chen, H.; Chen, X. An efficient micropropagation protocol for direct organogenesis from leaf explants of an economically valuable plant, drumstick (Moringa oleifera Lam.). Ind. Crops Prod. 2017, 103, 59–63. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Mathur, M.; Yadav, S.; Katariya, P.K.; Kamal, R. In vitro propagation and biosynthesis of steroidal sapogenins from various morphogenetic stages of Moringa oleifera Lam., and their antioxidant potential. Acta Physiol. Plant. 2014, 36, 1749–1762. [Google Scholar] [CrossRef]
- Quiala, E.; Cañal, M.-J.; Meijón, M.; Rodriguez, R.; Chávez, M.; Valledor, L.; De Feria, M.; Barbón, R. Morphological and physiological responses of proliferating shoots of teak to temporary immersion and BA treatments. Plant Cell Tissue Organ Cult. 2012, 109, 223–234. [Google Scholar] [CrossRef]
- Shahzad, U.; Jaskani, M.J.; Ahmad, S.; Awan, F.S. Optimisation of the micro-cloning system of threatened Moringa oleifera LAM. Pak. J. Agric. Sci. 2014, 51, 449–457. [Google Scholar]
- Saini, R.K.; Shetty, N.P.; Giridhar, P.; Ravishankar, G.A. Rapid in vitro regeneration method for Moringa oleifera and performance evaluation of field grown nutritionally enriched tissue culture. 3 Biotech 2012, 2, 187–192. [Google Scholar] [CrossRef]
- Förster, N.; Mewis, I.; Ulrichs, C. Moringa oleifera—Establishment and multiplication of different ecotypes in vitro. Gesunde Pflanz. 2013, 65, 21–31. [Google Scholar] [CrossRef]

| Treatment | Effect | Photo |
|---|---|---|
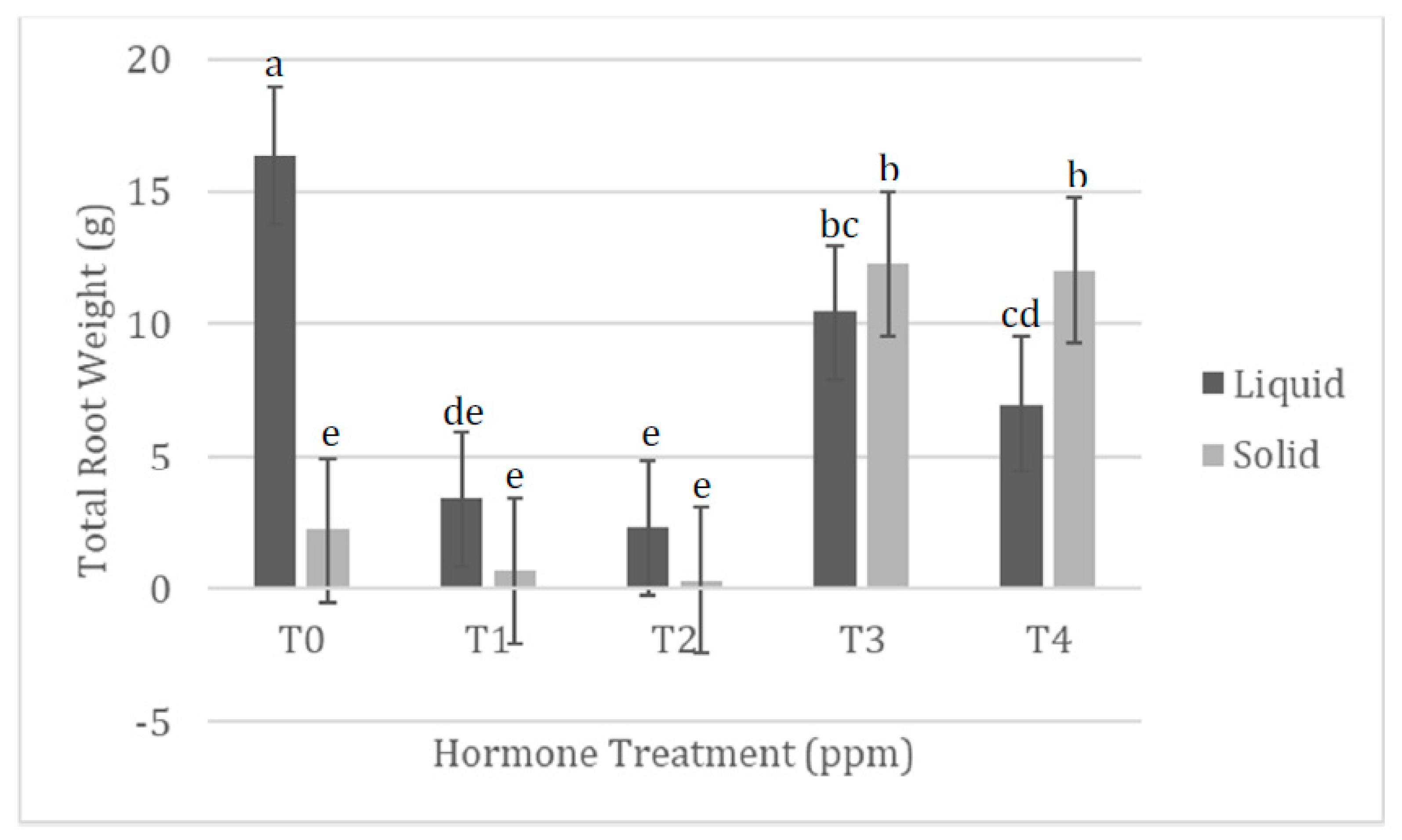
| 0.5 ppm NAA pre-culture No added cytokinins Solid media | Production of long fine roots and callus tissue. Green tissues are relics of the leaf tissue used as explants. Less tissue in terms of wet mass produced than TIS. |  |
| 0.5 ppm NAA pre-culture No added cytokinins TIS | Production of long fine roots and callus tissue. Green tissues are relics of the leaf tissue used as explants. More tissue in terms of wet mass produced than solidified medium treatment. |  |
| 0.5 ppm NAA pre-culture 0.5 ppm BA Solid media | Mostly production of callus. Production of some fine, but short roots. Slightly less callus wet mass production than TIS treatment. Similar results to 1.0 ppm BA treatment on solidified media. |  |
| 0.5 ppm NAA pre-culture 0.5 ppm BA TIS | Mostly production of callus. Production of some fine, but short roots. Slightly more callus wet mass production than solidified treatment. Similar results to 1.0 ppm BA treatment on solidified media. | 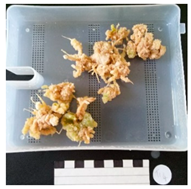 |
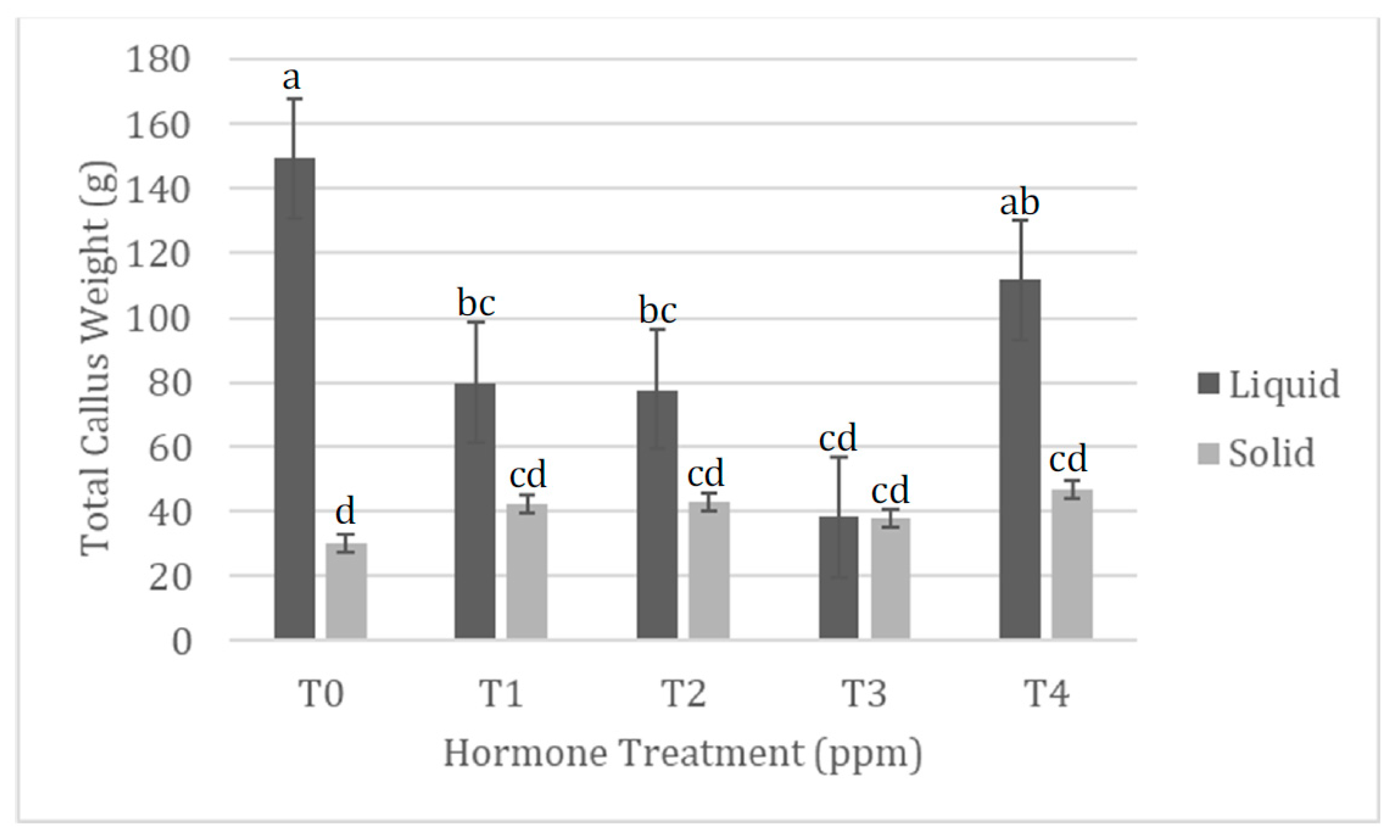
| 0.5 ppm NAA pre-culture 1.0 ppm BA Solid media | Mostly production of callus. Production of some fine, but short roots. Slightly less callus wet mass production than TIS treatment. Similar results to 0.5 ppm BA treatment on solidified media. |  |
| 0.5 ppm NAA pre-culture 1.0 ppm BA TIS | Mostly production of callus. Production of some fine, but short roots. Slightly more callus wet mass production than solidified treatment. Similar results to 0.5 ppm BA treatment on solidified media. |  |
| 0.5 ppm NAA pre-culture 0.25 ppm KT Solid media | Production of callus and long fine roots. Callus wet mass production similar to TIS treatment and 0.5 ppm KT treatment. Root wet mass slightly more than TIS treatment, but similar to 0.5 ppm KT treatment. |  |
| 0.5 ppm NAA pre-culture 0.25 ppm KT TIS | Production of callus and long fine roots. Callus wet mass production similar to solidified treatment, but less than 0.5 ppm KT treatment. Root wet mass slightly less than solidified treatment, and slightly more than 0.5 ppm KT treatment. |  |
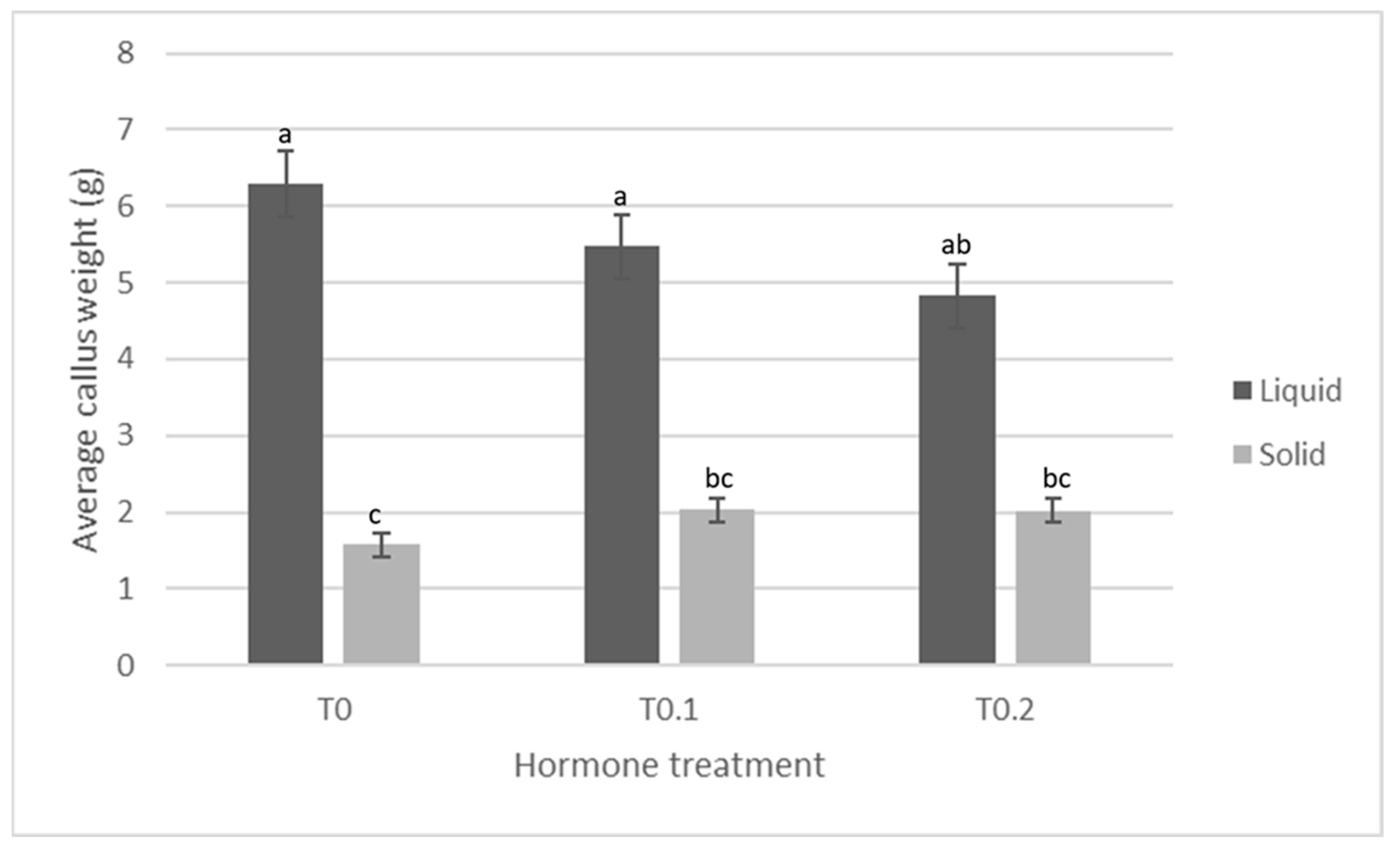
0.5 ppm NAA pre-culture 0.5 ppm KT Solid media | Production of callus and a few fine roots. Callus wet mass production less than TIS treatment, but similar to 0.25 ppm KT treatment. Root wet mass significantly more than TIS treatment, but similar to 0.25 ppm KT treatment. |  |
| 0.5 ppm NAA pre-culture 0.5 ppm KT TIS | Production of callus and some fine roots. Callus wet mass production significantly more than solidified treatment and 0.25 ppm KT treatment. Root wet mass significantly less than solidified treatment, and slightly less than 0.25 ppm KT treatment. |  |
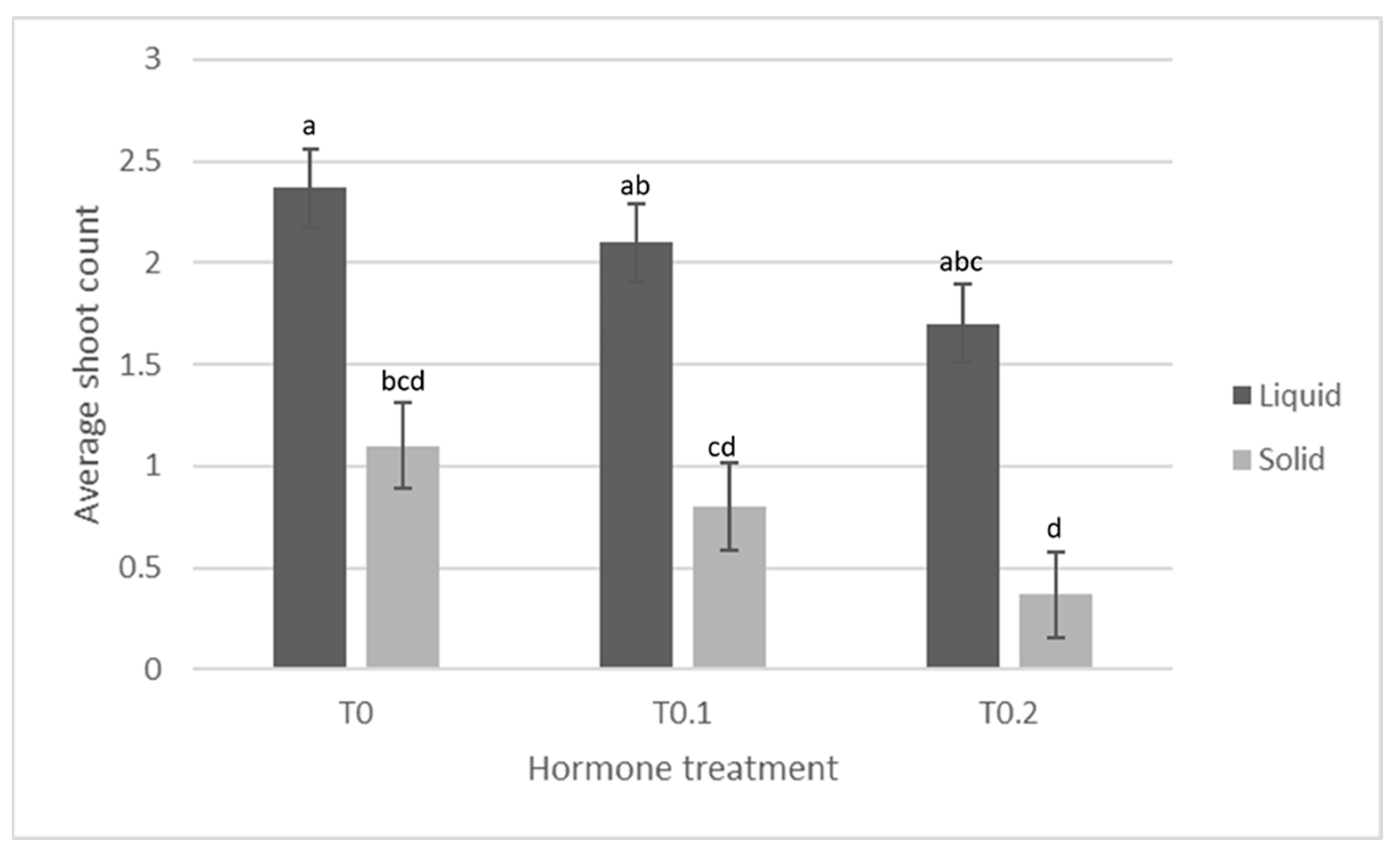
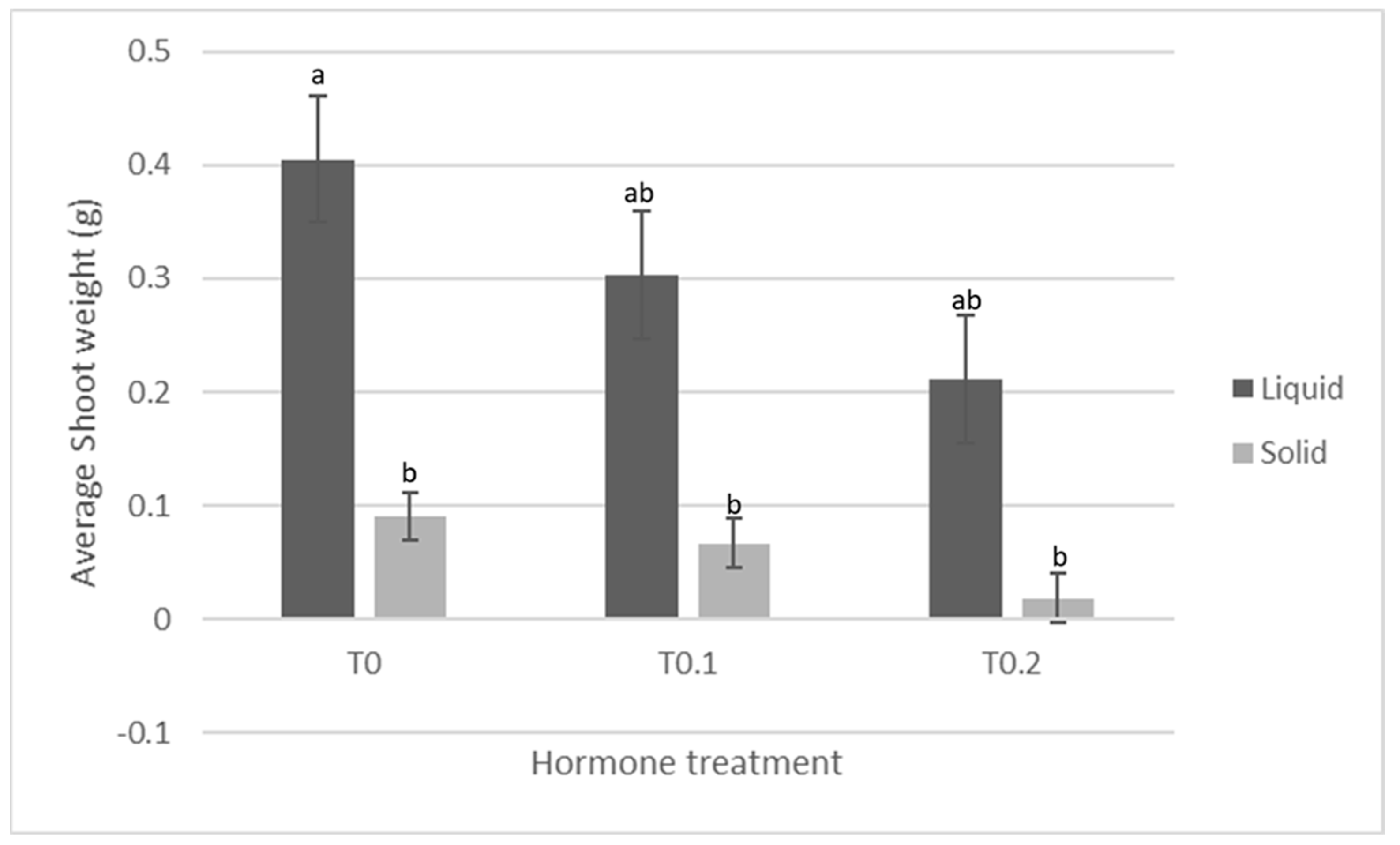
| 0 ppm BA pre-culture 0.1 ppm BA Solid media | Production of shoots and leaf material with wet mass significantly lower than TIS treatment, but similar to (slightly more than) 0.1 ppm and 0.2 ppm BA treatments. Callus wet mass production significantly less than TIS, but similar to other BA treatments. |  |
| 0 ppm BA pre-culture 0.1 ppm BA TIS | Production of shoots and leaf material with wet mass significantly higher than solidified treatment, but similar to (slightly more than) 0.1 ppm and 0.2 ppm BA treatments. Callus wet mass production significantly more than solidified treatment, but similar to (slightly more than) other BA treatments. |  |
| 0.1 ppm BA pre-culture 0.1 ppm BA Solid media | Production of shoots and leaf material with wet mass lower than TIS treatment, but similar to 0 ppm and 0.2 ppm BA treatments. Callus wet mass production significantly less than TIS, but similar to other BA treatments. |  |
| 0.1 ppm BA pre-culture 0.1 ppm BA TIS | Production of shoots and leaf material with wet mass higher than solidified treatment, but similar to 0 ppm and 0.2 ppm BA treatments. Callus wet mass production significantly more than solidified treatment, but similar to other BA treatments. |  |
| 0.2 ppm BA pre-culture 0.1 ppm BA Solid media | Production of some shoots and leaf material with wet mass lower than TIS treatment, but similar to (slightly lower than) 0 ppm and 0.1 ppm BA treatments. Callus wet mass production less than TIS, but similar to other BA treatments. |  |
| 0.2 ppm BA pre-culture 0.1 ppm BA TIS | Production of some shoots and leaf material with wet mass higher than solidified treatment, but similar to (slightly less than) 0 ppm and 0.1 ppm BA treatments. Callus wet mass production more than solidified treatment, but similar to other BA treatments. |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coetser, E.; du Toit, E.S.; Prinsloo, G. An Investigation into Using Temporary Immersion Bioreactors to Micropropagate Moringa oleifera Lam. Callus, Roots, and Shoots. Agronomy 2022, 12, 2672. https://doi.org/10.3390/agronomy12112672
Coetser E, du Toit ES, Prinsloo G. An Investigation into Using Temporary Immersion Bioreactors to Micropropagate Moringa oleifera Lam. Callus, Roots, and Shoots. Agronomy. 2022; 12(11):2672. https://doi.org/10.3390/agronomy12112672
Chicago/Turabian StyleCoetser, Elmien, Elsa S. du Toit, and Gerhard Prinsloo. 2022. "An Investigation into Using Temporary Immersion Bioreactors to Micropropagate Moringa oleifera Lam. Callus, Roots, and Shoots" Agronomy 12, no. 11: 2672. https://doi.org/10.3390/agronomy12112672
APA StyleCoetser, E., du Toit, E. S., & Prinsloo, G. (2022). An Investigation into Using Temporary Immersion Bioreactors to Micropropagate Moringa oleifera Lam. Callus, Roots, and Shoots. Agronomy, 12(11), 2672. https://doi.org/10.3390/agronomy12112672






