Psychological Aspects of Sensitive Skin: A Vicious Cycle
Abstract
1. Introduction
2. Physiological Contributors to SSS
Other Host Related Factors
3. Psychological Effects of Skin Diseases
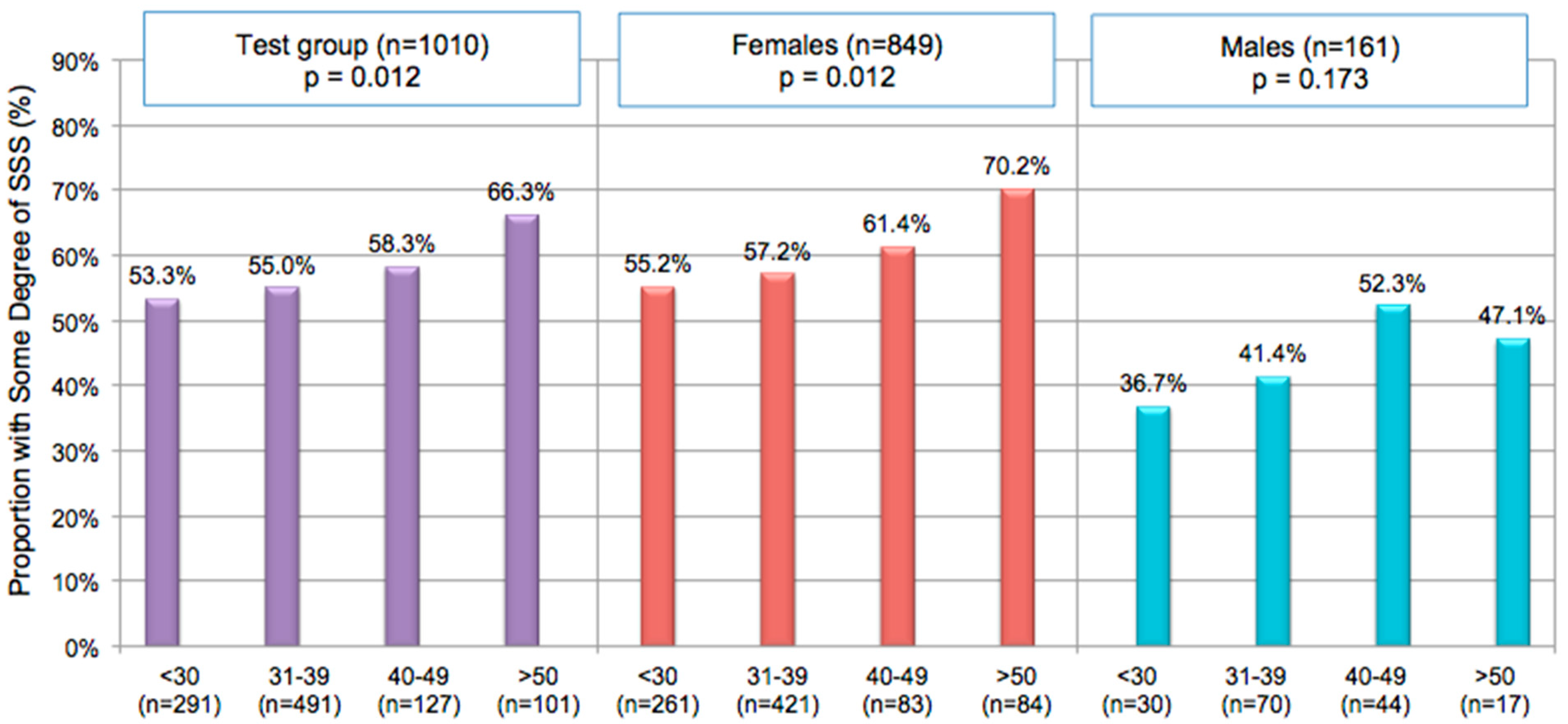
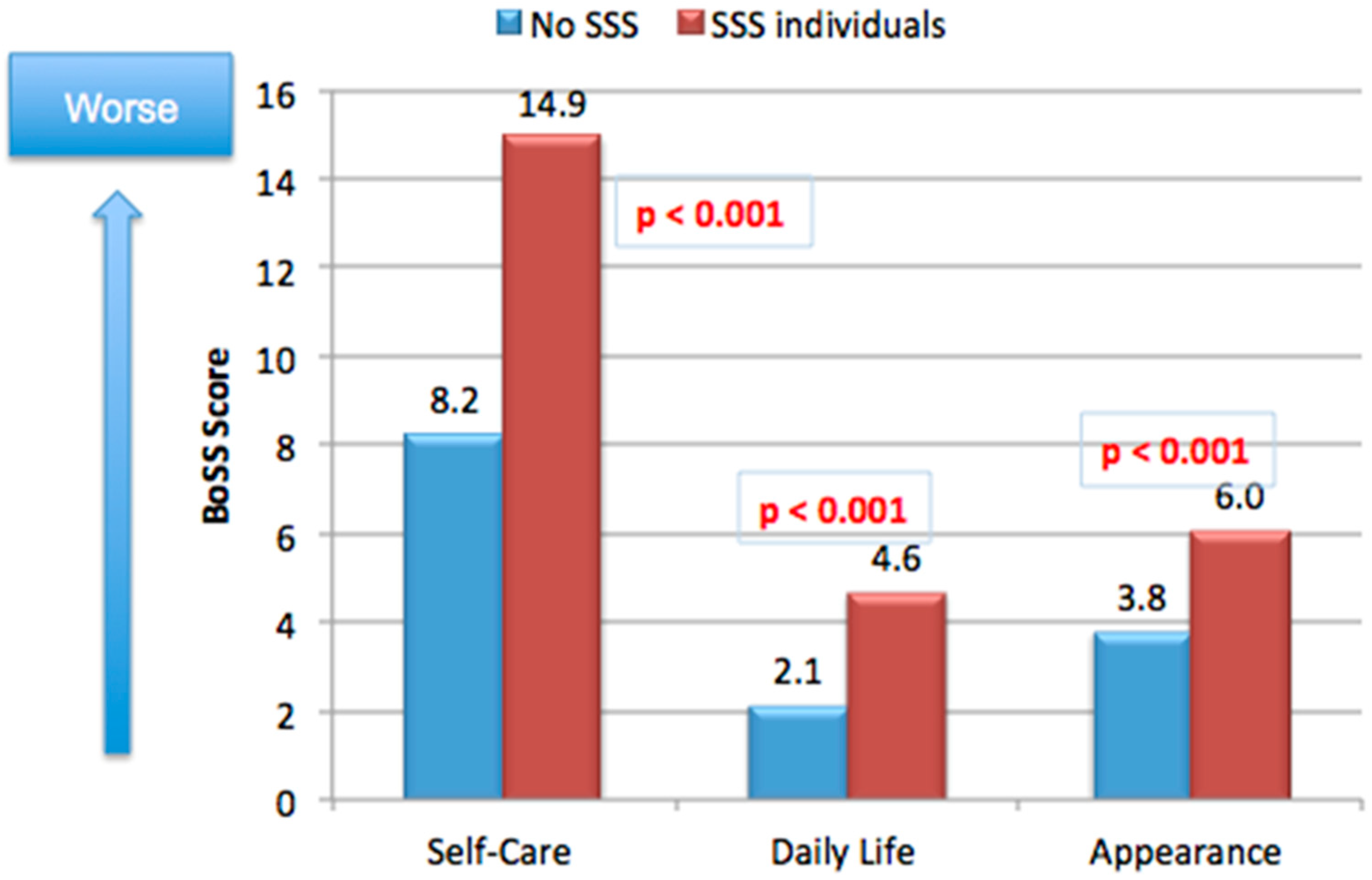
4. Impact of SSS on The Daily Life of Consumers
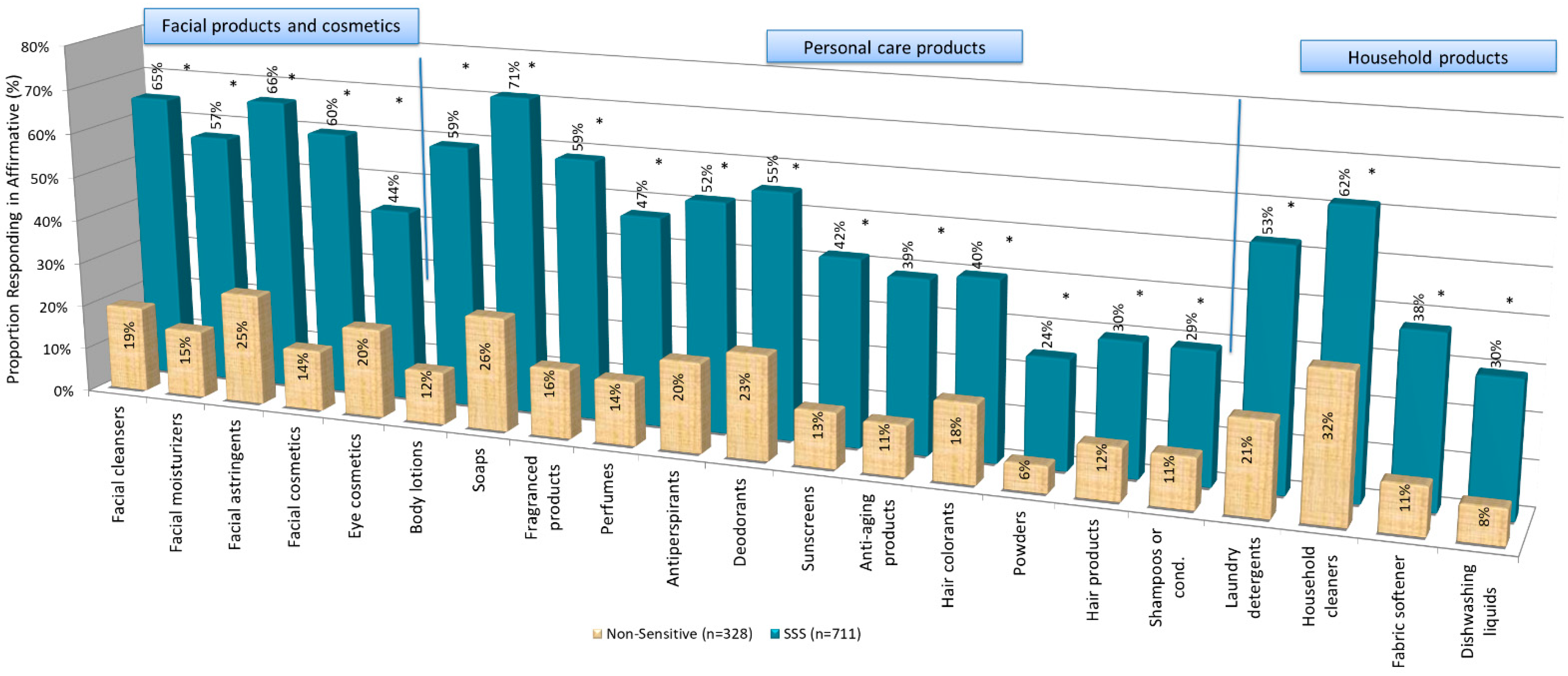
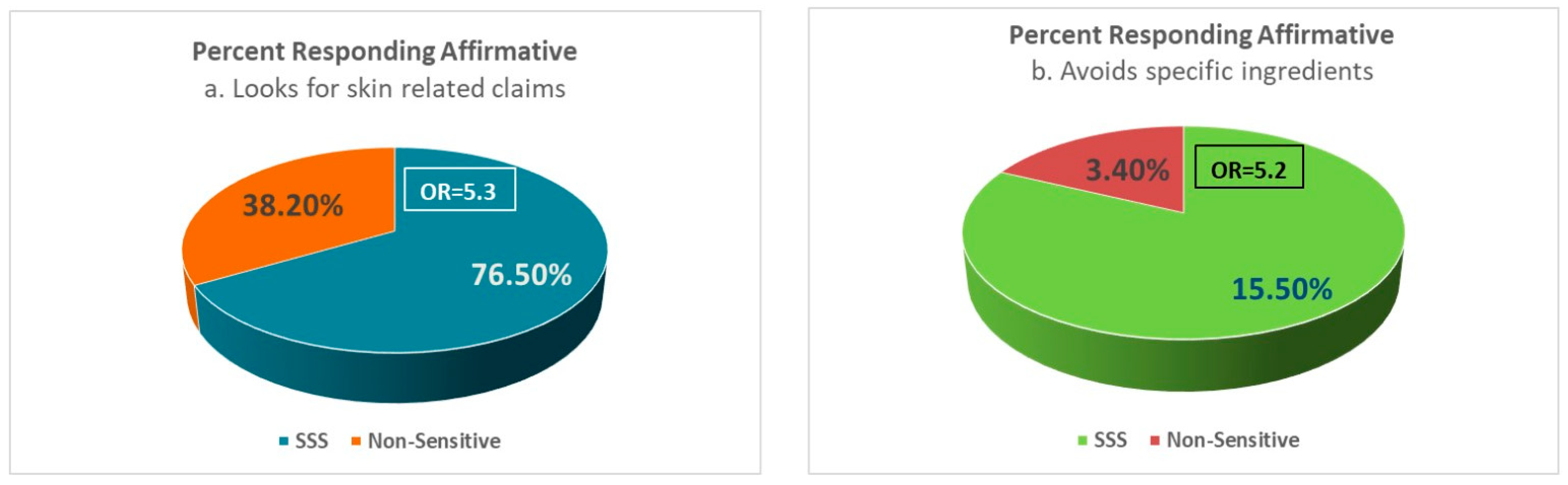
4.1. Consumers’ Behavior: Avoidance and Shopping Practices
4.2. Fatigue and Sleep Disorders
4.3. Stress, Anxiety and Depression
4.4. The Stress of COVID Containment
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farage, M.A. Understanding the Sensitive Skin Subject to Achieve a More Holistic Diagnosis. Cosmetics 2021, 8, 81. [Google Scholar] [CrossRef]
- Bataille, A.; Gall-Ianotto, C.L.; Genin, E.; Misery, L. Sensitive Skin: Lessons from Transcriptomic Studies. Front. Med. 2019, 6, 115. [Google Scholar] [CrossRef]
- Misery, L.; Ständer, S.; Szepietowski, J.C.; Reich, A.; Wallengren, J.; Evers, A.W.; Takamori, K.; Brenaut, E.; Gall-Ianotto, C.L.; Fluhr, J.; et al. Definition of Sensitive Skin: An Expert Position Paper from the Special Interest Group on Sensitive Skin of the International Forum for the Study of Itch. Acta Derm. Venereol. 2017, 97, 4–6. [Google Scholar] [CrossRef]
- Berardesca, E.; Farage, M.; Maibach, H. Sensitive skin: An overview. Int. J. Cosmet. Sci. 2013, 35, 2–8. [Google Scholar] [CrossRef]
- Farage, M.A. The Prevalence of Sensitive Skin. Front. Med. 2019, 6, 98. [Google Scholar] [CrossRef]
- Chen, W.; Dai, R.; Li, L. The prevalence of self-declared sensitive skin: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2019, 34, 1779–1788. [Google Scholar] [CrossRef]
- Misery, L.; Jourdan, E.; Abadie, S.; Ezzedine, K.; Brenaut, E.; Huet, F.; Sayag, M.; Taieb, C. Development and validation of a new tool to assess the Burden of Sensitive Skin (BoSS). J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2217–2223. [Google Scholar] [CrossRef]
- Cho, H.J.; Chung, B.Y.; Lee, H.B.; Kim, H.O.; Park, C.W.; Lee, C.H. Quantitative study of stratum corneum ceramides contents in patients with sensitive skin. J. Dermatol. 2012, 39, 295–300. [Google Scholar] [CrossRef]
- Cua, A.B.; Wilhelm, K.P.; Maibach, H.I. Cutaneous sodium lauryl sulphate irritation potential: Age and regional variability. Br. J. Dermatol. 1990, 123, 607–613. [Google Scholar] [CrossRef]
- Pons-Guiraud, A. Sensitive skin: A complex and multifactorial syndrome. J. Cosmet. Dermatol. 2004, 3, 145–148. [Google Scholar] [CrossRef]
- Misery, L.; Weisshaar, E.; Brenaut, E.; Evers, A.; Huet, F.; Ständer, S.; Reich, A.; Berardesca, E.; Serra-Baldrich, E.; Wallengren, J.; et al. Pathophysiology and management of sensitive skin: Position paper from the special interest group on sensitive skin of the International Forum for the Study of Itch (IFSI). J. Eur. Acad. Dermatol. Venereol. 2020, 34, 222–229. [Google Scholar] [CrossRef]
- Roussaki-Schulze, A.V.; Zafiriou, E.; Nikoulis, D.; Klimi, E.; Rallis, E.; Zintzaras, E. Objective biophysical findings in patients with sensitive skin. Drugs Exp. Clin. Res. 2005, 31, 17–24. [Google Scholar]
- Misery, L.; Loser, K.; Ständer, S. Sensitive skin. J. Eur. Acad. Dermatol. Venereol. 2016, 30 (Suppl. 1), 2–8. [Google Scholar] [CrossRef]
- Buhé, V.; Vié, K.; Guéré, C.; Natalizio, A.; Lhéritier, C.; Gall-Ianotto, C.; Huet, F.; Talagas, M.; Lebonvallet, N.; Marcorelles, P.; et al. Pathophysiological Study of Sensitive Skin. Acta Derm. Venereol. 2016, 96, 314–318. [Google Scholar] [CrossRef]
- Ehnis-Pérez, A.; Torres-Álvarez, B.; Cortés-García, D.; Hernández-Blanco, D.; Fuentes-Ahumada, C.; Castanedo-Cázares, J.P. Relationship between transient receptor potential vanilloid-1 expression and the intensity of sensitive skin symptoms. J. Cosmet. Dermatol. 2016, 15, 231–237. [Google Scholar] [CrossRef]
- Kueper, T.; Krohn, M.; Haustedt, L.O.; Hatt, H.; Schmaus, G.; Vielhaber, G. Inhibition of TRPV1 for the treatment of sensitive skin. Exp. Dermatol. 2010, 19, 980–986. [Google Scholar] [CrossRef]
- Mikkelsen, C.S.; Holmgren, H.R.; Kjellman, P.; Heidenheim, M.; Kappinnen, A.; Bjerring, P.; Huldt-Nystrøm, T. Rosacea: A Clinical Review. Dermatol. Rep. 2016, 8, 6387. [Google Scholar] [CrossRef]
- Chang, A.L.S.; Raber, I.; Xu, J.; Li, R.; Spitale, R.; Chen, J.; Kiefer, A.K.; Tian, C.; Eriksson, N.K.; Hinds, D.A.; et al. Assessment of the genetic basis of rosacea by genome-wide association study. J. Investig. Dermatol. 2015, 135, 1548–1555. [Google Scholar] [CrossRef]
- Kim, Y.R.; Cheon, H.I.; Misery, L.; Taieb, C.; Lee, Y.W. Sensitive skin in Korean population: An epidemiological approach. Skin Res. Technol. 2018, 24, 229–234. [Google Scholar] [CrossRef]
- Brenaut, E.; Misery, L.; Taieb, C. Sensitive Skin in the Indian Population: An Epidemiological Approach. Front. Med. 2019, 6, 29. [Google Scholar] [CrossRef]
- Wang, X.Y.; Liu, Y.Y.; Liu, Y.X.; Ma, W.W.; Zhang, J.W.; Liu, Z.J.; Liu, J.; Zhou, B.R.; Xu, Y. A predictive model for differential diagnosis between rosacea and sensitive skin: A cross-sectional study. Chin. Med. J. 2020, 133, 2132–2134. [Google Scholar] [CrossRef]
- Willis, C.M.; Shaw, S.; De Lacharriere, O.; Baverel, M.; Reiche, L.; Jourdain, R.; Bastien, P.; Wilkinson, J.D. Sensitive skin: An epidemiological study. Br. J. Dermatol. 2001, 145, 258–263. [Google Scholar] [CrossRef]
- Misery, L.; Jourdan, E.; Huet, F.; Brenaut, E.; Cadars, B.; Virassamynaïk, S.; Sayag, M.; Taieb, C. Sensitive skin in France: A study on prevalence, relationship with age and skin type and impact on quality of life. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 791–795. [Google Scholar] [CrossRef]
- Farage, M.A.; Bowtell, P.; Katsarou, A. Self-diagnosed sensitive skin in women with clinically diagnosed atopic dermatitis. Clin. Med. Dermatol. 2008, 2, 21–28. [Google Scholar]
- Farage, M.A.; Jiang, Y.; Tiesman, J.P.; Fontanillas, P.; Osborne, R. Genome-Wide Association Study Identifies Loci Associated with Sensitive Skin. Cosmetics 2020, 7, 49. [Google Scholar] [CrossRef]
- Misery, L.; Cochener, B.; Brenaut, E.; Séité, S.; Taieb, C. Association of sensitive skin with sensitive corneas and sensitive eyelids. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1358–1362. [Google Scholar] [CrossRef]
- Misery, L.; Duboc, H.; Coffin, B.; Brenaut, E.; Huet, F.; Taieb, C. Association between two painful and poorly understood conditions: Irritable bowel and sensitive skin syndromes. Eur. J. Pain 2019, 23, 160–166. [Google Scholar] [CrossRef]
- Huet, F.; Misery, L. Sensitive skin is a neuropathic disorder. Exp. Dermatol. 2019, 28, 1470–1473. [Google Scholar] [CrossRef]
- Farage, M.A. Perceptions of Sensitive Skin with Age. In Textbook of Aging Skin, 2nd ed.; Farage, M.A., Miller, K.W., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Robinson, M.K. Population differences in acute skin irritation responses. Race, sex, age, sensitive skin and repeat subject comparisons. Contact Dermat. 2002, 46, 86–93. [Google Scholar] [CrossRef]
- Lejman, E.; Stoudemayer, T.; Grove, G.; Kligman, A.M. Age differences in poison ivy dermatitis. Contact Dermat. 1984, 11, 163–167. [Google Scholar] [CrossRef]
- Grove, G.L.; Duncan, S.; Kligman, A.M. Effect of ageing on the blistering of human skin with ammonium hydroxide. Br. J. Dermatol. 1982, 107, 393–400. [Google Scholar] [CrossRef]
- Misery, L.; Sibaud, V.; Merial-Kieny, C.; Taieb, C. Sensitive skin in the American population: Prevalence, clinical data, and role of the dermatologist. Int. J. Dermatol. 2011, 50, 961–967. [Google Scholar] [CrossRef]
- Xiao, X.; Qiao, L.; Ye, R.; Zuo, F. Nationwide Survey and Identification of Potential Stress Factor in Sensitive Skin of Chinese Women. Clin. Cosmet. Investig. Dermatol. 2020, 13, 867–874. [Google Scholar] [CrossRef]
- Farage, M.A. Does sensitive skin differ between men and women? Cutan. Ocul. Toxicol. 2010, 29, 153–163. [Google Scholar] [CrossRef]
- Farage, M.A.; Mandl, C.P.; Berardesca, E.; Maibach, H.I. Sensitive Skin in China. J. Cosmet. Dermatol. Sci. Appl. 2012, 2, 184–195. [Google Scholar] [CrossRef][Green Version]
- Farage, M.A. Sensitive skin in the genital area. Front. Med. 2019, 6, 142–154. [Google Scholar] [CrossRef]
- Farage, M.A.; Neill, S.; MacLean, A.B. Physiological changes associated with the menstrual cycle: A review. Obstet. Gynecol. Surv. 2009, 64, 58–72. [Google Scholar] [CrossRef]
- Falcone, D.; Richters, R.J.; Uzunbajakava, N.E.; Van Erp, P.E.; Van De Kerkhof, P.C. Sensitive skin and the influence of female hormone fluctuations: Results from a cross-sectional digital survey in the Dutch population. Eur. J. Dermatol. 2017, 27, 42–48. [Google Scholar] [CrossRef]
- Barankin, B.; DeKoven, J. Psychosocial effect of common skin diseases. Can. Fam. Physician 2002, 48, 712–716. [Google Scholar]
- Hong, J.; Koo, B.; Koo, J. The psychosocial and occupational impact of chronic skin disease. Dermatol. Ther. 2008, 21, 54–59. [Google Scholar] [CrossRef]
- Yew, Y.W.; Kuan, A.H.Y.; Ge, L.; Yap, C.W.; Heng, B.H. Psychosocial impact of skin diseases: A population-based study. PLoS ONE 2020, 15, e0244765. [Google Scholar] [CrossRef]
- Dalgard, F.J.; Gieler, U.; Tomas-Aragones, L.; Lien, L.; Poot, F.; Jemec, G.B.E.; Misery, L.; Szabo, C.; Linder, D.; Sampogna, F.; et al. The psychological burden of skin diseases: A cross-sectional multicenter study among dermatological out-patients in 13 European countries. J. Investig. Dermatol. 2015, 135, 984–991. [Google Scholar] [CrossRef]
- Costeris, C.; Petridou, M.; Ioannou, Y. Psychological Impact of Skin Disorders on Patients’ Self-esteem and Perceived Social Support. J. Dermatol. Ski. Sci. 2021, 3, 14–22. [Google Scholar]
- Misery, L.; Myon, E.; Martin, N.; Consoli, S.; Boussetta, S.; Nocera, T.; Taieb, C. Sensitive skin: Psychological effects and seasonal changes. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 620–628. [Google Scholar] [CrossRef]
- Polena, H.; Chavagnac-Bonneville, M.; Misery, L.; Sayag, M. Burden of Sensitive Skin (BoSS) Questionnaire and Current Perception Threshold: Use as Diagnostic Tools for Sensitive Skin Syndrome. Acta Derm. Venereol. 2021, 101, adv00606. [Google Scholar] [CrossRef]
- Farage, M.A. Perceptions of sensitive skin: Changes in perceived severity and associations with environmental causes. Contact Dermat. 2008, 59, 226–232. [Google Scholar] [CrossRef]
- Misery, L.; Morisset, S.; Séité, S.; Brenaut, E.; Ficheux, A.S.; Fluhr, J.W.; Delvigne, V.; Taieb, C. Relationship between sensitive skin and sleep disorders, fatigue, dust, sweating, food, tobacco consumption or female hormonal changes: Results from a worldwide survey of 10 743 individuals. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1371–1376. [Google Scholar] [CrossRef]
- Farage, M.A. How do perceptions of sensitive skin differ at different anatomical sites? An epidemiological study. Clin. Exp. Dermatol. 2009, 34, e521–e530. [Google Scholar] [CrossRef]
- Farage, M.A.; Nusair, T.L.; Hanseman, D.; Sherman, S.N.; Tsevat, J. The Farage Quality of Life Measure for Consumer Products: Development and Initial Implementation. Appl. Res. Qual. Life 2010, 5, 1–25. [Google Scholar] [CrossRef]
- Farage, M.A.; Rodenberg, C.; Chen, J. Translation and Validation of the Farage Quality of Life (FQoL) Instrument for Consumer Products into Traditional Chinese. Glob. J. Health Sci. 2013, 5, 1–12. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Adique, A.; Sarkar, P.; Shenai, V.; Sampath, M.; Lai, R.; Qi, J.; Wang, M.; Farage, M.A. The Impact of Routine Skin Care on the Quality of Life. Cosmetics 2020, 7, 59. [Google Scholar] [CrossRef]
- Verhoeven, E.W.; Kraaimaat, F.W.; van de Kerkhof, P.C.; van Weel, C.; Duller, P.; van der Valk, P.G.; van den Hoogen, H.J.; Bor, J.H.; Schers, H.J.; Evers, A.W. Prevalence of physical symptoms of itch, pain and fatigue in patients with skin diseases in general practice. Br. J. Dermatol. 2007, 156, 1346–1349. [Google Scholar] [CrossRef]
- Misery, L.; Shourick, J.; Taieb, C. Prevalence and characterization of fatigue in patients with skin diseases. Acta Derm. Venereol. 2020, 100, adv00327. [Google Scholar] [CrossRef]
- Mostaghimi, L.; Hetzel, S. Insomnia and other sleep complaints in inflammatory versus noninflammatory skin disorders: An observational case-control study. Int. J. Dermatol. 2019, 58, 976–981. [Google Scholar] [CrossRef]
- Schmelz, M. Itch Processing in the Skin. Front. Med. 2019, 6, 167. [Google Scholar] [CrossRef]
- Halioua, B.; Misery, L.; Seite, S.; Delvigne, V.; Chelli, C.; Taieb, J.; Taieb, C. Influence of Skin Subjective Symptoms on Sleep Quality in Patients with Cutaneous Disorders: A Study of 2871 Subjects. Clin. Cosmet. Investig. Dermatol. 2021, 14, 143–152. [Google Scholar] [CrossRef]
- Osman, O.T.; Mufaddel, A.; Almugaddam, F.; Augusterfer, E.F. The psychiatric aspects of skin disorders. Expert Rev. Dermatol. 2011, 6, 195–209. [Google Scholar] [CrossRef]
- Verhoeven, E.W.; de Klerk, S.; Kraaimaat, F.W.; van de Kerkhof, P.C.; de Jong, E.M.; Evers, A.W. Biopsychosocial mechanisms of chronic itch in patients with skin diseases: A review. Acta Derm. Venereol. 2008, 88, 211–218. [Google Scholar]
- Huynh, T.T. Burden of Disease: The Psychosocial Impact of Rosacea on a Patient’s Quality of Life. Am. Health Drug Benefits 2013, 6, 348–354. [Google Scholar]
- Reich, A.; Wójcik-Maciejewicz, A.; Slominski, A.T. Stress and the skin. G. Ital. Dermatol. Venereol. 2010, 145, 213–219. [Google Scholar]
- Blount, B.W.; Pelletier, A.L. Rosacea: A common, yet commonly overlooked, condition. Am. Fam. Physician 2002, 66, 435–440. [Google Scholar]
- Saint-Martory, C.; Roguedas-Contios, A.M.; Sibaud, V.; Degouy, A.; Schmitt, A.M.; Misery, L. Sensitive skin is not limited to the face. Br. J. Dermatol. 2008, 158, 130–133. [Google Scholar] [CrossRef]
- Misery, L.; Jean-Decoster, C.; Mery, S.; Georgescu, V.; Sibaud, V. A new ten-item questionnaire for assessing sensitive skin: The Sensitive Scale-10. Acta Derm. Venereol. 2014, 94, 635–639. [Google Scholar] [CrossRef]
- Kluger, N.; Floc’h, C.L.; Niore, M.; Delvigne, V.; Dantec, G.L.; Taieb, C. Self-Reported Skin Sensation by People Who Have Experienced Containment During COVID-19 Pandemic. Clin. Cosmet. Investig. Dermatol. 2020, 13, 943–947. [Google Scholar] [CrossRef]
| Epidermal | |
| [5,8,9,10] | Reduced barrier integrity |
| Decrease in ceramide and sphingolipid | |
| Increased penetration of potential irritants | |
| Decreased protection of nerve endings | |
| Vascular | |
| [12,13] | Increase in vascular activity |
| Intense vascular reaction to to methyl nicotinate | |
| Greater reactions to standard allergens | |
| Lower alkali resistance | |
| Neurosensorial | |
| [11,14,15,16] | Decrease of intraepidermal nerve fiber density |
| Reduced peptidergic C-fiber density | |
| Increase in Transient Receptor Potential Vanilloid-1 (TRPV1) |
| Skin Condition |
|---|
| Rosacea [17,20,21] |
| Acne [19,20] |
| Atopic dermatitis (eczema) [5,19,20] |
| Atopic [22,23,24] |
| Blushing [19] |
| Seborrheic dermatitis (dandruff) [19,20,25] |
| Psoriasis [20] |
| Vitiligo [20] |
| Contact dermatitis [25] |
| Freckles [25] |
| Sensitivity of the corneas and eyelids [26] |
| Irritable bowel syndrome [26] |
| SSS Subjects | Non-Sensitive Subjects | p Value | Ref | |||
|---|---|---|---|---|---|---|
| Total Responding | Factor Causes Irritation (%) | Total Responding | Factor Causes Irritation (%) | |||
| Environmental | ||||||
| Humid Weather a | 641 | 48% | 295 | 13% | <0.0005 | [47] |
| Dry weather a | 664 | 78% | 297 | 54% | <0.0005 | |
| Hot weather a | 659 | 66% | 295 | 32% | <0.0005 | |
| Cold weather a | 675 | 87% | 303 | 70% | <0.0005 | |
| Sun a | 673 | 82% | 306 | 66% | <0.0005 | |
| Wind a | 654 | 71% | 292 | 53% | <0.0005 | |
| Air conditioning b | 63 | 13% | 22 | 5% | <0.001 | [33] |
| Temperature variation b | 210 | 47% | 104 | 19% | <0.001 | |
| Water b | 69 | 15% | 33 | 6% | <0.001 | |
| Pollution b | 3249 | 63% | 1823 | 33% | <0.001 | [48] |
| Dust b | 2990 | 58% | 1633 | 29% | <0.001 | |
| Lifestyle and habits | ||||||
| Rough fabrics a | 666 | 71% | 293 | 43% | <0.0005 | [47] |
| Cosmetics b | 2989 | 58% | 1250 | 22% | <0.001 | [48] |
| Sweating b | 2814 | 54% | 1496 | 27% | <0.001 | |
| Tobacco smoke b | 2055 | 40% | 1135 | 20% | <0.001 | |
| Food b | 2262 | 44% | 950 | 17% | <0.001 | |
| Sensitive Skin in the Genital Area | Hot Weather | Cold Weather | Rough Fabric | Dry Weather | Stress | Humid Weather | Menstrual Cycle a | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥50 years old | |||||||||||||||||||
| Total sensitive responders | 59 | 59 | 64 | 60 | 62 | 60 | 23 | ||||||||||||
| Factor causes irritation (%) | 88% | b | 86% | 86% | c | 72% | 58% | 47% | 30% | d | |||||||||
| 40–49 years old | |||||||||||||||||||
| Total sensitive responders | 68 | 70 | 70 | 67 | 68 | 66 | 42 | ||||||||||||
| Factor causes irritation (%) | 57% | 79% | 71% | 73% | 44% | e | 39% | 52% | |||||||||||
| 31–39 years old | |||||||||||||||||||
| Total sensitive responders | 256 | 261 | 255 | 257 | 256 | 245 | 229 | ||||||||||||
| Factor causes irritation (%) | 64% | 86% | 71% | 79% | 62% | 44% | 62% | ||||||||||||
| ≤30 years old | |||||||||||||||||||
| Total sensitive responders | 151 | 151 | 150 | 152 | 150 | 147 | 137 | ||||||||||||
| Factor causes irritation (%) | 59% | 82% | 75% | 76% | 65% | 45% | 65% | ||||||||||||
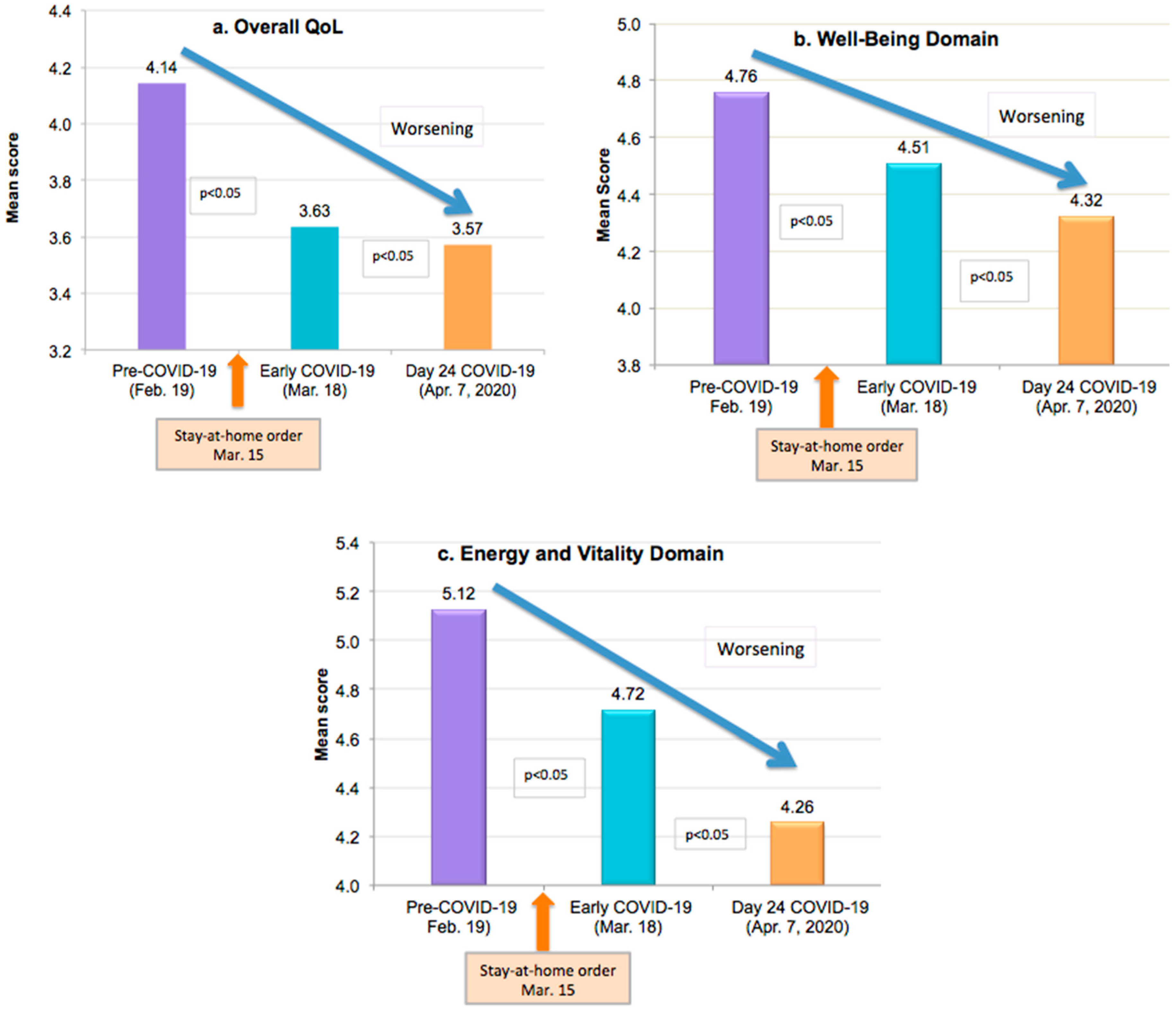
| FQoLTM | Evaluation Timepoint in 2020 (Mean Scores) | |||||
|---|---|---|---|---|---|---|
| Domains and Subdomains | Pre-COVID-19 (19 February) | Early COVID-19 (18 March) | Day 24 COVID-19 (7 April) | |||
| Overall QoL | 4.14 | * | 3.63 | * | 3.57 | * |
| Well-Being Domain | 4.76 | * | 4.51 | * | 4.32 | * |
| Emotion subdomain | 4.88 | * | 4.44 | * | 4.35 | * |
| Self-Image subdomain | 4.37 | * | 4.32 | * | 4.05 | * |
| Self-Competence subdomain | 5.44 | * | 5.14 | * | 4.93 | * |
| Energy and Vitality domain | 5.12 | * | 4.72 | * | 4.26 | * |
| Personal Pleasure subdomain | 5.27 | * | 4.76 | * | 4.22 | * |
| Physical State subdomain | 4.53 | * | 4.38 | * | 4.18 | * |
| Routine Activity subdomain | 5.71 | * | 5.16 | * | 4.51 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farage, M.A. Psychological Aspects of Sensitive Skin: A Vicious Cycle. Cosmetics 2022, 9, 78. https://doi.org/10.3390/cosmetics9040078
Farage MA. Psychological Aspects of Sensitive Skin: A Vicious Cycle. Cosmetics. 2022; 9(4):78. https://doi.org/10.3390/cosmetics9040078
Chicago/Turabian StyleFarage, Miranda A. 2022. "Psychological Aspects of Sensitive Skin: A Vicious Cycle" Cosmetics 9, no. 4: 78. https://doi.org/10.3390/cosmetics9040078
APA StyleFarage, M. A. (2022). Psychological Aspects of Sensitive Skin: A Vicious Cycle. Cosmetics, 9(4), 78. https://doi.org/10.3390/cosmetics9040078






