Correlation of TP53 Genetic Alterations with p53 Immunohistochemical Expression and Their Prognostic Significance in DLBCL
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patient Cohort and Sample Collection
2.2. DNA Extraction and Targeted Next-Generation Sequencing (tNGS)
2.3. OncoScan Copy Number Analysis
2.4. Immunohistochemistry (IHC)
2.5. Statistical Analysis
3. Results
3.1. TP53 Genetic Alterations Identified by tNGS
3.2. TP53 Copy Number Variation (CNV) by OncoScan Array
3.3. Correlation Between TP53 Genetic Alterations and p53 IHC Expression
3.4. p53 IHC Expression Across Genetic Alteration Types
3.5. Correlation of TP53 Genetic Alterations and p53 IHC with Clinical Features
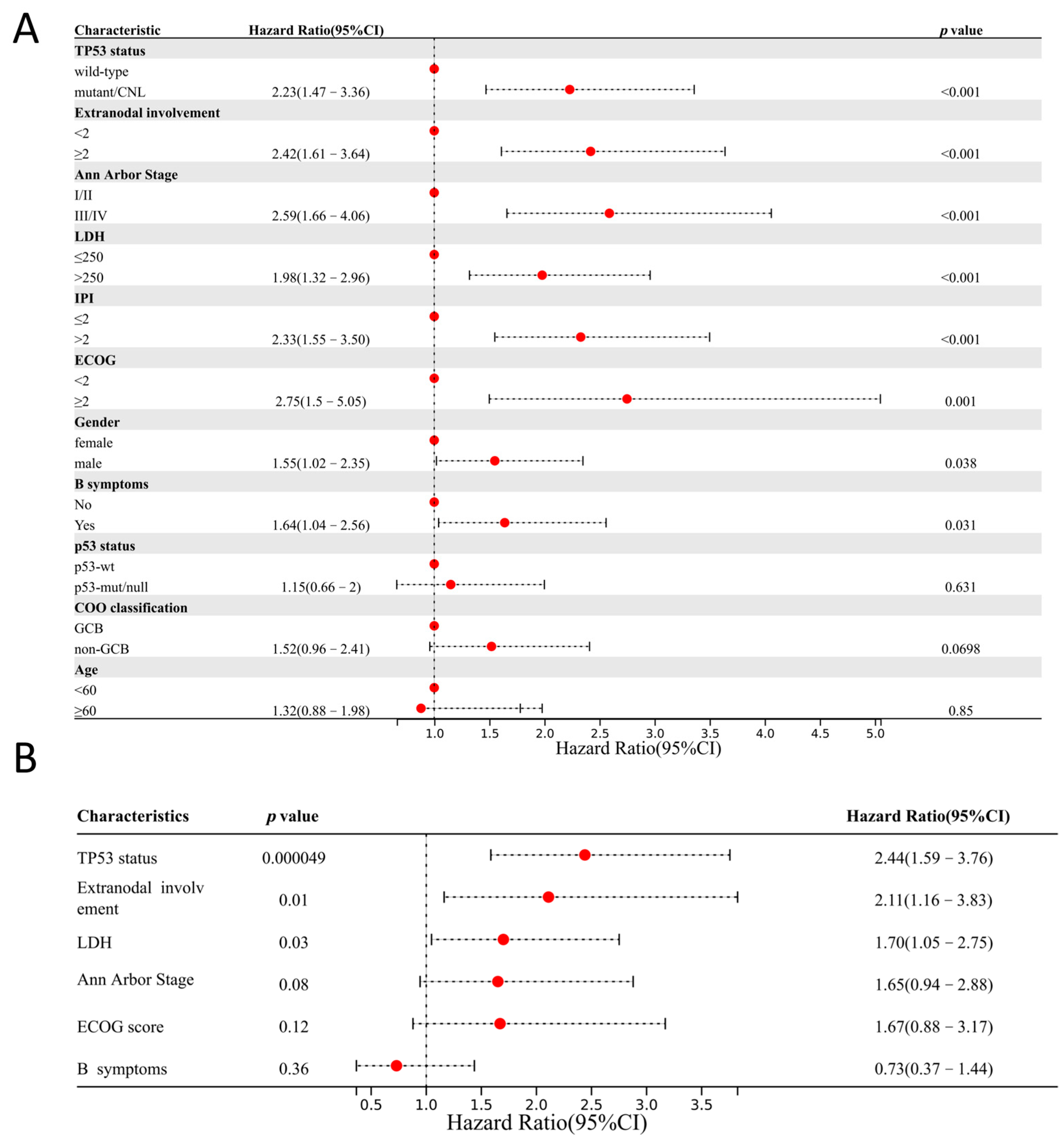
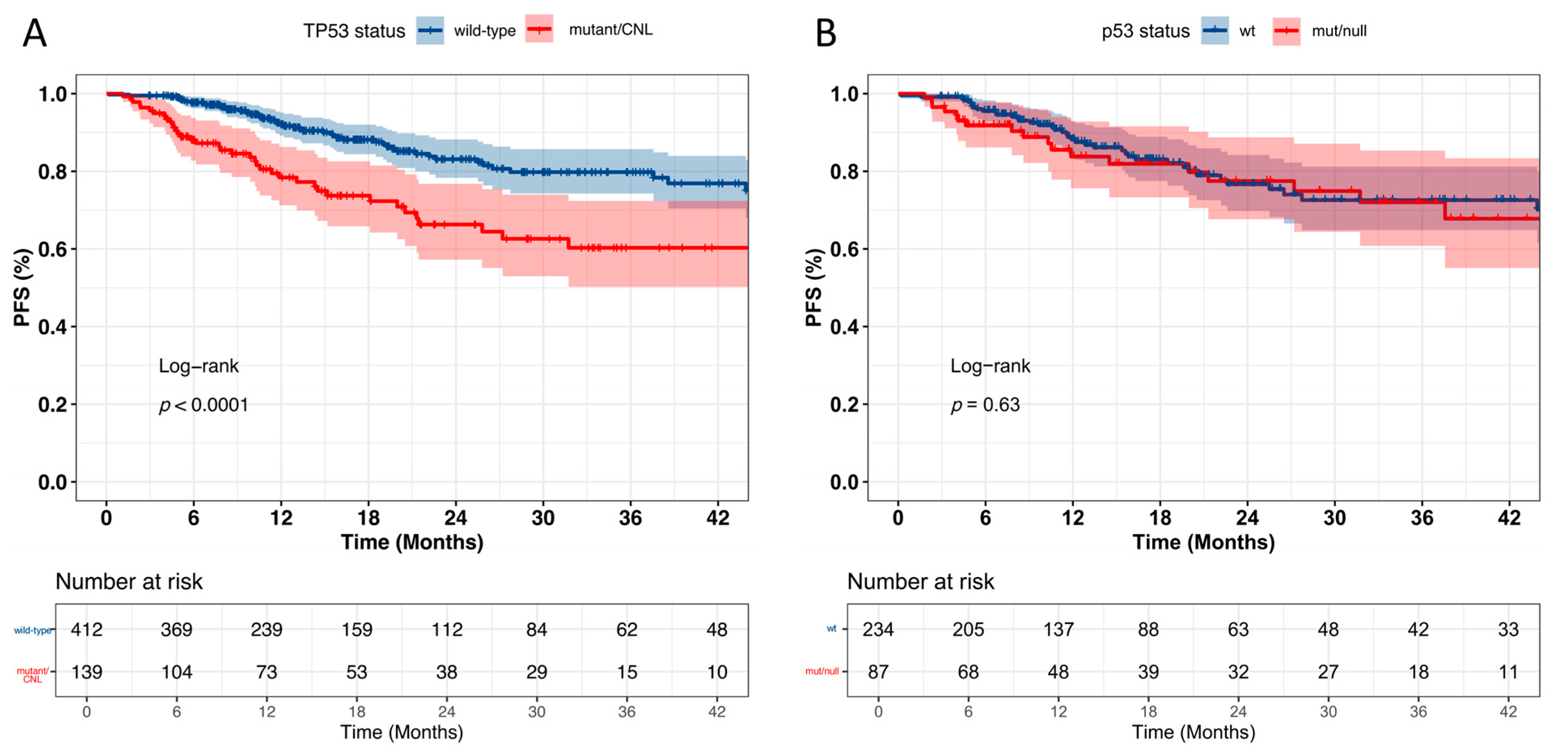
3.6. Prognostic Significance of TP53 Alterations and p53 IHC Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levine, A.J.; Oren, M. The first 30 years of p53: Growing ever more complex. Nat. Rev. Cancer 2009, 9, 749–758. [Google Scholar] [CrossRef]
- Levine, A.J. p53: 800 million years of evolution and 40 years of discovery. Nat. Rev. Cancer 2020, 20, 471–480. [Google Scholar] [CrossRef]
- Vousden, K.H.; Prives, C. Blinded by the Light: The Growing Complexity of p53. Cell 2009, 137, 413–431. [Google Scholar] [CrossRef]
- Olivier, M.; Hollstein, M.; Hainaut, P. TP53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harb. Perspect. Biol. 2010, 2, a001008. [Google Scholar] [CrossRef] [PubMed]
- Xu-Monette, Z.Y.; Wu, L.; Visco, C.; Tai, Y.C.; Tzankov, A.; Liu, W.M.; Montes-Moreno, S.; Dybkaer, K.; Chiu, A.; Orazi, A.; et al. Mutational profile and prognostic significance of TP53 in diffuse large B-cell lymphoma patients treated with R-CHOP: Report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood 2012, 120, 3986–3996. [Google Scholar] [CrossRef]
- Landsburg, D.J.; Morrissette, J.J.; Nasta, S.D.; Barta, S.K.; Schuster, S.J.; Svoboda, J.; Chong, E.A.; Bagg, A. TP53 mutations predict for poor outcomes in patients with newly diagnosed aggressive B-cell lymphomas in the current era. Blood Adv. 2023, 7, 7243–7253. [Google Scholar] [CrossRef] [PubMed]
- Chiappella, A.; Diop, F.; Agostinelli, C.; Novo, M.; Nassi, L.; Evangelista, A.; Ciccone, G.; Di Rocco, A.; Martelli, M.; Melle, F.; et al. Prognostic impact of TP53 mutation in newly diagnosed diffuse large B-cell lymphoma patients treated in the FIL-DLCL04 trial. Br. J. Haematol. 2022, 196, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Zhang, W.L.; Tan, R.; Zhong, T.T.; Zhang, M.R.; Fang, X.S. Progress in deciphering the role of p53 in diffuse large B-cell lymphoma: Mechanisms and therapeutic targets. Am. J. Cancer Res. 2024, 14, 3280–3293. [Google Scholar] [CrossRef]
- Schmitz, R.; Wright, G.W.; Huang, D.W.; Johnson, C.A.; Phelan, J.D.; Wang, J.Q.; Roulland, S.; Kasbekar, M.; Young, R.M.; Shaffer, A.L.; et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018, 378, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Chapuy, B.; Stewart, C.; Dunford, A.J.; Kim, J.; Kamburov, A.; Redd, R.A.; Lawrence, M.S.; Roemer, M.G.M.; Li, A.J.; Ziepert, M.; et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 2018, 24, 679–690. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Z.; Tavana, O.; Gu, W. Understanding the complexity of p53 in a new era of tumor suppression. Cancer Cell 2024, 42, 946–967. [Google Scholar] [CrossRef]
- Brummer, T.; Zeiser, R. The role of the MDM2/p53 axis in antitumor immune responses. Blood 2024, 143, 2701–2709. [Google Scholar] [CrossRef] [PubMed]
- de Leval, L.; Alizadeh, A.A.; Bergsagel, P.L.; Campo, E.; Davies, A.; Dogan, A.; Fitzgibbon, J.; Horwitz, S.M.; Melnick, A.M.; Morice, W.G.; et al. Genomic profiling for clinical decision making in lymphoid neoplasms. Blood 2022, 140, 2193–2227. [Google Scholar] [CrossRef]
- Stiewe, T.; Haran, T.E. How mutations shape p53 interactions with the genome to promote tumorigenesis and drug resistance. Drug Resist. Updat. 2018, 38, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Joerger, A.C.; Stiewe, T.; Soussi, T. TP53: The unluckiest of genes? Cell Death Differ. 2025, 32, 219–224. [Google Scholar] [CrossRef]
- Kennedy, M.C.; Lowe, S.W. Mutant p53: It’s not all one and the same. Cell Death Differ. 2022, 29, 983–987. [Google Scholar] [CrossRef]
- Robles, A.I.; Harris, C.C. Clinical outcomes and correlates of TP53 mutations and cancer. Cold Spring Harb. Perspect. Biol. 2010, 2, a001016. [Google Scholar] [CrossRef]
- Lu, T.X.; Young, K.H.; Xu, W.; Li, J.Y. TP53 dysfunction in diffuse large B-cell lymphoma. Crit. Rev. Oncol. Hematol. 2016, 97, 47–55. [Google Scholar] [CrossRef]
- Kim, K.M.; Ahn, A.R.; Park, H.S.; Jang, K.Y.; Moon, W.S.; Kang, M.J.; Ha, G.W.; Lee, M.R.; Chung, M.J. Clinical significance of p53 protein expression and TP53 variation status in colorectal cancer. BMC Cancer 2022, 22, 940. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Grote, I.; Bartels, S.; Kandt, L.; Christgen, H.; Lehmann, U.; Gluz, O.; Graeser, M.; Kates, R.; Harbeck, N.; et al. p53 Expression in Luminal Breast Cancer Correlates with TP53 Mutation and Primary Endocrine Resistance. Mod. Pathol. 2023, 36, 100100. [Google Scholar] [CrossRef]
- Hwang, H.J.; Nam, S.K.; Park, H.; Park, Y.; Koh, J.; Na, H.Y.; Kwak, Y.; Kim, W.H.; Lee, H.S. Prediction of TP53 mutations by p53 immunohistochemistry and their prognostic significance in gastric cancer. J. Pathol. Transl. Med. 2020, 54, 378–386. [Google Scholar] [CrossRef]
- Guedes, L.B.; Almutairi, F.; Haffner, M.C.; Rajoria, G.; Liu, Z.; Klimek, S.; Zoino, R.; Yousefi, K.; Sharma, R.; De Marzo, A.M.; et al. Analytic, Preanalytic, and Clinical Validation of p53 IHC for Detection of TP53 Missense Mutation in Prostate Cancer. Clin. Cancer Res. 2017, 23, 4693–4703. [Google Scholar] [CrossRef]
- Kang, E.Y.; Cheasley, D.; LePage, C.; Wakefield, M.J.; da Cunha Torres, M.; Rowley, S.; Salazar, C.; Xing, Z.; Allan, P.; Bowtell, D.D.L.; et al. Refined cut-off for TP53 immunohistochemistry improves prediction of TP53 mutation status in ovarian mucinous tumors: Implications for outcome analyses. Mod. Pathol. 2021, 34, 194–206. [Google Scholar] [CrossRef]
- Vermij, L.; Léon-Castillo, A.; Singh, N.; Powell, M.E.; Edmondson, R.J.; Genestie, C.; Khaw, P.; Pyman, J.; McLachlin, C.M.; Ghatage, P.; et al. p53 immunohistochemistry in endometrial cancer: Clinical and molecular correlates in the PORTEC-3 trial. Mod. Pathol. 2022, 35, 1475–1483. [Google Scholar] [CrossRef]
- de Haan, L.M.; de Groen, R.A.L.; de Groot, F.A.; Noordenbos, T.; van Wezel, T.; van Eijk, R.; Ruano, D.; Diepstra, A.; Koens, L.; Nicolae-Cristea, A.; et al. Real-world routine diagnostic molecular analysis for TP53 mutational status is recommended over p53 immunohistochemistry in B-cell lymphomas. Virchows Arch. 2024, 485, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Luo, D.; Zhang, L.; Li, Q.; Fan, J.; Zhang, J.; Huang, B.; Yang, M.; Nie, X.; Chang, X.; et al. Accurate interpretation of p53 immunohistochemical patterns is a surrogate biomarker for TP53 alterations in large B-cell lymphoma. BMC Cancer 2023, 23, 1008. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Medeiros, L.J.; Bueso-Ramos, C.E.; Tang, G.; Wang, S.; Oki, Y.; Desai, P.; Khoury, J.D.; Miranda, R.N.; Tang, Z.; et al. P53 expression correlates with poorer survival and augments the negative prognostic effect of MYC rearrangement, expression or concurrent MYC/BCL2 expression in diffuse large B-cell lymphoma. Mod. Pathol. 2017, 30, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.C.A.; Bahmad, H.F.; Aljamal, A.A.; Delgado, R.; Salami, A.; Guillot, C.; Castellano-Sánchez, A.A.; Medina, A.M.; Sriganeshan, V. Prognostic Significance of p53 and p63 in Diffuse Large B-Cell Lymphoma: A Single-Institution Experience. Curr. Oncol. 2023, 30, 1314–1331. [Google Scholar] [CrossRef]
- Bouroumeau, A.; Bussot, L.; Bonnefoix, T.; Fournier, C.; Chapusot, C.; Casasnovas, O.; Martin, L.; McLeer, A.; Col, E.; David-Boudet, L.; et al. c-MYC and p53 expression highlight starry-sky pattern as a favourable prognostic feature in R-CHOP-treated diffuse large B-cell lymphoma. J. Pathol. Clin. Res. 2021, 7, 604–615. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, Y.; Wang, L.; Zhang, H.; Ren, B.; Zheng, J.; Xia, Q.; Liu, Y. TP53 mutation and immunohistochemical p53 expression characteristics in diffuse large B-cell lymphoma. Front. Oncol. 2025, 15, 1550207. [Google Scholar] [CrossRef]
- Van Loo, P.; Nordgard, S.H.; Lingjærde, O.C.; Russnes, H.G.; Rye, I.H.; Sun, W.; Weigman, V.J.; Marynen, P.; Zetterberg, A.; Naume, B.; et al. Allele-specific copy number analysis of tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 16910–16915. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.W.; Huang, D.W.; Phelan, J.D.; Coulibaly, Z.A.; Roulland, S.; Young, R.M.; Wang, J.Q.; Schmitz, R.; Morin, R.D.; Tang, J.; et al. A Probabilistic Classification Tool for Genetic Subtypes of Diffuse Large B Cell Lymphoma with Therapeutic Implications. Cancer Cell 2020, 37, 551–568.e514. [Google Scholar] [CrossRef]
- Tessier-Cloutier, B.; Kortekaas, K.E.; Thompson, E.; Pors, J.; Chen, J.; Ho, J.; Prentice, L.M.; McConechy, M.K.; Chow, C.; Proctor, L.; et al. Major p53 immunohistochemical patterns in in situ and invasive squamous cell carcinomas of the vulva and correlation with <em>TP53</em> mutation status. Mod. Pathol. 2020, 33, 1595–1605. [Google Scholar] [CrossRef]
- Köbel, M.; Piskorz, A.M.; Lee, S.; Lui, S.; LePage, C.; Marass, F.; Rosenfeld, N.; Mes Masson, A.M.; Brenton, J.D. Optimized p53 immunohistochemistry is an accurate predictor of TP53 mutation in ovarian carcinoma. J. Pathol. Clin. Res. 2016, 2, 247–258. [Google Scholar] [CrossRef]
- Peroja, P.; Pedersen, M.; Mantere, T.; Nørgaard, P.; Peltonen, J.; Haapasaari, K.M.; Böhm, J.; Jantunen, E.; Turpeenniemi-Hujanen, T.; Rapakko, K.; et al. Mutation of TP53, translocation analysis and immunohistochemical expression of MYC, BCL-2 and BCL-6 in patients with DLBCL treated with R-CHOP. Sci. Rep. 2018, 8, 14814. [Google Scholar] [CrossRef]
- Du, K.X.; Wu, Y.F.; Hua, W.; Duan, Z.W.; Gao, R.; Liang, J.H.; Li, Y.; Yin, H.; Wu, J.Z.; Shen, H.R.; et al. Identify truly high-risk TP53-mutated diffuse large B cell lymphoma patients and explore the underlying biological mechanisms. Cell Commun. Signal. 2024, 22, 401. [Google Scholar] [CrossRef]
- Schiefer, A.I.; Kornauth, C.; Simonitsch-Klupp, I.; Skrabs, C.; Masel, E.K.; Streubel, B.; Vanura, K.; Walter, K.; Migschitz, B.; Stoiber, D.; et al. Impact of Single or Combined Genomic Alterations of TP53, MYC, and BCL2 on Survival of Patients with Diffuse Large B-Cell Lymphomas: A Retrospective Cohort Study. Medicine 2015, 94, e2388. [Google Scholar] [CrossRef]
- Dodero, A.; Guidetti, A.; Marino, F.; Tucci, A.; Barretta, F.; Re, A.; Balzarotti, M.; Carniti, C.; Monfrini, C.; Chiappella, A.; et al. Dose-adjusted EPOCH and rituximab for the treatment of double expressor and double-hit diffuse large B-cell lymphoma: Impact of TP53 mutations on clinical outcome. Haematologica 2022, 107, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Young, K.H.; Leroy, K.; Møller, M.B.; Colleoni, G.W.; Sánchez-Beato, M.; Kerbauy, F.R.; Haioun, C.; Eickhoff, J.C.; Young, A.H.; Gaulard, P.; et al. Structural profiles of TP53 gene mutations predict clinical outcome in diffuse large B-cell lymphoma: An international collaborative study. Blood 2008, 112, 3088–3098. [Google Scholar] [CrossRef] [PubMed]
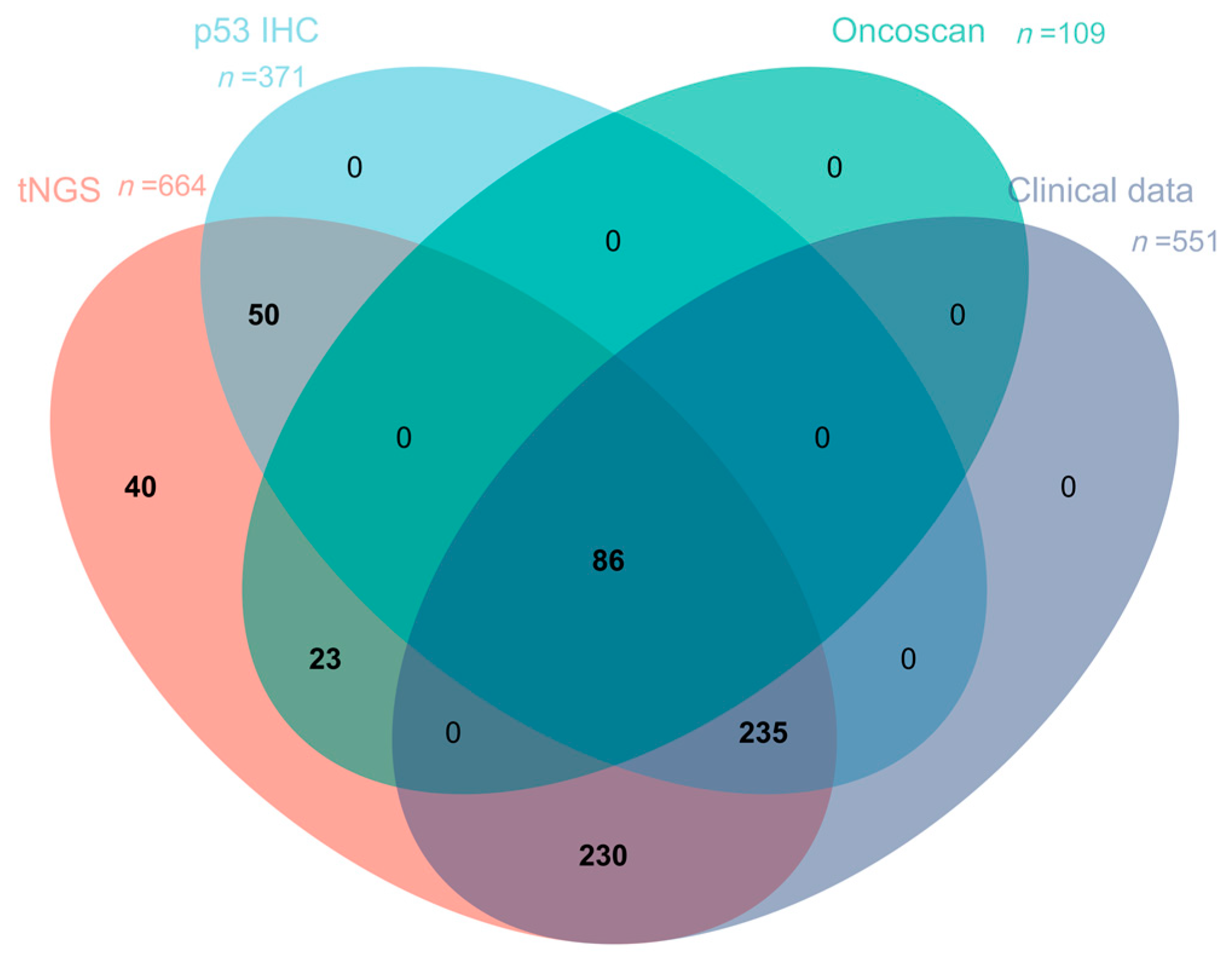




| tNGS (n = 664) | p53 IHC (n = 371) | OncoScan (n = 109) | |
|---|---|---|---|
| p53 IHC | 371 | -- | 86 |
| OncoScan | 109 | 86 | -- |
| Clinical Data | 551 | 321 | 109 |
| p53 Protein Expression Status | ||||
|---|---|---|---|---|
| TP53 Gene Status | p53-Mut (%) | p53-Null (%) | p53-wt (%) | |
| mutant/CNL (n = 96) | 66 (68.8) | 10 (10.4) | 20 (20.8) | |
| missense (n = 65) | 54 (83.1) | 2 (3.1) | 9 (13.8) | |
| nonsense (n = 7) | 0 (0) | 1 (14.3) | 6 (85.7) | |
| frameshift (n = 6) | 0 (0) | 4 (66.7) | 2 (23.3) | |
| in-frame (n = 2) | 2 (100) | 0 (0) | 0 (0) | |
| splice (n = 3) | 1 (33.3) | 2 (66.7) | 0(0) | |
| CNL (n = 1) | 0 (0) | 0 (0) | 1 (100) | |
| CNL + missense (n = 6) | 6 (100) | 0 (0) | 0 (0) | |
| CNL + frameshift (n = 2) | 0 (0) | 1 (50) | 1 (50) | |
| missense + frameshift (n = 2) | 2 (100) | 0 (0) | 0 (0) | |
| missense + nonsense (n = 1) | 0 (0) | 0 (0) | 1 (100) | |
| splice + nonsense (n = 1) | 1 (100) | 0 (0) | 0 (0) | |
| wild type (n = 275) | 12 (4.4) | 11 (4) | 252 (91.6) | |
| total (n = 371) | 78 (21) | 21 (5.7) | 272 (73.3) | |
| Type | Protein Change | Allele Frequency | p53 IHC | |
|---|---|---|---|---|
| 1 | missense | p.E11GN | 21.33% | 10% |
| 2 | missense | p.S215T | 31.90% | 20% |
| 3 | missense | p.S215R | 31.44% | 20% |
| 4 | missense | p.V272A | 32.85% | 30% |
| 5 | missense | p.R248Q | 15.64% | 5% |
| 6 | missense | p.R248W | 10.40% | 10% |
| 7 | missense | p.I195T | 19.07% | 40% |
| 8 | missense | p.G245S | 33.62% | 50% |
| 9 | missense | p.S240G | 30.03% | 30% |
| 10 | missense nonsense | p.M246L p.C229* | 31.80% 5.17% | 10% |
| 11 | frameshift | p.V73Rfs*76 | 49.59% | 10% |
| 12 | CNL frameshift frameshift | CNL p.A39Sfs*4 p.Q38Kfs*5 | 54.75% 76.57% 74.30% | 5% |
| 13 | frameshift | p.R306Efs*3 | 53.95% | 10% |
| 14 | nonsense | p.W53* | 22.55% | 30% |
| 15 | nonsense | p.R306* | 26.78% | 5% |
| 16 | nonsense | p.R306* | 20.94% | 5% |
| 17 | nonsense | p.R306* | 71.41% | 10% |
| 18 | nonsense | p.C124* | 53.48% | 10% |
| 19 | nonsense | p.R342* | 69.69% | 20% |
| 20 | CNL | CNL* | 53.85% | 5% |
| Characteristics | Mutant/CNL (n = 139) | Wild Type (n = 412) | p Value |
|---|---|---|---|
| p53 status | <0.001 | ||
| p53-mut/null | 64 (46.04%) | 18 (4.37%) | |
| p53-wt | 16 (11.51%) | 223 (54.13%) | |
| NA | 59 (42.45%) | 171 (41.50%) | |
| Gender | 0.61 | ||
| female | 61 (43.88%) | 193 (46.84%) | |
| male | 78 (56.12%) | 219 (53.16%) | |
| Age | 0.11 | ||
| <60 | 89 (64.03%) | 230 (55.82%) | |
| ≥60 | 50 (35.97%) | 182 (44.18%) | |
| COO classification | 0.16 | ||
| GCB | 59 (42.45%) | 143 (34.71%) | |
| non-GCB | 78 (56.12%) | 256 (62.13%) | |
| NA | 2 (1.43%) | 13 (3.16%) | |
| Primary site | 0.73 | ||
| extranodal | 87 (62.59%) | 249 (60.44%) | |
| nodal | 52 (37.41%) | 163 (39.56%) | |
| Extranodal involvement | 0.80 | ||
| <2 | 104 (74.82%) | 301 (73.06%) | |
| ≥2 | 35 (25.18%) | 110 (26.70%) | |
| NA | 0 (0%) | 1 (0.24%) | |
| Ann Arbor Stage | 0.96 | ||
| I/II | 72 (51.80%) | 210 (50.97%) | |
| III/IV | 67 (48.20%) | 201 (48.79%) | |
| NA | 0 (0%) | 1 (0.24%) | |
| LDH | 0.99 | ||
| ≤250 | 88 (63.31%) | 265 (64.32%) | |
| >250 | 50 (35.97%) | 147 (35.68%) | |
| NA | 1 (0.72%) | 0 (0%) | |
| ECOG score | 0.04 | ||
| <2 | 128 (92.09%) | 392 (95.15%) | |
| ≥2 | 11 (7.91%) | 13 (3.15%) | |
| NA | 0 (0%) | 7 (1.70%) | |
| B symptoms | <0.01 | ||
| Yes | 41 (29.50%) | 76 (18.45%) | |
| No | 96 (69.06%) | 330 (80.09%) | |
| NA | 2 (1.44%) | 6 (1.46%) | |
| IPI | 0.35 | ||
| <2 | 98 (70.50%) | 309 (75.00%) | |
| ≥2 | 41 (29.50%) | 103 (25.00%) | |
| Therapeutic response | <0.001 | ||
| CR | 95 (68.34%) | 340 (82.52%) | |
| non-CR | 44 (31.66%) | 72 (17.48%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Hu, Z.; Ren, M.; Bao, L.; Wei, R.; Tian, T.; Zhu, X.; Bai, Q.; Yu, B.; Li, X.; et al. Correlation of TP53 Genetic Alterations with p53 Immunohistochemical Expression and Their Prognostic Significance in DLBCL. Curr. Oncol. 2025, 32, 488. https://doi.org/10.3390/curroncol32090488
Chen C, Hu Z, Ren M, Bao L, Wei R, Tian T, Zhu X, Bai Q, Yu B, Li X, et al. Correlation of TP53 Genetic Alterations with p53 Immunohistochemical Expression and Their Prognostic Significance in DLBCL. Current Oncology. 2025; 32(9):488. https://doi.org/10.3390/curroncol32090488
Chicago/Turabian StyleChen, Chen, Zijuan Hu, Min Ren, Longlong Bao, Ran Wei, Tian Tian, Xiaoli Zhu, Qianming Bai, Baohua Yu, Xiaoqiu Li, and et al. 2025. "Correlation of TP53 Genetic Alterations with p53 Immunohistochemical Expression and Their Prognostic Significance in DLBCL" Current Oncology 32, no. 9: 488. https://doi.org/10.3390/curroncol32090488
APA StyleChen, C., Hu, Z., Ren, M., Bao, L., Wei, R., Tian, T., Zhu, X., Bai, Q., Yu, B., Li, X., & Zhou, X. (2025). Correlation of TP53 Genetic Alterations with p53 Immunohistochemical Expression and Their Prognostic Significance in DLBCL. Current Oncology, 32(9), 488. https://doi.org/10.3390/curroncol32090488





