The Clinical Characteristics, Treatment, and Prognosis of Lung Cancer in Young Patients in the New Era of Cancer Treatment: A Retrospective and Comprehensive Analysis
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Patients
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Molecular Features
3.3. Outcomes of the Overall Population
3.4. Treatment Modalities and Outcomes in NSCLC Patients
3.5. Outcomes in Young Patients with SCLC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Li, C.; Lei, S.; Ding, L.; Xu, Y.; Wu, X.; Wang, H.; Zhang, Z.; Gao, T.; Zhang, Y.; Li, L. Global burden and trends of lung cancer incidence and mortality. Chin. Med. J. 2023, 136, 1583–1590. [Google Scholar] [CrossRef]
- Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Li, L.; Wei, W.; He, J. Lung cancer incidence and mortality in China: Updated statistics and an overview of temporal trends from 2000 to 2016. J. Natl. Cancer Cent. 2022, 2, 139–147. [Google Scholar] [CrossRef]
- Peng, Y.; Cui, H.; Xu, Y.; Liu, D.; Song, Y.; Duan, H. Morbidity situation of lung cancer in China—Japan Friendship Hospital from 2005 to 2014. Chin. Gen. Pract. 2016, 19, 565–569. [Google Scholar]
- Li, W.; Liang, H.; Wang, W.; Liu, J.; Liu, X.; Lao, S.; Liang, W.; He, J. Global cancer statistics for adolescents and young adults: Population based study. J. Hematol. Oncol. 2024, 17, 99. [Google Scholar] [CrossRef]
- Cho, J.-W.; Hong, M.H.; Ha, S.-J.; Kim, Y.-J.; Cho, B.C.; Lee, I.; Kim, H.R. Genome-wide identification of differentially methylated promoters and enhancers associated with response to anti-PD-1 therapy in non-small cell lung cancer. Exp. Mol. Med. 2020, 52, 1550–1563. [Google Scholar] [CrossRef]
- Yoneyama, R.; Saji, H.; Kato, Y.; Kudo, Y.; Shimada, Y.; Kimura, M.; Hagiwara, M.; Kakihana, M.; Miyajima, K.; Kajiwara, N.; et al. Clinicopathological characteristics and treatment strategies for young lung cancer patients. Ann. Transl. Med. 2019, 7, 100. [Google Scholar] [CrossRef]
- Bratova, M.; Brat, K.; Hurdalkova, K.; Barinova, M.; Drosslerova, M.; Kultan, J.; Wanke, M.; Koubkova, L.; Krejci, J.; Svaton, M. Lung Cancer Versus “Young Cancer”: Is Non-Small Cell Lung Cancer in Young Patients a Different Entity? J. Adolesc. Young Adult Oncol. 2022, 11, 451–458. [Google Scholar] [CrossRef]
- West, H.J. Young Patients with Lung Cancer—An Understudied Population. JAMA Oncol. 2016, 2, 321. [Google Scholar] [CrossRef][Green Version]
- Galvez-Nino, M.; Ruiz, R.; Pinto, J.A.; Roque, K.; Mantilla, R.; Raez, L.E.; Mas, L. Lung Cancer in the Young. Lung 2020, 198, 195–200. [Google Scholar] [CrossRef]
- Sacher, A.G.; Dahlberg, S.E.; Heng, J.; Mach, S.; Jänne, P.A.; Oxnard, G.R. Association Between Younger Age and Targetable Genomic Alterations and Prognosis in Non–Small-Cell Lung Cancer. JAMA Oncol. 2016, 2, 313–320. [Google Scholar] [CrossRef]
- Arnold, B.N.; Thomas, D.C.; Rosen, J.E.; Salazar, M.C.; Blasberg, J.D.; Boffa, D.J.; Detterbeck, F.C.; Kim, A.W. Lung Cancer in the Very Young: Treatment and Survival in the National Cancer Data Base. J. Thorac. Oncol. 2016, 11, 1121–1131. [Google Scholar] [CrossRef]
- Oliveira, I.; Mota, P.; Almodovar, T. Lung cancer in young patients: Natural history, biology and prognosis. Pulmonology 2022, 28, 80–81. [Google Scholar] [CrossRef]
- Viñal, D.; Martínez, D.; Higuera, O.; de Castro, J. Genomic profiling in non-small-cell lung cancer in young patients. A systematic review. ESMO Open 2021, 6, 100045. [Google Scholar] [CrossRef]
- Ruiz, R.; Galvez-Nino, M.; Roque, K.; Montes, J.; Nuñez, M.; Raez, L.; Sánchez-Gambetta, S.; Jaúregui, S.; Viale, S.; Smith, E.S.; et al. Genomic landscape of lung cancer in the young. Front. Oncol. 2022, 12, 910117. [Google Scholar] [CrossRef]
- Tian, P.; Liu, Y.; Zeng, H.; Tang, Y.; Lizaso, A.; Ye, J.; Shao, L.; Li, Y. Unique molecular features and clinical outcomes in young patients with non-small cell lung cancer harboring ALK fusion genes. J. Cancer Res. Clin. Oncol. 2020, 146, 935–944. [Google Scholar] [CrossRef]
- Jin, M.-Z.; Jin, W.-L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 166. [Google Scholar] [CrossRef]
- Fane, M.; Weeraratna, A.T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer 2019, 20, 89–106. [Google Scholar] [CrossRef]
- Chen, A.C.Y.; Jaiswal, S.; Martinez, D.; Yerinde, C.; Ji, K.; Miranda, V.; Fung, M.E.; Weiss, S.A.; Zschummel, M.; Taguchi, K.; et al. The aged tumor microenvironment limits T cell control of cancer. Nat. Immunol. 2024, 25, 1033–1045. [Google Scholar] [CrossRef]
- Li, P. Age-dependent genomic characteristics and their impact on immunotherapy in lung adenocarcinoma. J. Cancer Res. Clin. Oncol. 2023, 149, 2997–3007. [Google Scholar] [CrossRef]
- He, C.-H.; Shih, J.-F.; Lai, S.-L.; Chen, Y.-M. Non–small cell lung cancer in the very young: Higher EGFR/ALK mutation proportion than the elder. J. Chin. Med. Assoc. 2020, 83, 461–465. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Shi, F.; Zhang, C.; Jiao, Q.; Zhu, H. Better cancer specific survival in young small cell lung cancer patients especially with AJCC stage III. Oncotarget 2017, 8, 34923–34934. [Google Scholar] [CrossRef]
- Frcs, P.G. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forth-coming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zeng, Z.; Jin, Y.; Yang, L.; Fan, T.; Wang, Z.; Pan, Y.; Yang, Y.; Yao, M.; Li, Y.; et al. Distinct immune microenvironment of lung adenocarcinoma in never-smokers from smokers. Cell Rep. Med. 2023, 4, 101078. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, X.; Shen, F.; Zhao, D.; Shi, Y.; Zhang, H.; Liu, J.; Gao, X.; Chen, M.; Zhao, J.; et al. Osimertinib versus comparator first-generation epidermal growth factor receptor tyrosine kinase inhibitors as first-line treatment in patients with advanced EGFR-mutated non-small cell lung cancer: A Chinese, multicenter, real-world cohort study. Transl. Lung Cancer Res. 2023, 12, 2229–2244. [Google Scholar] [CrossRef]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR -Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.W.; Ou, S.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in Untreated ALK -Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef]
- Zhao, M.; Shao, T.; Shao, H.; Zhou, C.; Tang, W. Identifying optimal ALK inhibitors in first- and second-line treatment of patients with advanced ALK-positive non-small-cell lung cancer: A systematic review and network meta-analysis. BMC Cancer 2024, 24, 186. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xu, Y.; Zhao, J.; Liu, X.; Liu, X.; Zhang, D.; Shi, Y.; Zhang, L.; Zhong, W.; Wang, M. Comparison of Chemotherapy Plus Pem-brolizumab vs. Chemo-therapy Alone in EGFR-Mutant Non-small-Cell Lung Cancer Patients. Clin. Lung Cancer 2023, 24, 278–286. [Google Scholar] [CrossRef]
- Voruganti, T.; Soulos, P.R.; Mamtani, R.; Presley, C.J.; Gross, C.P. Association Between Age and Survival Trends in Advanced Non–Small Cell Lung Cancer After Adoption of Immunotherapy. JAMA Oncol. 2023, 9, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Shah, Y.; Verma, A.; Marderstein, A.R.; White, J.; Bhinder, B.; Garcia Medina, J.S.; Elemento, O. Pan-cancer analy-sis reveals molecular patterns associated with age. Cell Rep. 2021, 37, 110100. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.Z.; Huang, Y.L.; Wu, C.C.; Tang, E.K.; Chen, C.S.; Mar, G.Y.; Yen, Y.; Wu, M.T. Assessment of Selection Criteria for Low-Dose Lung Screening CT Among Asian Ethnic Groups in Taiwan: From Mass Screening to Specific Risk-Based Screening for Non-Smoker Lung Cancer. Clin. Lung Cancer 2016, 17, e45–e56. [Google Scholar] [CrossRef] [PubMed]

| Variable | Overall Population (N = 343) | NSCLC Group (N = 309) | SCLC Group (N = 34) | p * |
|---|---|---|---|---|
| Sex | 0.019 | |||
| Men | 159 (46.4) | 137 (44.3) | 22 (64.7) | |
| Women | 184 (53.6) | 172 (55.7) | 12 (35.3) | |
| Age, years | 0.896 | |||
| 36–45 | 259 (75.5) | 232 (75.1) | 27 (79.4) | |
| 26–35 | 63 (18.4) | 58 (18.8) | 5 (14.7) | |
| ≤25 | 21 (6.1) | 19 (6.1) | 2 (5.9) | |
| Smoking history | 0.001 | |||
| Never smokers | 253 (73.8) | 238 (77) | 15 (44.1) | |
| Current/former smokers | 90 (26.2) | 71 (23) | 19 (55.9) | |
| Smoking index (pack year) | 13.5 ± 6.2 | 12.0 ± 5.7 | 20.4 ± 11.2 | 0.010 |
| Family history of cancer | 95 (27.7) | 86 (27.8) | 9 (26.5) | 0.523 |
| Family history of lung cancer | 52 (15.2) | 45 (14.6) | 6 (17.6) | 0.393 |
| ECOG | 0.488 | |||
| 0–1 | 317 (92.4) | 286 (92.6) | 31 (91.2) | |
| ≥2 | 26 (7.6) | 23 (7.4) | 3 (8.8) | |
| Type | 0.001 | |||
| Central lung cancer | 109 (31.8) | 86 (27.8) | 30 (88.2) | |
| Peripheral lung cancer | 234 (68.2) | 223 (72.2) | 4 (11.8) | |
| Histology | / | |||
| NSCLC | 309 (90.1) | 309 | / | |
| Adenocarcinoma | 247 (72.0) | 247 (79.9) | ||
| Squamous cell carcinoma | 27 (7.9) | 27 (8.7) | ||
| Lung carcinoid | 22 (6.4) | 22 (7.1) | ||
| Adenosquamous carcinoma | 7 (2.0) | 7 (2.3) | ||
| Large cell carcinoma | 2 (0.6) | 2 (0.6) | ||
| NOS | 2 (0.6) | 2 (0.6) | ||
| Pulmonary sarcomatoid carcinoma | 1 (0.3) | 1 (0.3) | ||
| Pulmonary mucoepidermoid carcinoma | 1 (0.3) | 1 (0.3) | ||
| SCLC | 34 (9.9) | / | 34 | |
| Staging | 0.001 | |||
| I | 72 (21.0) | 70 (22.7) | 2 (5.9) | |
| II | 22 (6.4) | 22 (7.1) | 0 (0) | |
| III | 64 (18.7) | 50 (16.2) | 14 (41.2) | |
| IV | 185 (53.9) | 167 (54) | 18 (52.9) | |
| Distant metastasis | 0.697 | |||
| Pleural | 97 (52.4) | 90 | 7 | |
| Bone | 91 (49.2) | 85 | 6 | |
| Intrapulmonary | 75 (40.5) | 69 | 6 | |
| Brain | 46 (24.9) | 42 | 4 | |
| Pericardium | 34 (18.4) | 33 | 1 | |
| Liver | 24 (13.0) | 18 | 6 | |
| Adrenal gland | 17 (9.2) | 14 | 2 | |
| Leptomeningeal | 3 (1.6) | 3 | 0 | |
| Others # | 14 (7.6) | 13 | 1 | |
| SUVmax (n = 117) | 9.3 ± 6.3 | 9.2 ± 6.4 | 10.8 ± 5.1 | 0.338 |
| Molecular Characteristics | Overall NSCLC (N = 309) | Adenocarcinoma (N = 247) | Non-Adenocarcinoma (N = 62) | p * |
|---|---|---|---|---|
| Driver gene positive | 161 (52.1) | 152 (61.5) | 9 (14.5) | <0.001 |
| EGFR mutation | 111 (35.9) | 105 (42.5) | 6 (9.7) | <0.001 |
| exon 19 del | 64 (20.7) | 61 (24.7) | 3 (4.8) | |
| exon 21 L858R | 28 (9.1) | 26 (10.5) | 2 (3.2) | |
| exon 20 ins | 14 (4.5) | 13 (5.3) | 1 (1.6) | |
| exon 18 G719X | 2 (0.6) | 2 (0.8) | 0 | |
| exon 21 L861Q | 1 (0.3) | 1 (0.4) | 0 | |
| exon 18 E709X | 1 (0.3) | 1 (0.4) | 0 | |
| exon 18 G719A | 1 (0.3) | 1 (0.4) | 0 | |
| ALK rearrangement | 44 (14.2) | 41 (16.6) | 3 (4.8) | <0.001 |
| EML4-ALK fusion | 26 (8.4) | 23 (9.3) | 3 (4.8) | |
| HIP1-ALK fusion | 1 (0.3) | 1 (0.4) | 0 | |
| KLC1-ALK fusion | 1 (0.3) | 1 (0.4) | 0 | |
| Unknown | 16 (5.2) | 16 (6.4) | 0 | |
| ROS1 rearrangement | 6 (1.9) | 6 (2.4) | 0 | 0.612 |
| c-MET exon14 skipping or c-MET amplification | 3 (1.0) | 3 (1.2) | 0 | 0.438 |
| RET rearrangement | 3 (1.0) | 3 (1.2) | 0 | 0.716 |
| No detectable driver mutations | 58 (18.8) | 47 (19.0) | 11 (17.7) | 0.849 |
| Driver gene unknown | 90 (29.1) | 48 (19.4) | 42 (67.7) | <0.001 |
| Other gene mutations detected | / | |||
| TP53 | 25 (8.1) | 22 (8.9) | 3 (4.8) | |
| HER-2 | 7 (2.3) | 6 (2.4) | 1 (1.6) | |
| KRAS G12D | 3 (1.0) | 3 (1.2) | 0 | |
| BRCA | 2 (0.6) | 2 (0.8) | 0 | |
| MET exon21skipping | 2 (0.6) | 2 (0.8) | 0 | |
| MET protein overexpression | 1 (0.3) | 1 (0.4) | 0 | |
| MET gene fusion | 1 (0.3) | 1 (0.4) | 0 | |
| BRAF-V600E | 1 (0.3) | 1 (0.4) | 0 | |
| NTRK1 | 1 (0.3) | 0 | 1 (1.6) | |
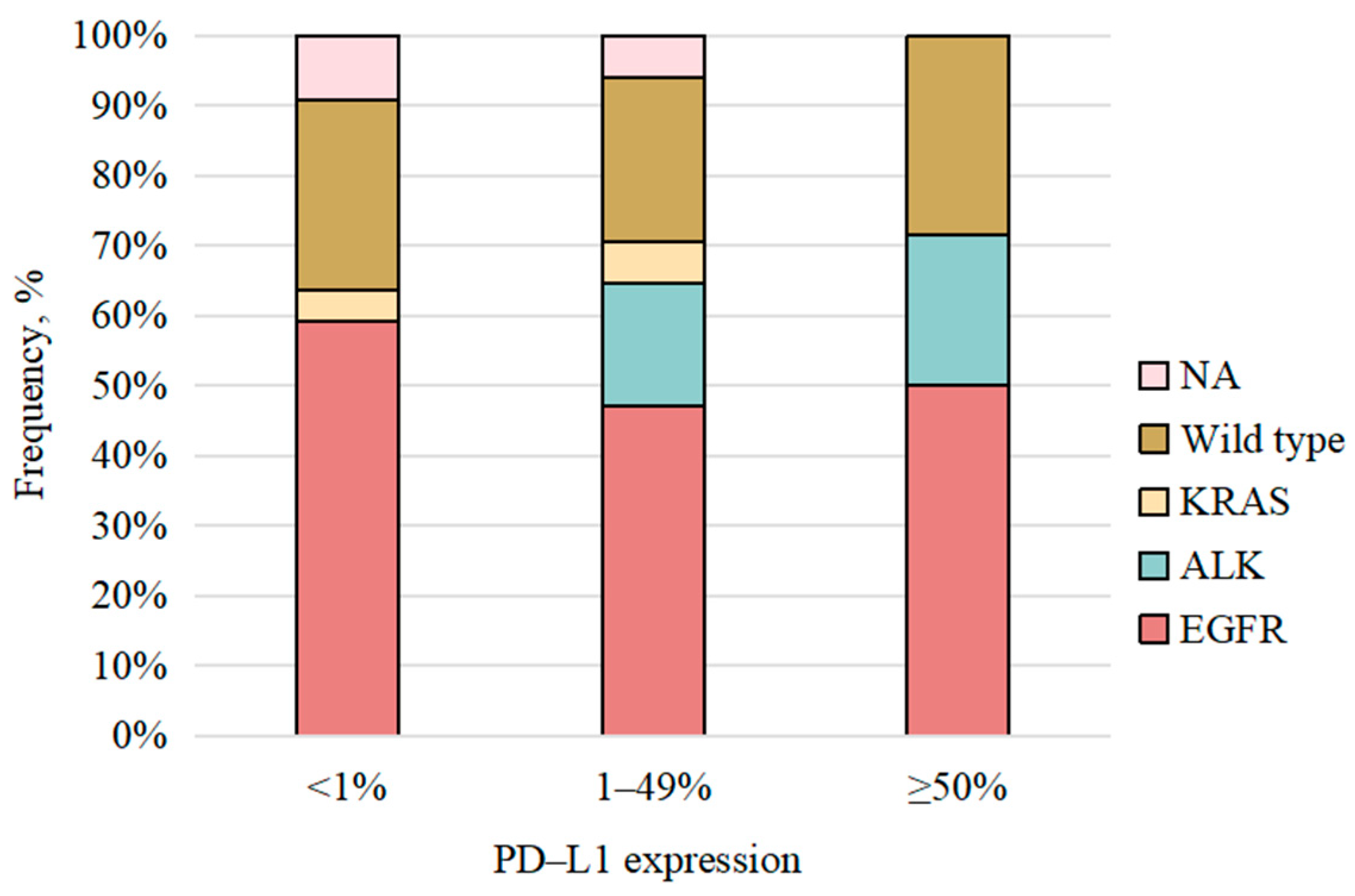
| PD-L1 expression | 0.480 | |||
| <1% | 24 (7.8) | 21 (8.5) | 3 (4.8) | |
| 1–49% | 17 (5.5) | 14 (5.7) | 3 (4.8) | |
| ≥50% | 14 (4.5) | 13 (5.3) | 1 (1.6) | |
| Unknown | 254 (82.2) | 199 (80.6) | 55 (88.7) | |
| tTMB(Muts/Mb), median (Q1–Q3) | 22 (7.1) | 2.19 (1.60, 4.82) | NA | / |
| Therapy | n (%) |
|---|---|
| Surgery | 128 (41.4) |
| Adjuvant therapy | 68 (53.1) |
| Neoadjuvant therapy | 8 (6.3) |
| Postoperative recurrence | 40 (31.3) |
| Radical radiotherapy | 76 (24.6) |
| First line therapies in advanced stage | |
| Target therapy | 116 (37.5) |
| C only | 58 (18.8) |
| C + ICIs | 24 (7.8) |
| C + Beva | 22 (7.1) |
| Best supportive care | 3 (1.0) |
| Variable | Univariate Analyses | Multivariate Analyses | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Sex | ||||
| men | re | re | ||
| women | 0.562 (0.359–0.880) | 0.012 | 0.556 (0.355–0.870) | 0.010 |
| Family history of lung cancer | ||||
| No | re | re | ||
| Yes | 2.240 (1.424–4.114) | 0.001 | 2.643 (1.543–4.528) | 0.001 |
| Smoking history | ||||
| Never smokers | re | |||
| Current/former smokers | 1.406 (0.836–2.366) | 0.199 | ||
| ECOG | ||||
| 0–1 | re | |||
| ≥2 | 1.063 (0.562–2.008) | 0.852 | ||
| Histology | ||||
| squamous cell carcinoma | re | |||
| non-squamous NSCLC | 0.918 (0.288–2.933) | 0.886 | ||
| PD-L1 expression | ||||
| Negative | re | |||
| positive | 0.866 (0.206–3.634) | 0.844 | ||
| NA | 1.526 (0.478–4.868) | 0.475 | ||
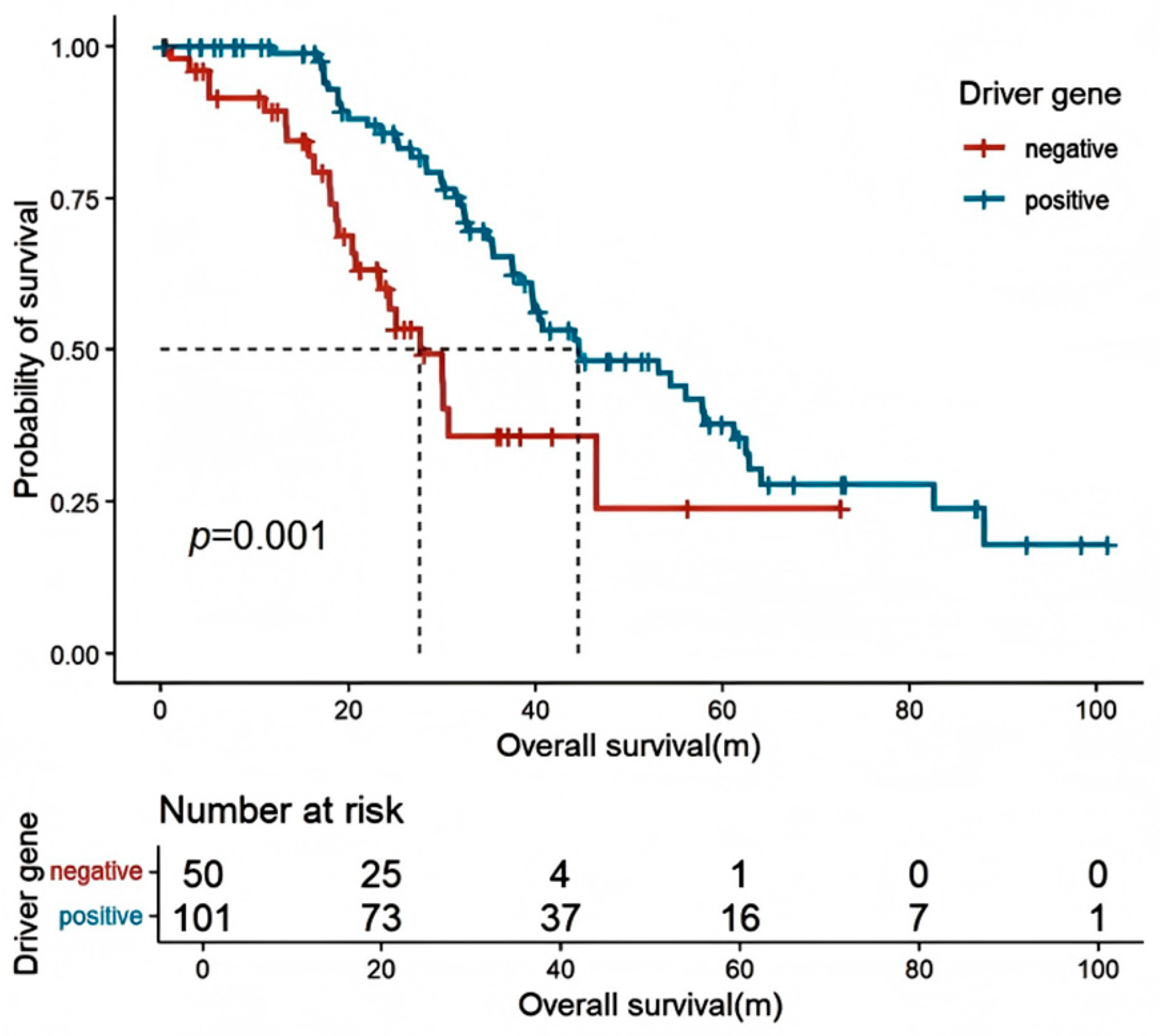
| Driver gene mutation | ||||
| No detectable | re | re | ||
| positive | 0.564 (0.361–0.881) | 0.012 | 0.590 (0.373–0.935) | 0.025 |
| NA | 0.566 (0.305–1.052) | 0.072 | ||
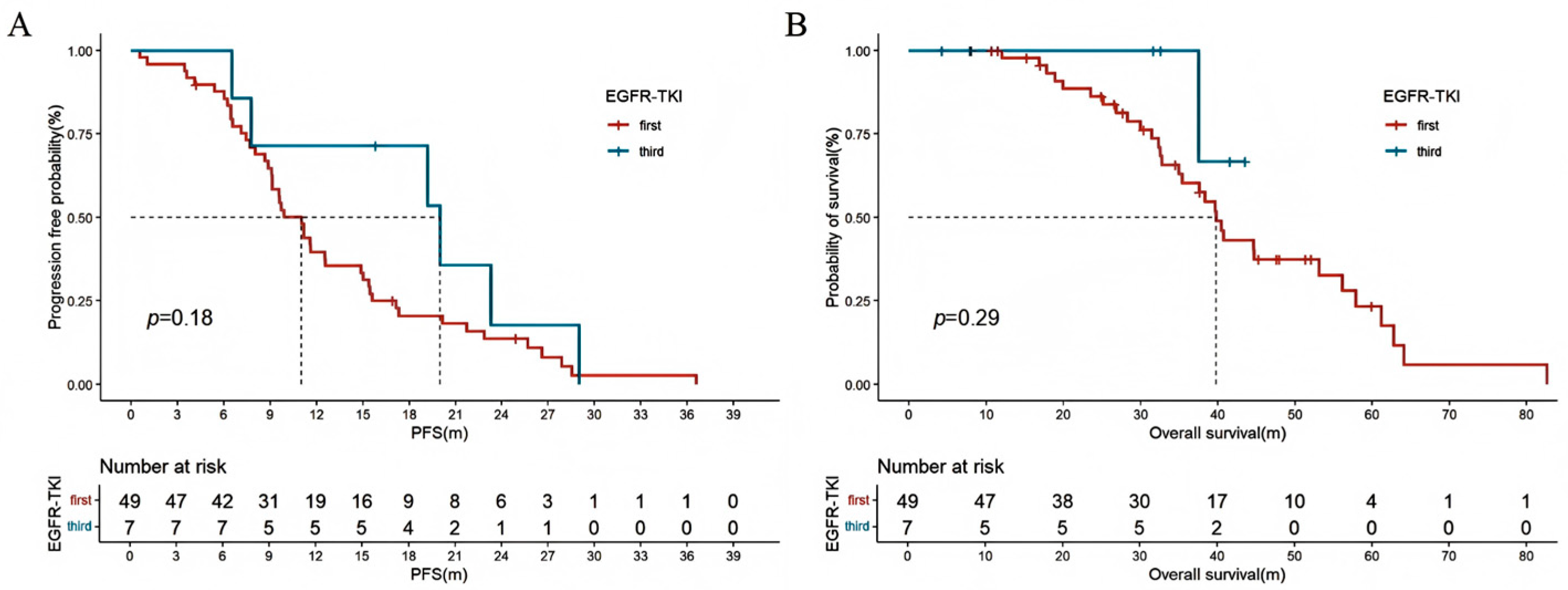
| EGFR-TKI | ||||
| no | re | |||
| First | 1.149 (0.729–1.812) | 0.550 | ||
| Third | 0.390 (0.054–2.837) | 0.352 | ||
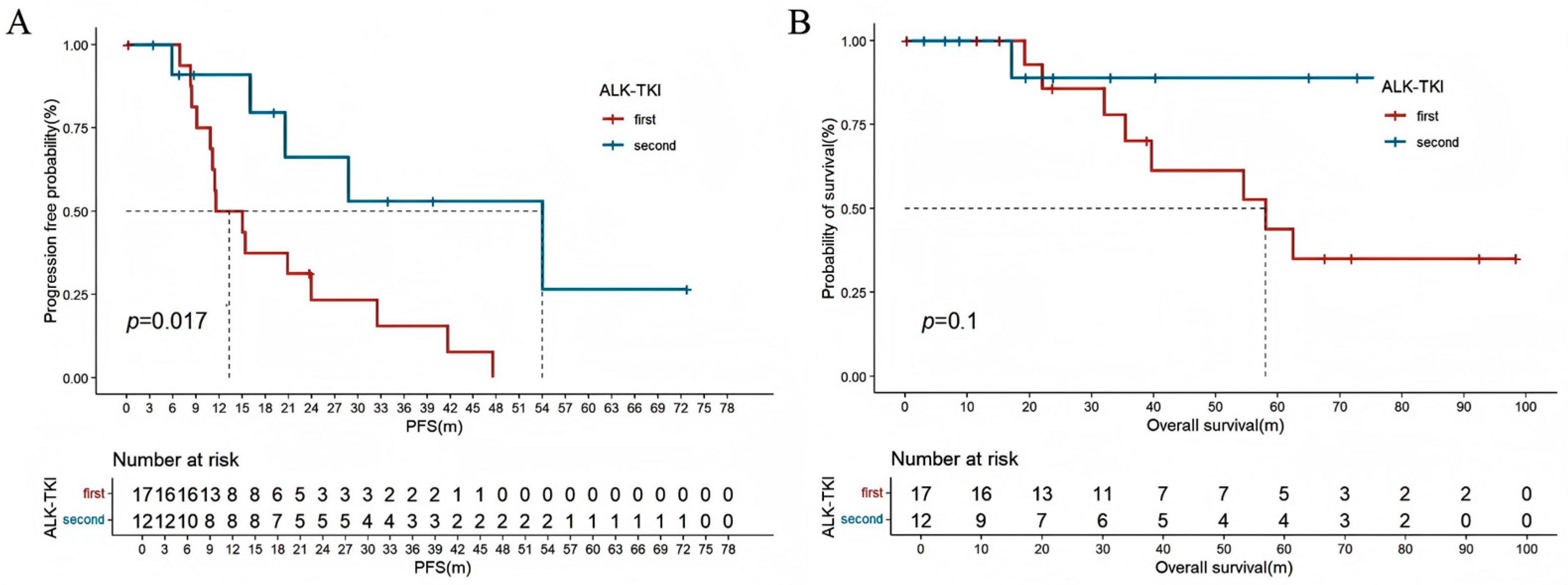
| ALK-TKI | ||||
| no | re | re | ||
| First | 0.499 (0.239–1.042) | 0.064 | ||
| Second | 0.132 (0.018–0.954) | 0.045 | 0.181 (0.025–0.317) | 0.041 |
| Immunotherapy | ||||
| no | re | |||
| yes | 1.106 (0.478–2.562) | 0.813 | ||
| Radical radiotherapy | ||||
| no | re | |||
| yes | 0.791 (0.510–1.226) | 0.294 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Li, S.; Yu, S.; Liu, Y.; Peng, Z.; Zhang, H.; Gao, X.; Liu, X.; Chen, M.; Zhao, J.; et al. The Clinical Characteristics, Treatment, and Prognosis of Lung Cancer in Young Patients in the New Era of Cancer Treatment: A Retrospective and Comprehensive Analysis. Curr. Oncol. 2025, 32, 489. https://doi.org/10.3390/curroncol32090489
Feng X, Li S, Yu S, Liu Y, Peng Z, Zhang H, Gao X, Liu X, Chen M, Zhao J, et al. The Clinical Characteristics, Treatment, and Prognosis of Lung Cancer in Young Patients in the New Era of Cancer Treatment: A Retrospective and Comprehensive Analysis. Current Oncology. 2025; 32(9):489. https://doi.org/10.3390/curroncol32090489
Chicago/Turabian StyleFeng, Xiaoyi, Shengjie Li, Siyuan Yu, Yunxin Liu, Zhanxian Peng, Haoran Zhang, Xiaoxing Gao, Xiaoyan Liu, Minjiang Chen, Jing Zhao, and et al. 2025. "The Clinical Characteristics, Treatment, and Prognosis of Lung Cancer in Young Patients in the New Era of Cancer Treatment: A Retrospective and Comprehensive Analysis" Current Oncology 32, no. 9: 489. https://doi.org/10.3390/curroncol32090489
APA StyleFeng, X., Li, S., Yu, S., Liu, Y., Peng, Z., Zhang, H., Gao, X., Liu, X., Chen, M., Zhao, J., Zhong, W., Xu, Y., & Wang, M. (2025). The Clinical Characteristics, Treatment, and Prognosis of Lung Cancer in Young Patients in the New Era of Cancer Treatment: A Retrospective and Comprehensive Analysis. Current Oncology, 32(9), 489. https://doi.org/10.3390/curroncol32090489




