Safety of Combination TARE and SBRT in Hepatocellular Carcinoma: A Review of Literature & Single-Center Case Series
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
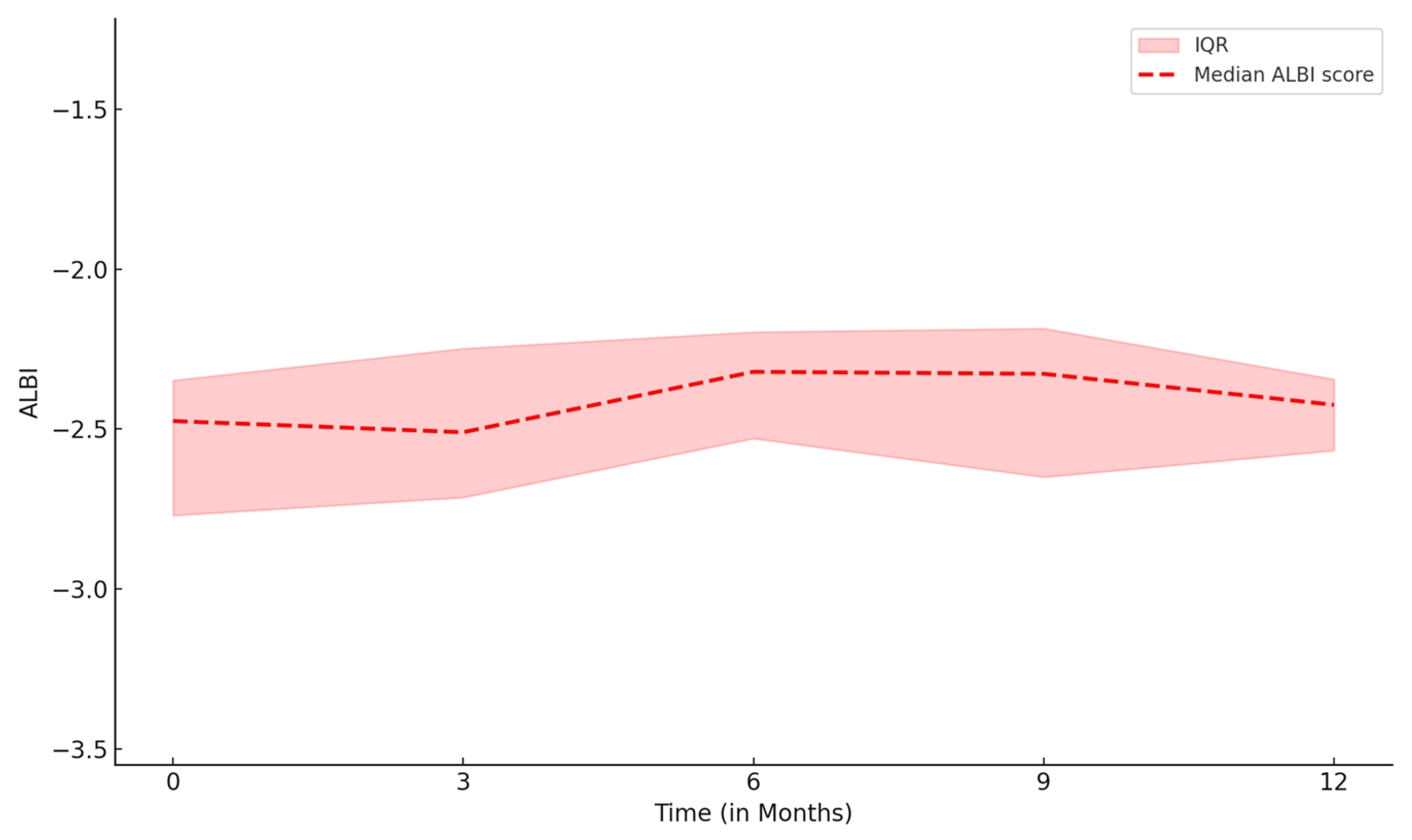
3.1. Patient Characteristics
3.2. Toxicity Following Combination Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| TARE | Transarterial radioembolization |
| SBRT | Stereotactic body radiation therapy |
| CP | Child-Pugh |
| TACE | Transarterial chemoembolization |
| ECOG | Eastern Cooperative Oncology Group |
| BCLC | Barcelona Clinic Liver Cancer |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rumgay, H.; Ferlay, J.; de Martel, C.; Georges, D.; Ibrahim, A.S.; Zheng, R.; Wei, W.; Lemmens, V.E.; Soerjomataram, I. Global, regional and national burden of primary liver cancer by subtype. Eur. J. Cancer 2022, 161, 108–118. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Delis, S.G.; Dervenis, C. Selection criteria for liver resection in patients with hepatocellular carcinoma and chronic liver disease. World J. Gastroenterol. 2008, 14, 3452–3460. [Google Scholar] [CrossRef]
- Gkika, E.; Schultheiss, M.; Bettinger, D.; Maruschke, L.; Neeff, H.P.; Schulenburg, M.; Adebahr, S.; Kirste, S.; Nestle, U.; Thimme, R.; et al. Excellent local control and tolerance profile after stereotactic body radiotherapy of advanced hepatocellular carcinoma. Radiat. Oncol. 2017, 12, 116. [Google Scholar] [CrossRef]
- Qiu, H.; Moravan, M.J.; Milano, M.T.; Usuki, K.Y.; Katz, A.W. SBRT for Hepatocellular Carcinoma: 8-Year Experience from a Regional Transplant Center. J. Gastrointest. Cancer 2018, 49, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.J.; Dawson, L.A. Advances in Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. Semin. Radiat. Oncol. 2017, 27, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Culleton, S.; Jiang, H.; Haddad, C.R.; Kim, J.; Brierley, J.; Brade, A.; Ringash, J.; Dawson, L.A. Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother. Oncol. 2014, 111, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.H.; Chun, S.J.; Chung, J.H.; Kim, E.; Kang, J.K.; Jang, W.I.; Moon, J.E.; Roquette, I.; Mirabel, X.; Kimura, T.; et al. Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma: Meta-Analysis and International Stereotactic Radiosurgery Society Practice Guidelines. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 337–351. [Google Scholar] [CrossRef]
- Kim, E.; Sher, A.; Abboud, G.; Schwartz, M.; Facciuto, M.; Tabrizian, P.; Knešaurek, K.; Fischman, A.; Patel, R.; Nowakowski, S.; et al. Radiation segmentectomy for curative intent of unresectable very early to early stage hepatocellular carcinoma (RASER): A single-centre, single-arm study. Lancet Gastroenterol. Hepatol. 2022, 7, 843–850. [Google Scholar] [CrossRef]
- de Bettencourt, M.; Harris, A.; Stang, K.; Cottler, S.; Refaat, T.; Molvar, C.; Thomas, T. Stereotactic body radiotherapy and yttrium-90 in the treatment of hepatocellular carcinoma: A comparison of outcomes and costs. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, e36–e37. [Google Scholar] [CrossRef]
- Hong, K.; Akinwande, O.; Bodei, L.; Chamarthy, M.R.K.; Devlin, P.M.; Elman, S.; Ganguli, S.; Kennedy, A.S.; Koo, S.J.; Ouhib, Z.; et al. ACR–ABS–ACNM–ASTRO–SIR–SNMMI practice parameter for selective internal radiation therapy or radioembolization for treatment of liver malignancies. Brachytherapy 2021, 20, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.G.; Abdelmaksoud, M.H.; Chang, D.T.; Eclov, N.C.; Chung, M.P.; Koong, A.C.; Louie, J.D.; Sze, D.Y. Safety of 90Y radioembolization in patients who have undergone previous external beam radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Hardy-Abeloos, C.; Lazarev, S.; Ru, M.; Kim, E.; Fischman, A.; Moshier, E.; Rosenzweig, K.; Buckstein, M. Safety and Efficacy of Liver Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma After Segmental Transarterial Radioembolization. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 968–976. [Google Scholar] [CrossRef]
- Liu, J.; Ladbury, C.; Amini, A.; Glaser, S.; Kessler, J.; Lee, A.; Chen, Y.-J. Combination of yttrium-90 radioembolization with stereotactic body radiation therapy in the treatment of portal vein tumor thrombosis. Radiat. Oncol. J. 2021, 39, 113–121. [Google Scholar] [CrossRef]
- Dezarn, W.A.; Cessna, J.T.; DeWerd, L.A.; Feng, W.; Gates, V.L.; Halama, J.; Kennedy, A.S.; Nag, S.; Sarfaraz, M.; Sehgal, V.; et al. Recommendations of the American Association of Physicists in Medicine on dosimetry, imaging, and quality assurance procedures for 90Y microsphere brachytherapy in the treatment of hepatic malignancies. Med. Phys. 2011, 38, 4824–4845. [Google Scholar] [CrossRef]
- Miften, M.; Vinogradskiy, Y.; Moiseenko, V.; Grimm, J.; Yorke, E.; Jackson, A.; Tomé, W.A.; Haken, R.K.T.; Ohri, N.; Romero, A.M.; et al. Radiation Dose-Volume Effects for Liver SBRT. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 196–205. [Google Scholar] [CrossRef]
- Pan, C.C.; Kavanagh, B.D.; Dawson, L.A.; Li, X.A.; Das, S.K.; Miften, M.; Haken, R.K.T. Radiation-associated liver injury. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76 (Suppl. S3), S94–S100. [Google Scholar] [CrossRef]
- Whitmill, M.; Young, M.; Tan, X.; Zhang, X.; Thapa, D.; Kim, H.P.; Danquah, F.; Moon, A.M.; Tepper, J.E.; Yanagihara, T.K. Oncologic outcomes and rates of hepatotoxicity following stereotactic body radiotherapy for hepatocellular carcinoma. Hepatoma Res. 2024, 10, 35. [Google Scholar] [CrossRef]
- Salem, R.; Thurston, K.G. Radioembolization with 90Yttrium microspheres: A state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J. Vasc. Interv. Radiol. 2006, 17, 1251–1278. [Google Scholar] [CrossRef] [PubMed]
- Afrasiabi, A.; du Pisanie, J.L.; Gholami, B.; Wang, H.; Gad, S.; Kokabi, N. Post-Y90 PET Dosimetry. PET Clin. 2025, 20, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, R.J.; Sato, K.T.; Atassi, B.; Ryu, R.K.; Nemcek, A.A.; Kulik, L., Jr.; Geschwind, J.-F.; Murthy, R.; Rilling, W.; Liu, D.; et al. Radioembolization with 90Y microspheres: Angiographic and technical considerations. Cardiovasc. Interv. Radiol. 2007, 30, 571–592. [Google Scholar] [CrossRef]
- Llovet, J.M.; Lencioni, R. mRECIST for HCC: Performance and novel refinements. J. Hepatol. 2020, 72, 288–306. [Google Scholar] [CrossRef]
- Wong, T.C.L.; Chiang, C.-L.; Lee, A.-S.; Lee, V.H.F.; Yeung, C.S.Y.; Ho, C.H.M.; Cheung, T.-T.; Ng, K.K.; Chok, S.-H.; Chan, A.C.; et al. Better survival after stereotactic body radiation therapy following transarterial chemoembolization in nonresectable hepatocellular carcinoma: A propensity score matched analysis. Surg. Oncol. 2019, 28, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.M.; Ryoo, B.-Y.; Lee, S.J.; Kim, J.H.; Shin, J.H.; An, J.H.; Lee, H.C.; Lim, Y.S. Efficacy and Safety of Transarterial Chemoembolization Plus External Beam Radiotherapy vs Sorafenib in Hepatocellular Carcinoma With Macroscopic Vascular Invasion: A Randomized Clinical Trial. JAMA Oncol. 2018, 4, 661–669. [Google Scholar] [CrossRef] [PubMed]
| Count (%)/Median (Range) | |
|---|---|
| Age, year * | 66.5 (40, 87) |
| Male sex | 9 (75%) |
| Cirrhosis etiology | |
| HCV/alcohol | 1 (8.3%) |
| MASLD ¥/alcohol | 2 (16.6%) |
| MASLD/HCV | 1 (8.3%) |
| MASLD | 2 (16.6%) |
| HCV | 3 (25%) |
| HCV/HBV | 1 (8.3%) |
| No cirrhosis | 2 (16.6%) |
| Prior liver directed therapies: | |
| None | 8 (66.7%%) |
| RFA ** | 1 (8.3%) |
| TARE | 2 (16.7%) |
| TACE/TACE | 1 (8.3%) |
| First treatment in sequential treatment | |
| TARE | 9 (75%) |
| SBRT | 3 (25%) |
| ECOG: | |
| 0 | 7 (58.3%) |
| 1 | 4 (33.3%) |
| 2 | 1 (8.3%) |
| Baseline BCLC class | |
| A | 4 (33.3%) |
| B | 3 (25%) |
| C | 5 (41.6%) |
| Baseline CP | |
| A | 12 (100%) |
| Baseline ALBI grade | |
| 1 | 5 (41.7%) |
| 2 | 7 (58.3%) |
| Baseline tumor burden: | |
| Single lesion <2 cm | 0 |
| Single lesion >2 cm | 2 (16.6%) |
| 2–3 lesions all <3 cm | 4 (33.3%) |
| Multifocal | 6 (50%) |
| Portal vein invasion | 0 |
| Metastatic spread | 0 |
| Time between TARE and SBRT (months) | 6.5 (1.5 to 24) |
| Treatment to the same lesion | |
| Yes | 3 (25%) |
| No | 9 (75%) |
| Y90 delivery method | |
| Glass | 11 (91.6%) |
| Resin | 1 (0.4%) |
| Y90 segment dose (Gy) | 215.6 (93,538) |
| Y90 tumor dose (Gy) | 520.25 (220, 1892) |
| SBRT dose (Gy) | 45 (25, 45) |
| Gender | Age | Cirrhosis Etiology | Response | First Seg Treated | Second Seg Treated | Same Lesion Targeted | Baseline CTP Class | Baseline BCLC | Baseline ECOG | Baseline ALBI Grade | Y90 Segment Dose | Y90 Umor Dose | SBRT Dose Total (Gy) | Toxicities After Last Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | 67 | NAFLD/HCV | SBRT: SD TARE:PR | 7 | 7 | Yes | A | A | 0 | 1 | 143 | 377 | 45 | Grade 2 Cr 1 ↑ Grade 1 ALP 2 ↑ |
| Female | 54 | NAFLD | SBRT:SD TARE:CR | 6 | 4 | No | A | B | 0 | 2 | 160 | 385 | 45 | Grade 1 AST 3 ↑ Grade 2 ALP ↑ Late Grade 1 Bili 4 ↑ and Alb 5 ↓ |
| Male | 69 | HCV | SBRT: PR TARE: CR | 7 | 4 | No | A | B | 0 | 1 | 220 | 387 | 45 | Grade 1 AST ↑, Grade 1 ALT 6 ↑ |
| Male | 66 | HCV | TARE:PR SBRT: PR | 5 | 2/3 | No | A | B | 1 | 1 | 538 | 1204 | 45 | None |
| Male | 55 | HCV/alcohol | TARE:PR SBRT: SD | 4 | 7 | No | A | A | 1 | 2 | 102 | 226 | 45 | None |
| Female | 50 | HCV/Alcohol | TARE: PD SBRT: SD | 5 | Portal nodes | No | A | A | 0 | 1 | 292.7 | 959.5 | 25 | Grade 2 Alb ↓ grade 1 AST ↑ |
| Male | 66 | HCV/HBV | TARE: PR SBRT: PR | 6/7 | 5 | No | A | C | 1 | 1 | 177.9 | 342.3 | 45 | none |
| Male | 68 | HCV | TARE: SD SBRT: CR | 4a | 4a | Yes | A | B | 0 | 1 | 93 | 220 | 45 | Grade 1 LFT 7 ↑ |
| Male | 72 | HCV | TARE:PR SBRT:SD | 8 | 7/8 | No | A | A | 1 | 2 | 284.6 | 671.5 | 45 | Grade 1 INR 8 ↑ |
| Male | 82 | NAFLD, alcohol | TARE:SD SBRT:PR | 2/4 | 7 | No | A | B | 2 | 1 | 316 | 653.5 | 45 | Grade 1 ALP ↑ |
| Female | 40 | NAFLD | TARE: PD SBRT: CR | 3 and 6 | 5 | No | A | A | 0 | 2 | 211.2 | 888 | 45 | None |
| Male | 87 | NAFLD, alcohol | TARE:PR SBRT:CR | 8 | 8 | Yes | A | A | 1 | 2 | 439 | 1892 | 45 | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gholami, B.; Afrasiabi, A.; Moon, A.M.; Yanagihara, T.K.; Wang, H.; Gad, S.; Villalobos, A.; Mauro, D.M.; Yu, H.; du Pisanie, J.L.; et al. Safety of Combination TARE and SBRT in Hepatocellular Carcinoma: A Review of Literature & Single-Center Case Series. Curr. Oncol. 2025, 32, 487. https://doi.org/10.3390/curroncol32090487
Gholami B, Afrasiabi A, Moon AM, Yanagihara TK, Wang H, Gad S, Villalobos A, Mauro DM, Yu H, du Pisanie JL, et al. Safety of Combination TARE and SBRT in Hepatocellular Carcinoma: A Review of Literature & Single-Center Case Series. Current Oncology. 2025; 32(9):487. https://doi.org/10.3390/curroncol32090487
Chicago/Turabian StyleGholami, Bahareh, Ali Afrasiabi, Andrew M. Moon, Ted K. Yanagihara, Hui Wang, Sandra Gad, Alex Villalobos, David M. Mauro, Hyeon Yu, Johannes L. du Pisanie, and et al. 2025. "Safety of Combination TARE and SBRT in Hepatocellular Carcinoma: A Review of Literature & Single-Center Case Series" Current Oncology 32, no. 9: 487. https://doi.org/10.3390/curroncol32090487
APA StyleGholami, B., Afrasiabi, A., Moon, A. M., Yanagihara, T. K., Wang, H., Gad, S., Villalobos, A., Mauro, D. M., Yu, H., du Pisanie, J. L., & Kokabi, N. (2025). Safety of Combination TARE and SBRT in Hepatocellular Carcinoma: A Review of Literature & Single-Center Case Series. Current Oncology, 32(9), 487. https://doi.org/10.3390/curroncol32090487





