Hepatic Artery Infusion Chemotherapy for Hepatocellular Carcinoma: Clinical Advancements
Abstract
1. Introduction
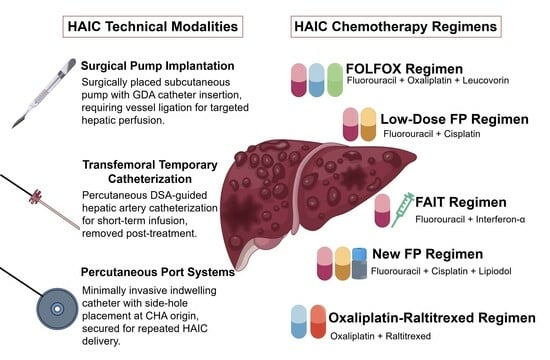
2. Definition and Technical Modalities
2.1. Definition
2.2. Technical Modalities
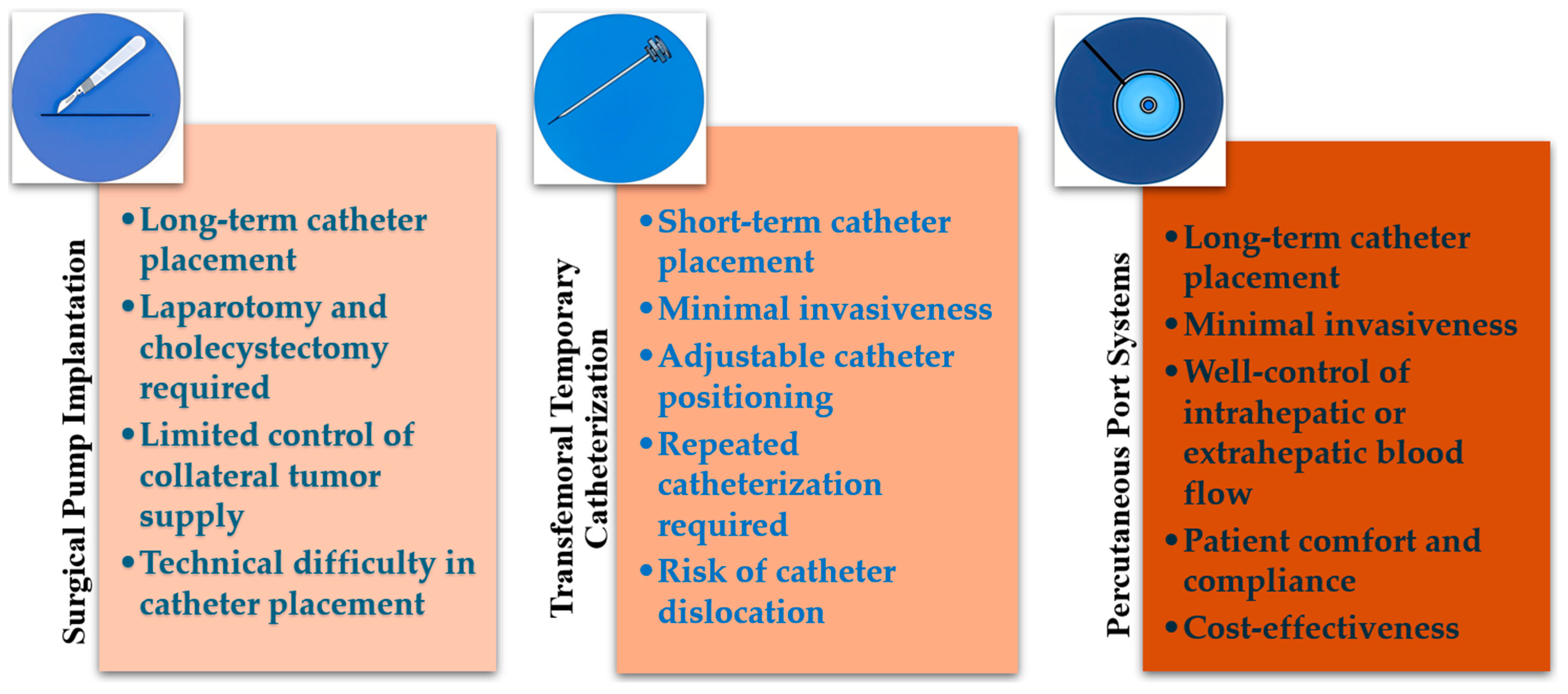
2.2.1. Surgical Pump Implantation
2.2.2. Transfemoral Temporary Catheterization
2.2.3. Percutaneous Port Systems
3. Pharmacological Rationale and Chemotherapeutic Agents
3.1. Pharmacological Rationale
3.2. Drug Selection
4. Patient Selection and Preprocedural Evaluation
4.1. Patient Selection
4.2. Preprocedural Evaluation
5. HAIC Chemotherapy Regimens and Outcomes
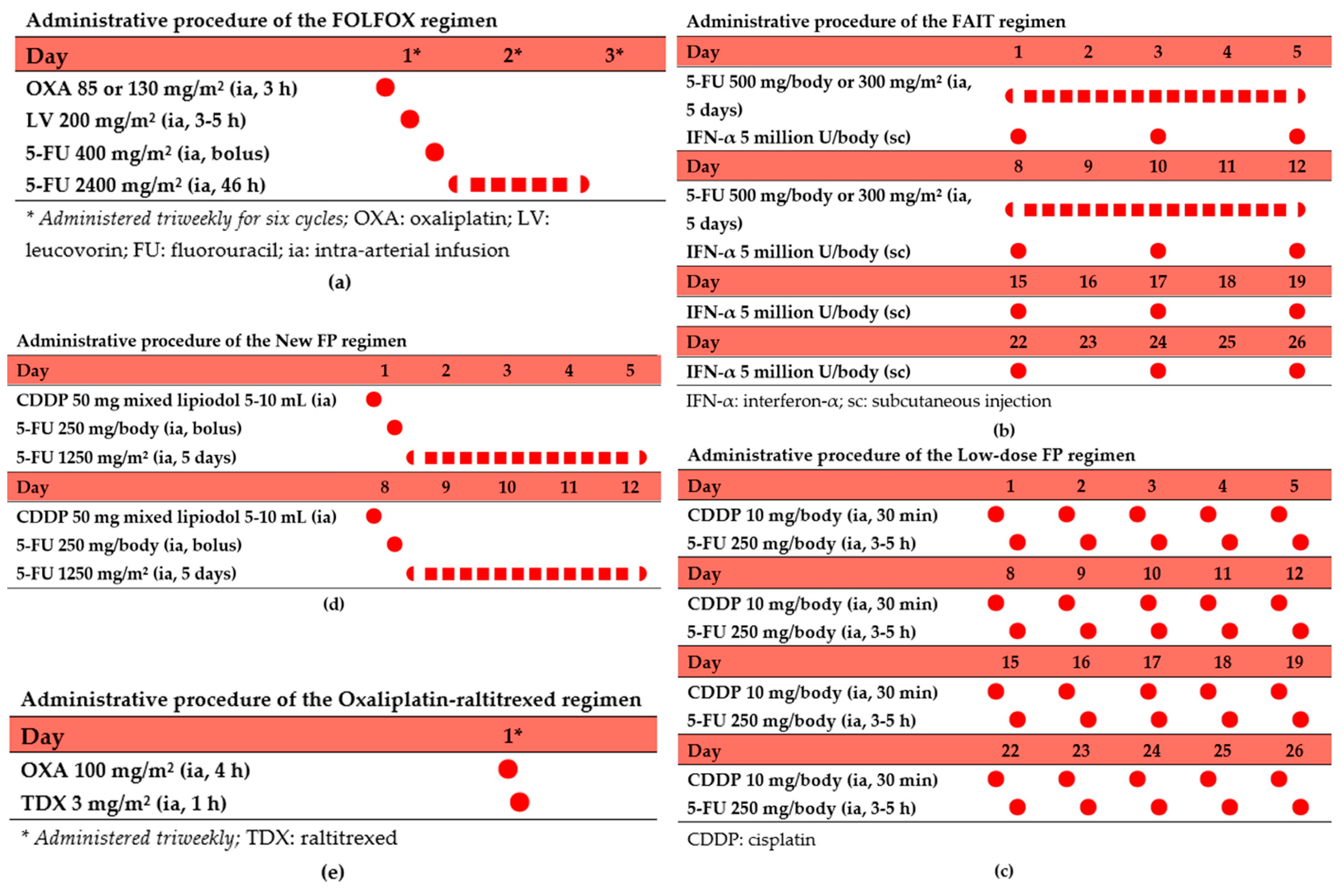
5.1. FOLFOX Regimen
5.2. Low-Dose FP Regimen
5.3. FAIT Regimen
5.4. New FP Regimen
5.5. Oxaliplatin–Raltitrexed Regimen
6. Adverse Events and Their Management
6.1. Chemotherapy-Induced AEs
6.2. HAIC Procedure-Related AEs
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| TACE | Transarterial chemoembolization |
| HAIC | Hepatic artery infusion chemotherapy |
| GDA | Gastroduodenal artery |
| AEs | Adverse events |
| ECOG | Eastern Cooperative Oncology Group |
| ORR | Objective response rates |
| OS | Overall survival |
| PFS | Progression-free survival |
| PVTT | Portal vein tumor thrombosis |
References
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Chen, W.; Zheng, R.; Zhang, S.; Ji, J.S.; Zou, X.; Xia, C.; Sun, K.; Yang, Z.; Li, H.; et al. Changing cancer survival in China during 2003–15: A pooled analysis of 17 population-based cancer registries. Lancet Glob. Health 2018, 6, e555–e567. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2021, 76, 681–693. [Google Scholar] [CrossRef]
- Vogel, A.; Martinelli, E.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.; Meyer, T.; Nault, J.-C.; Neumann, U.; Ricke, J.; et al. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann. Oncol. 2021, 32, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Takemura, N.; Yamashita, T.; Watadani, T.; Kaibori, M.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Aikata, H.; et al. Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2021 version (5th JSH-HCC Guidelines). Hepatol. Res. 2023, 53, 383–390. [Google Scholar] [CrossRef]
- Ueshima, K.; Komemushi, A.; Aramaki, T.; Iwamoto, H.; Obi, S.; Sato, Y.; Tanaka, T.; Matsueda, K.; Moriguchi, M.; Saito, H.; et al. Clinical Practice Guidelines for Hepatic Arterial Infusion Chemotherapy with a Port System Proposed by the Japanese Society of Interventional Radiology and Japanese Society of Implantable Port Assisted Treatment. Liver Cancer 2022, 11, 407–425. [Google Scholar] [CrossRef]
- Zhao, M.; Guo, Z.; Zou, Y.-H.; Li, X.; Yan, Z.-P.; Chen, M.-S.; Fan, W.-J.; Li, H.-L.; Yang, J.-J.; Chen, X.-M.; et al. Arterial chemotherapy for hepatocellular carcinoma in China: Consensus recommendations. Hepatol. Int. 2023, 18, 4–31. [Google Scholar] [CrossRef]
- Ueshima, K.; Ogasawara, S.; Ikeda, M.; Yasui, Y.; Terashima, T.; Yamashita, T.; Obi, S.; Sato, S.; Aikata, H.; Ohmura, T.; et al. Hepatic Arterial Infusion Chemotherapy versus Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer 2020, 9, 583–595. [Google Scholar] [CrossRef]
- Leal, J.N.; Kingham, T.P. Hepatic Artery Infusion Chemotherapy for Liver Malignancy. Surg. Oncol. Clin. N. Am. 2015, 24, 121–148. [Google Scholar] [CrossRef]
- Van Nieuwenhove, Y.; Aerts, M.; Neyns, B.; Delvaux, G. Techniques for the placement of hepatic artery catheters for regional chemotherapy in unresectable liver metastases. Eur. J. Surg. Oncol. 2007, 33, 336–340. [Google Scholar] [CrossRef]
- Zheng, K.; Wang, X. Techniques and status of hepatic arterial infusion chemotherapy for primary hepatobiliary cancers. Ther. Adv. Med Oncol. 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Thiels, C.A.; D’Angelica, M.I. Hepatic artery infusion pumps. J. Surg. Oncol. 2020, 122, 70–77. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, H.; Wang, Z.; Cong, W.; Zeng, M.; Zhou, W.; Bie, P.; Liu, L.; Wen, T.; Kuang, M.; et al. Guidelines for the Diagnosis and Treatment of Primary Liver Cancer (2022 Edition). Liver Cancer 2023, 12, 405–444. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, S.; Itano, S.; Nagamatsu, H.; Akiyoshi, J.; Kurogi, J.; Tajiri, N.; Kajiwara, M.; Sata, M. Temporary Indwelling Catheter System via the Left Brachial Artery: Evaluation in 83 Patients with Hepatic Tumors. Am. J. Roentgenol. 2007, 188, 652–658. [Google Scholar] [CrossRef]
- Arai, Y.; Takeuchi, Y.; Inaba, Y.; Yamaura, H.; Sato, Y.; Aramaki, T.; Matsueda, K.; Seki, H. Percutaneous Catheter Placement for Hepatic Arterial Infusion Chemotherapy. Tech. Vasc. Interv. Radiol. 2007, 10, 30–37. [Google Scholar] [CrossRef]
- Tanaka, T.; Arai, Y.; Inaba, Y.; Matsueda, K.; Aramaki, T.; Takeuchi, Y.; Kichikawa, K. Radiologic Placement of Side-hole Catheter with Tip Fixation for Hepatic Arterial Infusion Chemotherapy. J. Vasc. Interv. Radiol. 2003, 14, 63–68. [Google Scholar] [CrossRef]
- Ganaha, F.; Sadaoka, S.; Yamada, T. Continuous arterial infusion strategies using implanted ports. Tech. Vasc. Interv. Radiol. 2002, 5, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.A.; Waggershauser, T.; Sittek, H.; Reiser, M.F. Liver Intraarterial Chemotherapy: Use of the Femoral Artery for Percutaneous Implantation of Catheter-Port Systems. Radiology 2000, 215, 294–299. [Google Scholar] [CrossRef]
- Meyblum, L.; Faron, M.; Deschamps, F.; Kobe, A.; Bonnet, B.; Boileve, A.; Gelli, M.; Boige, V.; Hollebecque, A.; Durand-Labrunie, J.; et al. Safety and efficacy of percutaneous arterial port Implantation for Hepatic Arterial Infusion Chemotherapy. Eur. Radiol. 2024, 35, 1022–1033. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, X.; Wang, X.; Cao, G.; Wang, X.; Yang, R. Evaluation of percutaneous unilateral trans-femoral implantation of side-hole port-catheter system with coil only fixed-catheter-tip for hepatic arterial infusion chemotherapy. Cancer Imaging 2019, 19, 15. [Google Scholar] [CrossRef]
- Matsumoto, T.; Yamagami, T.; Yoshimatsu, R.; Morishita, H.; Kitamura, N.; Sato, O.; Hasebe, T. Hepatic Arterial Infusion Chemotherapy by the Fixed-Catheter-Tip Method: Retrospective Comparison of Percutaneous Left Subclavian and Femoral Port-Catheter System Implantation. Am. J. Roentgenol. 2014, 202, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, T.; Yoshimatsu, R.; Matsumoto, T.; Nishimura, T. Evaluation of non-target arterial patency after implantation of hepatic arterial catheter using a modified implantation technique with the fixed catheter tip method. Clin. Radiol. 2009, 64, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Breedis, C.; Young, G. The Blood Supply of Neoplasms in the Liver. Am. J. Pathol. 1954, 30, 969–985. [Google Scholar] [PubMed]
- Barber, F.D.; Mavligit, G.; Kurzrock, R. Hepatic arterial infusion chemotherapy for metastatic colorectal cancer: A concise overview. Cancer Treat. Rev. 2004, 30, 425–436. [Google Scholar] [CrossRef]
- Liang, B.; Xiong, F.; Wu, H.; Wang, Y.; Dong, X.; Cheng, S.; Feng, G.; Zhou, G.; Xiong, B.; Liang, H.; et al. Effect of Transcatheter Intraarterial Therapies on the Distribution of Doxorubicin in Liver Cancer in a Rabbit Model. PLoS ONE 2013, 8, e76388. [Google Scholar] [CrossRef]
- Ikeda, M.; Morizane, C.; Ueno, M.; Okusaka, T.; Ishii, H.; Furuse, J. Chemotherapy for hepatocellular carcinoma: Current status and future perspectives. Ultrasound Med. Biol. 2017, 48, 103–114. [Google Scholar] [CrossRef]
- Ensminger, W.D.; Gyves, J.W. Clinical pharmacology of hepatic arterial chemotherapy. Semin. Oncol. 1983, 10, 176–182. [Google Scholar]
- Obi, S.; Sato, S.; Kawai, T. Current Status of Hepatic Arterial Infusion Chemotherapy. Liver Cancer 2015, 4, 188–199. [Google Scholar] [CrossRef]
- Nishikawa, H.; Osaki, Y.; Kita, R.; Kimura, T. Hepatic Arterial Infusion Chemotherapy for Advanced Hepatocellular Carcinoma in Japan. Cancers 2012, 4, 165–183. [Google Scholar] [CrossRef]
- Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin. Mol. Hepatol. 2022, 28, 583–705. [Google Scholar] [CrossRef]
- D’avola, D.; Granito, A.; de la Torre-Aláez, M.; Piscaglia, F. The importance of liver functional reserve in the non-surgical treatment of hepatocellular carcinoma. J. Hepatol. 2022, 76, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Lyu, N.; Wang, X.; Li, J.-B.; Lai, J.-F.; Chen, Q.-F.; Li, S.-L.; Deng, H.-J.; He, M.; Mu, L.-W.; Zhao, M. Arterial Chemotherapy of Oxaliplatin Plus Fluorouracil Versus Sorafenib in Advanced Hepatocellular Carcinoma: A Biomolecular Exploratory, Randomized, Phase III Trial (FOHAIC-1). J. Clin. Oncol. 2022, 40, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Zhu, X.; Fu, S.; Cao, G.; Li, W.-Q.; Xu, L.; Chen, H.; Wu, D.; Yang, R.; Wang, K.; et al. Sorafenib Plus Hepatic Arterial Infusion Chemotherapy versus Sorafenib for Hepatocellular Carcinoma with Major Portal Vein Tumor Thrombosis: A Randomized Trial. Radiology 2022, 303, 455–464. [Google Scholar] [CrossRef]
- Murakami, E.; Aikata, H.; Miyaki, D.; Nagaoki, Y.; Katamura, Y.; Kawaoka, T.; Takaki, S.; Hiramatsu, A.; Waki, K.; Takahashi, S.; et al. Hepatic arterial infusion chemotherapy using 5-fluorouracil and systemic interferon-α for advanced hepatocellular carcinoma in combination with or without three-dimensional conformal radiotherapy to venous tumor thrombosis in hepatic vein or inferior vena cava. Hepatol. Res. 2011, 42, 442–453. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Li, Q.; Zou, R.; Shen, J.; Fang, W.; Tan, G.; Zhou, Y.; Wu, X.; Xu, L.; Wei, W.; et al. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma with Portal Vein Invasion: A Randomized Clinical Trial. JAMA Oncol. 2019, 5, 953–960. [Google Scholar] [CrossRef]
- Tang, H.-H.; Zhang, M.-Q.; Zhang, Z.-C.; Fan, C.; Jin, Y.; Wang, W.-D. The Safety and Efficacy of Hepatic Arterial Infusion Chemotherapy Combined with PD-(L)1 Inhibitors and Molecular Targeted Therapies for the Treatment of Intermediate and Advanced Hepatocellular Carcinoma Unsuitable for Transarterial Chemoembolization. J. Hepatocell. Carcinoma 2023, 10, 2211–2221. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, N.; Feng, Y.; Zhang, Y.; Zhang, T.; Wang, L. Tyrosine Kinase Inhibitors Plus Anti-PD-1 Antibodies with Hepatic Arterial Infusion Chemotherapy or Transarterial Chemoembolization for Unresectable Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2023, 10, 1735–1748. [Google Scholar] [CrossRef]
- Lai, Z.; He, M.; Bu, X.; Xu, Y.; Huang, Y.; Wen, D.; Li, Q.; Xu, L.; Zhang, Y.; Wei, W.; et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: A biomolecular exploratory, phase II trial. Eur. J. Cancer 2022, 174, 68–77. [Google Scholar] [CrossRef]
- Zhang, T.-Q.; Geng, Z.-J.; Zuo, M.-X.; Li, J.-B.; Huang, J.-H.; Huang, Z.-L.; Wu, P.-H.; Gu, Y.-K. Camrelizumab (a PD-1 inhibitor) plus apatinib (an VEGFR-2 inhibitor) and hepatic artery infusion chemotherapy for hepatocellular carcinoma in Barcelona Clinic Liver Cancer stage C (TRIPLET): A phase II study. Signal Transduct. Target. Ther. 2023, 8, 1–7. [Google Scholar] [CrossRef]
- You, H.; Liu, X.; Guo, J.; Lin, Y.; Zhang, Y.; Li, C. Hepatic arterial infusion chemotherapy and sequential ablation treatment in large hepatocellular carcinoma. Int. J. Hyperth. 2022, 39, 1097–1105. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, P.-J.; Guo, J.-H.; Chen, H.; Xu, H.-F.; Liu, P.; Yang, R.-J.; Zhu, X. Chemoembolization alone vs combined chemoembolization and hepatic arterial infusion chemotherapy in inoperable hepatocellular carcinoma patients. World J. Gastroenterol. 2015, 21, 10443–10452. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, K.; Kudo, M.; Tanaka, M.; Kumada, T.; Chung, H.; Hagiwara, S.; Inoue, T.; Yada, N.; Kitai, S. Phase I/II Study of Sorafenib in Combination with Hepatic Arterial Infusion Chemotherapy Using Low-Dose Cisplatin and 5-Fluorouracil. Liver Cancer 2015, 4, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Hatooka, M.; Kawaoka, T.; Aikata, H.; Inagaki, Y.; Morio, K.; Nakahara, T.; Murakami, E.; Tsuge, M.; Hiramatsu, A.; Imamura, M.; et al. Hepatic arterial infusion chemotherapy followed by sorafenib in patients with advanced hepatocellular carcinoma (HICS 55): An open label, non-comparative, phase II trial. BMC Cancer 2018, 18, 633. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Yokosuka, O.; Ogasawara, S.; Obi, S.; Izumi, N.; Aikata, H.; Nagano, H.; Hatano, E.; Sasaki, Y.; et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): A randomised, open label, phase 3 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 424–432. [Google Scholar] [CrossRef]
- Maruta, S.; Koshima, Y.; Tsuchiya, T.; Tamura, R.; Takahashi, M.; Ohshima, T.; Ooka, Y. Combination Therapy of Lenvatinib and Hepatic Arterial Infusion Chemotherapy Using Cisplatin with Lipiodol and 5-Fluorouracil: A Potential Breakthrough Therapy for Unresectable Advanced Hepatocellular Carcinoma. Cureus 2024, 16, e66185. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Yuan, B.; Peng, J.; Zhang, Q.; Yu, W.; Ge, N.; Weng, Z.; Huang, J.; Liu, W.; et al. Apatinib plus hepatic arterial infusion of oxaliplatin and raltitrexed for hepatocellular carcinoma with extrahepatic metastasis: Phase II trial. Nat. Commun. 2024, 15, 1–10. [Google Scholar] [CrossRef]
- He, M.-K.; Le, Y.; Li, Q.-J.; Yu, Z.-S.; Li, S.-H.; Wei, W.; Guo, R.-P.; Shi, M. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: A prospective non-randomized study. Chin. J. Cancer 2017, 36, 1–8. [Google Scholar] [CrossRef]
- Li, Q.-J.; He, M.-K.; Chen, H.-W.; Fang, W.-Q.; Zhou, Y.-M.; Xu, L.; Wei, W.; Zhang, Y.-J.; Guo, Y.; Guo, R.-P.; et al. Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin Versus Transarterial Chemoembolization for Large Hepatocellular Carcinoma: A Randomized Phase III Trial. J. Clin. Oncol. 2022, 40, 150–160. [Google Scholar] [CrossRef]
- Li, S.-H.; Mei, J.; Cheng, Y.; Li, Q.; Wang, Q.-X.; Fang, C.-K.; Lei, Q.-C.; Huang, H.-K.; Cao, M.-R.; Luo, R.; et al. Postoperative Adjuvant Hepatic Arterial Infusion Chemotherapy with FOLFOX in Hepatocellular Carcinoma with Microvascular Invasion: A Multicenter, Phase III, Randomized Study. J. Clin. Oncol. 2023, 41, 1898–1908. [Google Scholar] [CrossRef]
- Moriguchi, M.; Aramaki, T.; Nishiofuku, H.; Sato, R.; Asakura, K.; Yamaguchi, K.; Tanaka, T.; Endo, M.; Itoh, Y. Sorafenib versus Hepatic Arterial Infusion Chemotherapy as Initial Treatment for Hepatocellular Carcinoma with Advanced Portal Vein Tumor Thrombosis. Liver Cancer 2017, 6, 275–286. [Google Scholar] [CrossRef]
- Ando, E.; Tanaka, M.; Yamashita, F.; Kuromatsu, R.; Yutani, S.; Fukumori, K.; Sumie, S.; Yano, Y.; Okuda, K.; Sata, M. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer 2002, 95, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Niizeki, T.; Sumie, S.; Torimura, T.; Kurogi, J.; Kuromatsu, R.; Iwamoto, H.; Aino, H.; Nakano, M.; Kawaguchi, A.; Kakuma, T.; et al. Serum vascular endothelial growth factor as a predictor of response and survival in patients with advanced hepatocellular carcinoma undergoing hepatic arterial infusion chemotherapy. J. Gastroenterol. 2012, 47, 686–695. [Google Scholar] [CrossRef] [PubMed]
- The Liver Cancer Study Group of Japan; Nouso, K.; Miyahara, K.; Uchida, D.; Kuwaki, K.; Izumi, N.; Omata, M.; Ichida, T.; Kudo, M.; Ku, Y.; et al. Effect of hepatic arterial infusion chemotherapy of 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma in the Nationwide Survey of Primary Liver Cancer in Japan. Br. J. Cancer 2013, 109, 1904–1907. [Google Scholar] [CrossRef]
- Kasai, K.; Ushio, A.; Kasai, Y.; Sawara, K.; Miyamoto, Y.; Oikawa, K.; Kuroda, H.; Takikawa, Y.; Suzuki, K. Therapeutic efficacy of combination therapy with intra-arterial 5-fluorouracil and systemic pegylated interferon α-2b for advanced hepatocellular carcinoma with portal venous invasion. Cancer 2011, 118, 3302–3310. [Google Scholar] [CrossRef]
- Ota, H.; Nagano, H.; Sakon, M.; Eguchi, H.; Kondo, M.; Yamamoto, T.; Nakamura, M.; Damdinsuren, B.; Wada, H.; Marubashi, S.; et al. Treatment of hepatocellular carcinoma with major portal vein thrombosis by combined therapy with subcutaneous interferon-α and intra-arterial 5-fluorouracil; role of type 1 interferon receptor expression. Br. J. Cancer 2005, 93, 557–564. [Google Scholar] [CrossRef]
- Obi, S.; Yoshida, H.; Toune, R.; Unuma, T.; Kanda, M.; Sato, S.; Tateishi, R.; Teratani, T.; Shiina, S.; Omata, M. Combination therapy of intraarterial 5-fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma with portal venous invasion. Cancer 2006, 106, 1990–1997. [Google Scholar] [CrossRef] [PubMed]
- Monden, M.; Sakon, M.; Sakata, Y.; Ueda, Y.; Hashimura, E. FAIT Research Group 5-fluorouracil arterial infusion + interferon therapy for highly advanced hepatocellular carcinoma: A multicenter, randomized, phase II study. Hepatol. Res. 2011, 42, 150–165. [Google Scholar] [CrossRef]
- Niizeki, T.; Iwamoto, H.; Shirono, T.; Shimose, S.; Nakano, M.; Okamura, S.; Noda, Y.; Kamachi, N.; Hiroyuki, S.; Sakai, M.; et al. Clinical Importance of Regimens in Hepatic Arterial Infusion Chemotherapy for Advanced Hepatocellular Carcinoma with Macrovascular Invasion. Cancers 2021, 13, 4450. [Google Scholar] [CrossRef]
- Iwamoto, H.; Niizeki, T.; Nagamatsu, H.; Ueshima, K.; Tani, J.; Kuzuya, T.; Kasai, K.; Kooka, Y.; Hiraoka, A.; Sugimoto, R.; et al. The Clinical Impact of Hepatic Arterial Infusion Chemotherapy New-FP for Hepatocellular Carcinoma with Preserved Liver Function. Cancers 2022, 14, 4873. [Google Scholar] [CrossRef]
- Nagamatsu, H.; Sumie, S.; Niizeki, T.; Tajiri, N.; Iwamoto, H.; Aino, H.; Nakano, M.; Shimose, S.; Satani, M.; Okamura, S.; et al. Hepatic arterial infusion chemoembolization therapy for advanced hepatocellular carcinoma: Multicenter phase II study. Cancer Chemother. Pharmacol. 2016, 77, 243–250. [Google Scholar] [CrossRef]
- Iwamoto, H.; Niizeki, T.; Nagamatsu, H.; Ueshima, K.; Nomura, T.; Kuzuya, T.; Kasai, K.; Kooka, Y.; Hiraoka, A.; Sugimoto, R.; et al. Survival Benefit of Hepatic Arterial Infusion Chemotherapy over Sorafenib in the Treatment of Locally Progressed Hepatocellular Carcinoma. Cancers 2021, 13, 646. [Google Scholar] [CrossRef] [PubMed]
- Feng, A.-W.; Guo, J.-H.; Gao, S.; Kou, F.-X.; Liu, S.-X.; Liu, P.; Chen, H.; Wang, X.-D.; Xu, H.-F.; Cao, G.; et al. A randomized phase II trial of hepatic arterial infusion of oxaliplatin plus raltitrexed versus oxaliplatin plus 5-fluorouracil for unresectable colorectal cancer liver metastases. Front. Oncol. 2022, 12, 913017. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, K.; Liu, W.; Yu, W. Hepatic arterial infusion of oxaliplatin plus raltitrexed in patients with intermediate and advanced stage hepatocellular carcinoma: A phase II, single-arm, prospective study. Eur. J. Cancer 2020, 134, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Tanaka, M.; Shibata, J.; Ando, E.; Ogata, T.; Kinoshita, H.; Eriguchi, N.; Aoyagi, S.; Tanikawa, K. Hepatic arterial infusion chemotherapy with continuous low dose administration of cisplatin and 5-fluorouracil for multiple recurrence of hepatocellular carcinoma after surgical treatment. Oncol. Rep. 1999, 6, 587–678. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.W.; Jang, J.Y.; Lee, J.E.; Lee, S.H.; Kim, S.G.; Cha, S.; Kim, Y.S.; Cho, Y.D.; Kim, H.S.; Kim, B.S.; et al. The efficacy of hepatic arterial infusion chemotherapy as an alternative to sorafenib in advanced hepatocellular carcinoma. Asia-Pacific J. Clin. Oncol. 2012, 8, 164–171. [Google Scholar] [CrossRef]
- Yamasaki, T.; Kimura, T.; Kurokawa, F.; Aoyama, K.; Ishikawa, T.; Tajima, K.; Yokoyama, Y.; Takami, T.; Omori, K.; Kawaguchi, K.; et al. Prognostic factors in patients with advanced hepatocellular carcinoma receiving hepatic arterial infusion chemotherapy. J. Gastroenterol. 2005, 40, 70–78. [Google Scholar] [CrossRef]
- Ueshima, K.; Kudo, M.; Takita, M.; Nagai, T.; Tatsumi, C.; Ueda, T.; Kitai, S.; Ishikawa, E.; Yada, N.; Inoue, T.; et al. Hepatic Arterial Infusion Chemotherapy Using Low-Dose 5-Fluorouracil and Cisplatin for Advanced Hepatocellular Carcinoma. Oncology 2010, 78, 148–153. [Google Scholar] [CrossRef]
- Sakon, M.; Nagano, H.; Dono, K.; Nakamori, S.; Umeshita, K.; Yamada, A.; Kawata, S.; Imai, Y.; Iijima, S.; Monden, M. Combined intraarterial 5-fluorouracil and subcutaneous interferon-α therapy for advanced hepatocellular carcinoma with tumor thrombi in the major portal branches. Cancer 2002, 94, 435–442. [Google Scholar] [CrossRef]
- Enjoji, M.; Morizono, S.; Kotoh, K.; Kohjima, M.; Miyagi, Y.; Yoshimoto, T.; Nakamuta, M. Re-evaluation of antitumor effects of combination chemotherapy with interferon-α and 5-fluorouracil for advanced hepatocellular carcinoma. World J. Gastroenterol. 2005, 11, 5685–5687. [Google Scholar] [CrossRef]
- Nagano, H.; Wada, H.; Kobayashi, S.; Marubashi, S.; Eguchi, H.; Tanemura, M.; Tomimaru, Y.; Osuga, K.; Umeshita, K.; Doki, Y.; et al. Long-Term Outcome of Combined Interferon-α and 5-Fluorouracil Treatment for Advanced Hepatocellular Carcinoma with Major Portal Vein Thrombosis. Oncology 2011, 80, 63–69. [Google Scholar] [CrossRef]
- Yamashita, T.; Arai, K.; Sunagozaka, H.; Ueda, T.; Terashima, T.; Yamashita, T.; Mizukoshi, E.; Sakai, A.; Nakamoto, Y.; Honda, M.; et al. Randomized, Phase II Study Comparing Interferon Combined with Hepatic Arterial Infusion of Fluorouracil plus Cisplatin and Fluorouracil Alone in Patients with Advanced Hepatocellular Carcinoma. Oncology 2011, 81, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, W.-J.; Wang, H.-Y.; Wang, Y.-F.; Peng, B.-G.; Zhou, Q. Arterial infusion of 5-fluorouracil combined with subcutaneous injection of pegylated interferon alpha-2b in treating unresectable hepatocellular carcinoma with portal vein tumor thrombus. Med Oncol. 2015, 32. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Urabe, T.; Kobayashi, K. Combination Chemotherapy for Advanced Hepatocellular Carcinoma Complicated by Major Portal Vein Thrombosis. Oncology 2002, 62, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Ono, N.; Yodono, H.; Ichida, T.; Nakamura, H. Phase II study of hepatic arterial infusion of a fine-powder formulation of cisplatin for advanced hepatocellular carcinoma. Hepatol. Res. 2008, 38, 474–483. [Google Scholar] [CrossRef]
- Ikeda, M.; Okusaka, T.; Furuse, J.; Mitsunaga, S.; Ueno, H.; Yamaura, H.; Inaba, Y.; Takeuchi, Y.; Satake, M.; Arai, Y. A multi-institutional phase II trial of hepatic arterial infusion chemotherapy with cisplatin for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother. Pharmacol. 2013, 72, 463–470. [Google Scholar] [CrossRef]
- Ikeda, M.; Shimizu, S.; Sato, T.; Morimoto, M.; Kojima, Y.; Inaba, Y.; Hagihara, A.; Kudo, M.; Nakamori, S.; Kaneko, S.; et al. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: Randomized phase II trial. Ann. Oncol. 2016, 27, 2090–2096. [Google Scholar] [CrossRef]
- Zaizen, Y.; Nakano, M.; Fukumori, K.; Yano, Y.; Takaki, K.; Niizeki, T.; Kuwaki, K.; Fukahori, M.; Sakaue, T.; Yoshimura, S.; et al. Hepatic Arterial Infusion Chemotherapy with Cisplatin versus Sorafenib for Intrahepatic Advanced Hepatocellular Carcinoma: A Propensity Score-Matched Analysis. Cancers 2021, 13, 5282. [Google Scholar] [CrossRef]
- Ikeda, M.; Yamashita, T.; Ogasawara, S.; Kudo, M.; Inaba, Y.; Morimoto, M.; Tsuchiya, K.; Shimizu, S.; Kojima, Y.; Hiraoka, A.; et al. Multicenter Phase II Trial of Lenvatinib plus Hepatic Intra-Arterial Infusion Chemotherapy with Cisplatin for Advanced Hepatocellular Carcinoma: LEOPARD. Liver Cancer 2023, 13, 193–202. [Google Scholar] [CrossRef]
- Nakano, M.; Niizeki, T.; Nagamatsu, H.; Tanaka, M.; Kuromatsu, R.; Satani, M.; Okamura, S.; Iwamoto, H.; Shimose, S.; Shirono, T.; et al. Clinical effects and safety of intra-arterial infusion therapy of cisplatin suspension in lipiodol combined with 5-fluorouracil versus sorafenib, for advanced hepatocellular carcinoma with macroscopic vascular invasion without extra-hepatic spread: A prospective cohort study. Mol. Clin. Oncol. 2017, 7, 1013–1020. [Google Scholar] [CrossRef][Green Version]
- Allen, P.J.; Nissan, A.; Picon, A.I.; Kemeny, N.; Dudrick, P.; Ben-Porat, L.; Espat, J.; Stojadinovic, A.; Cohen, A.M.; Fong, Y.; et al. Technical Complications and Durability of Hepatic Artery Infusion Pumps for Unresectable Colorectal Liver Metastases: An Institutional Experience of 544 Consecutive Cases. J. Am. Coll. Surg. 2005, 201, 57–65. [Google Scholar] [CrossRef]
- Sharib, J.M.; Creasy, J.M.; Wildman-Tobriner, B.; Kim, C.; Uronis, H.; Hsu, S.D.; Strickler, J.H.; Gholami, S.; Cavnar, M.; Merkow, R.P.; et al. Hepatic Artery Infusion Pumps. Ann. Surg. 2022, 276, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Granito, A.; Facciorusso, A.; Sacco, R.; Bartalena, L.; Mosconi, C.; Cea, U.V.; Cappelli, A.; Antonino, M.; Modestino, F.; Brandi, N.; et al. TRANS-TACE: Prognostic Role of the Transient Hypertransaminasemia after Conventional Chemoembolization for Hepatocellular Carcinoma. J. Pers. Med. 2021, 11, 1041. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.-L.; Liu, L.-X.; Piao, D.-X.; Lin, Y.-X.; Zhao, J.-P.; Jiang, H.-C. Liver regional continuous chemotherapy: Use of femoral or subclavian artery for percutaneous implantation of catheter-port systems. World J. Gastroenterol. 2004, 10, 1659–1662. [Google Scholar] [CrossRef] [PubMed]
- Laface, C.; Laforgia, M.; Molinari, P.; Ugenti, I.; Gadaleta, C.D.; Porta, C.; Ranieri, G. Hepatic Arterial Infusion of Chemotherapy for Advanced Hepatobiliary Cancers: State of the Art. Cancers 2021, 13, 3091. [Google Scholar] [CrossRef]
| Study Design | Patient Population | Arm | Sample Size | Efficacy Outcomes | Author (Years) | References |
|---|---|---|---|---|---|---|
| RCT Phase II | Advanced HCC with major PVTT | HAIC (FOLFOX) + Sorafenib | 32 | OS: 16.3 months; ORR: 41%, PFS: 9.0 months | Zeng, K. (2022) | [33] |
| Sorafenib | 32 | OS: 6.5 months; ORR: 3%, PFS: 2.5 months | ||||
| RCT Phase III | HCC with PVI (Vp3 and Vp4) | HAIC (FOLFOX) + Sorafenib | 125 | OS: 13.37 months; ORR: 40.8%, PFS: 7.03 months | He, M. (2019) | [35] |
| Sorafenib | 122 | OS: 7.13 months; ORR: 2.46%, PFS: 2.6 months | ||||
| Retrospective Analysis | Intermediate and advanced HCC unsuitable for TACE | HAIC (FOLFOX) + PD-(L)1 Inhibitors + MTT | 55 | OS: 15.0 months, PFS: 10.0 months, ORR: 43.6%, DCR: 61.8% | Tang, H.-H. (2023) | [36] |
| Retrospective Analysis | Unresectable HCC suitable for HAIC or TACE | HAIC (FOLFOX) + TKIs + PD-(L)1 Inhibitors | 302 | OS: Not reached, PFS: 12.4 months, ORR: 33.1%, DCR: 77.8% | Yu, B. (2023) | [37] |
| TACE + TKIs + PD-(L)1 Inhibitors | 446 | OS: 13.8 months, PFS: 8.2 months, ORR: 7.8%; DCR: 47.1% | ||||
| Single-arm Phase II | Advanced HCC unsuitable for TACE | HAIC (FOLFOX) + Lenvatinib + Toripalimab | 36 | PFS at 6 months: 80.6%, median PFS: 10.4 months, median OS: 17.9 months | Lai, Z. (2022) | [38] |
| Single-arm Phase II | Intermediate and advanced HCC unsuitable for TACE | HAIC-FOLFOX + Camrelizumab + Apatinib | 35 | ORR: 77.1%, DCR: 97.1%, median PFS: 10.38 months | Zhang, T.-Q. (2023) | [39] |
| Retrospective Analysis | Large HCC | HAIC (FOLFOX) | 135 | OS: 14.5 months, PFS: 4.6 months, ORR: 33.1% | You, H. (2022) | [40] |
| HAIC (FOLFOX) and sequential ablation | 93 | OS: 22.2 months, PFS: 8.5 months, ORR: 51.4% | ||||
| RCT Phase II | Inoperable HCC without distant metastasis | Chemoembolization alone | 39 | ORR: 45.9%, mPFS: 4.5 months | Gao, S. (2015) | [41] |
| HAIC (FOLFOX) + Chemoembolization | 45 | ORR: 68.9%, mPFS: 8.0 months | ||||
| Single-arm Phase I/II | Advanced HCC | HAIC (Low-dose FP) + Sorafenib | 18 | ORR: 38.9%, DCR: 77.8%, TTP: 9.7 months, 1-year OS: 88.2% | Ueshima, K. (2015) | [42] |
| Single-arm Phase II | Advanced HCC | HAIC (Low-dose FP) followed by sorafenib if non-response | 55 | 1-year OS: 64.0%, 2-year OS: 48.3% | Hatooka, M. (2018) | [43] |
| RCT Phase III | Advanced HCC | Sorafenib | 103 | OS: 11.5 months | Kudo, M. (2018) | [44] |
| HAIC (Low-dose FP) + Sorafenib | 103 | OS: 11.8 months | ||||
| Retrospective Analysis | Unresectable HCC with prior systemic therapy | HAIC (New FP) + Lenvatinib | 6 | ORR: 83%, DCR: 100% | Maruta, S. (2024) | [45] |
| Single-arm Phase II | Advanced HCC with extrahepatic metastasis | HAIC (Oxaliplatin–raltitrexed) + Apatinib | 39 | ORR: 53.8%; PFS: 6.2 months, OS: 11.3 months, DCR: 89.7% | Chen, S. (2024) | [46] |
| Regimen | Study Design | Sample Size | Patient Population | OS (mo) | PFS (mo) | ORR (%) | DCR (%) | Severe * or Gr 3–4 AEs (%) | Major Gr 3–4 AEs (%) | Author (Years) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FOLFOX | RCT Phase III | 130 | Advanced HCC | 13.9 | 7.8 | 31.5 | - | 20.3 | Elevated AST (10.9); thrombocytopenia (10.9) | Lyu, N. (2022) | [32] |
| Prospective Phase II | 38 | Unresectable HCC | - | TTP: 5.87 | 54.1 | 83.8 | 34.2 | Vomiting (10.5); leukopenia (7.9) | He, M. (2017) | [47] | |
| RCT Phase III | 159 | Unresectable HCC | 23.1 | 9.6 | 46 .0 | 82 | 19.0 * | Elevated AST (17.8); elevated ALT (8.3) | Li, Q.-J. (2022) | [48] | |
| RCT Phase III | 157 | HCC with MVI post-op | 3-year OS rate: 80.4% | DFS: 20.3 | - | - | - | Pain (1.4) | Li, S.-H. (2023) | [49] | |
| Low-dose FP | Retrospective | 32 | Advanced HCC | 10.3 | TTF: 3.6 | 31.3 | 56.3 | - | Thrombocytopenia (25.0); neutropenia (12.5) | Moriguchi, M. (2017) | [50] |
| Retrospective | 48 | HCC with PVTT | 3-year OS rate: 25% | - | 48 | 77 | - | - | Ando, E. (2002) | [51] | |
| Retrospective | 71 | Advanced HCC | 10.2 | - | 35 | - | - | Leukocytopenia (13.0) | Niizeki, T. (2012) | [52] | |
| Nationwide Survey | 476 | Advanced HCC | 14 | - | 40.5 | - | - | - | Nouso, K. (2013) | [53] | |
| FAIT | Prospective Phase II | 59 | HCC with PVTT | 29.9 | 9.7 | 73 | 91.6 | - | Leucopenia (10.1); thrombocytopenia (8.4) | Kasai, K. (2012) | [54] |
| Prospective Phase II | 55 | HCC with major PVTT | 11.8 | - | 43.6 | 50.9 | 14.6 | Thrombocytopenia (9.1); leukopenia (5.5) | Ota, H. (2005) | [55] | |
| Retrospective | 116 | HCC with PVI | 6.9 | - | 52.6 | 54.3 | - | - | Obi, S. (2006) | [56] | |
| RCT Phase II | 30 | Advanced HCC | 8.4 | 3.5 | 26.7 | 63.3 | 51.6 * | Leukopenia (32.3); thrombocytopenia (29.0); neutropenia (29.0) | Monden, M. (2012) | [57] | |
| New FP | Retrospective | 99 | HCC with MVI without EHS | 24.7 | 8.8 | 76 | 88 | 26.2 * | Thrombocytopenia (8.1); cholangitis (6.1) | Niizeki, T. (2021) | [58] |
| Retrospective | 671 | HCC | 18 | - | 73 | - | - | - | Iwamoto, H. (2022) | [59] | |
| Prospective Phase II | 52 | HCC with PVTT | 27 | 8.6 | 75 | 96.2 | - | Renal dysfunction (4.0); CDDP allergy (4.0) | Nagamatsu, H. (2016) | [60] | |
| Retrospective | 644 | HCC | 12 | - | - | - | - | - | Iwamoto, H. (2021) | [61] | |
| Oxaliplatin–raltitrexed | RCT Phase II | 61 | Unresectable CRCLM | 13.1 | 4.6 | 22.4 | 71.4 | - | Abdominal pain (50.8) | Feng, A.-W. (2022) | [62] |
| Prospective Phase II | 39 | Intermediate and advanced HCC | 1-year OS rate: 43.2% | 5.2 | 46.2 | 79.5 | - | Elevated AST (12.8) | Chen, S. (2020) | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Li, Q.; Liang, B. Hepatic Artery Infusion Chemotherapy for Hepatocellular Carcinoma: Clinical Advancements. Curr. Oncol. 2025, 32, 313. https://doi.org/10.3390/curroncol32060313
Xu W, Li Q, Liang B. Hepatic Artery Infusion Chemotherapy for Hepatocellular Carcinoma: Clinical Advancements. Current Oncology. 2025; 32(6):313. https://doi.org/10.3390/curroncol32060313
Chicago/Turabian StyleXu, Wei, Qing Li, and Bin Liang. 2025. "Hepatic Artery Infusion Chemotherapy for Hepatocellular Carcinoma: Clinical Advancements" Current Oncology 32, no. 6: 313. https://doi.org/10.3390/curroncol32060313
APA StyleXu, W., Li, Q., & Liang, B. (2025). Hepatic Artery Infusion Chemotherapy for Hepatocellular Carcinoma: Clinical Advancements. Current Oncology, 32(6), 313. https://doi.org/10.3390/curroncol32060313