A Matched Case-Control Study Examining the Association Between Exposure to Depot Medroxyprogesterone Acetate and Cerebral Meningioma Using an Active Comparator
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Study Variables
2.3. Statistical Analysis
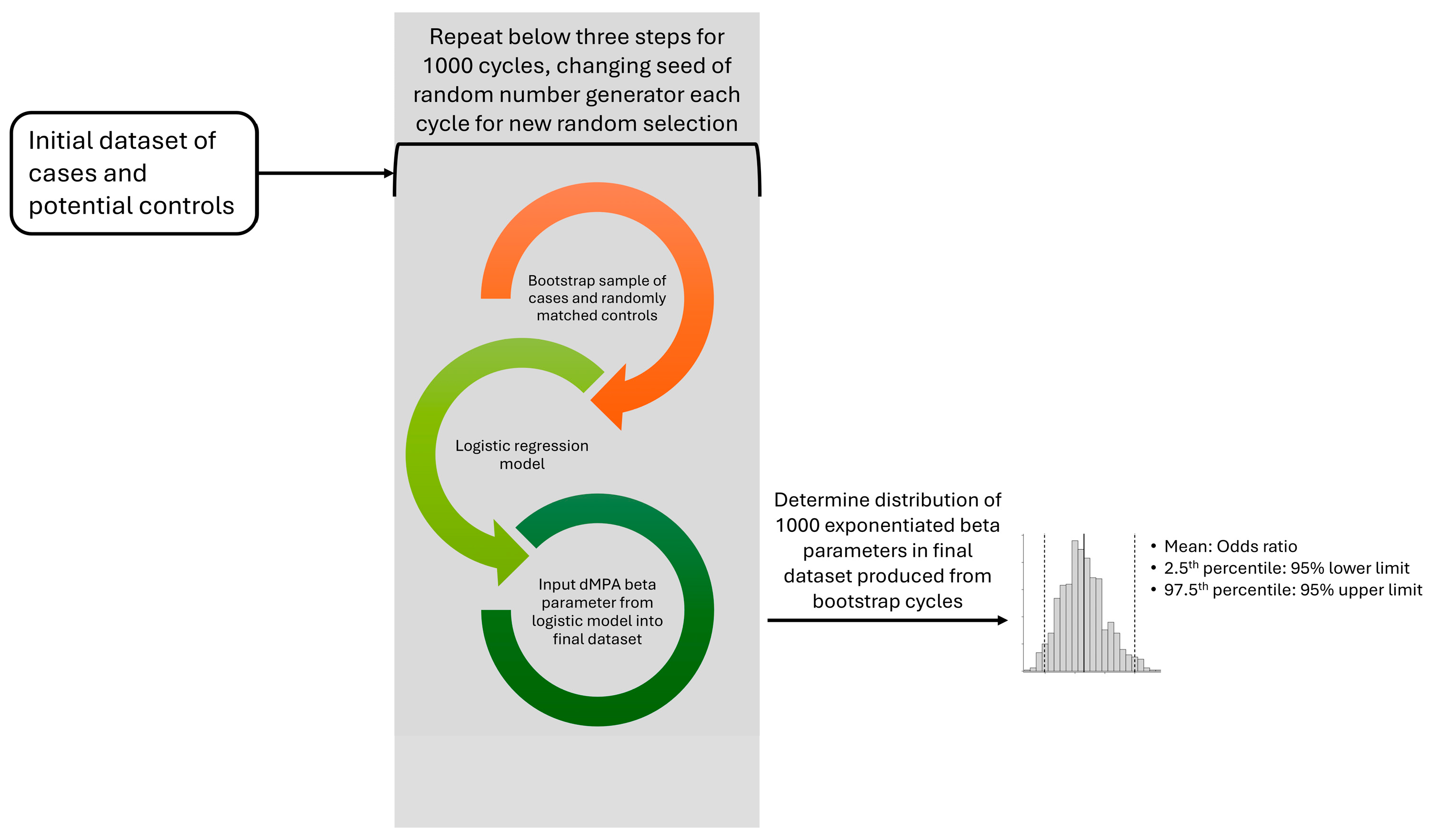
2.4. Bootstrapping
3. Results
4. Discussion
4.1. Principal Findings
4.2. Results in the Context of What Is Known
4.3. Implications
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MPA | Medroxyprogesterone acetate |
| dMPA | depot Medroxyprogesterone acetate |
| RUCA | Rural–Urban Commuting Area |
| OR | Odds ratio |
| CI | Confidence interval |
References
- NSFG—Listing C—Key Statistics from the National Survey of Family Growth. Available online: https://www.cdc.gov/nchs/nsfg/key_statistics/c-keystat.htm#contraception (accessed on 17 December 2024).
- Kurita, T. Cervix: Cell Biology. In Encyclopedia of Reproduction, 2nd ed.; Skinner, M.K., Ed.; Academic Press: Oxford, UK, 2018; pp. 347–352. ISBN 978-0-12-815145-7. [Google Scholar]
- Griffin, R.L. The Association between Medroxyprogesterone Acetate Exposure and Meningioma. Cancers 2024, 16, 3362. [Google Scholar] [CrossRef] [PubMed]
- Roland, N.; Neumann, A.; Hoisnard, L.; Duranteau, L.; Froelich, S.; Zureik, M.; Weill, A. Use of Progestogens and the Risk of Intracranial Meningioma: National Case-Control Study. BMJ 2024, 384, e078078. [Google Scholar] [CrossRef] [PubMed]
- Agopiantz, M.; Carnot, M.; Denis, C.; Martin, E.; Gauchotte, G. Hormone Receptor Expression in Meningiomas: A Systematic Review. Cancers 2023, 15, 980. [Google Scholar] [CrossRef] [PubMed]
- Hatiboglu, M.A.; Cosar, M.; Iplikcioglu, A.C.; Ozcan, D. Sex Steroid and Epidermal Growth Factor Profile of Giant Meningiomas Associated with Pregnancy. Surg. Neurol. 2008, 69, 356–362; discussion 362–363. [Google Scholar] [CrossRef] [PubMed]
- Guevara, P.; Escobar-Arriaga, E.; Saavedra-Perez, D.; Martinez-Rumayor, A.; Flores-Estrada, D.; Rembao, D.; Calderon, A.; Sotelo, J.; Arrieta, O. Angiogenesis and Expression of Estrogen and Progesterone Receptors as Predictive Factors for Recurrence of Meningioma. J. Neurooncol. 2010, 98, 379–384. [Google Scholar] [CrossRef]
- USDA. Rural-Urban Commuting Area Codes. Available online: https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/ (accessed on 17 December 2024).
- Ostrom, Q.T.; McCulloh, C.; Chen, Y.; Devine, K.; Wolinsky, Y.; Davitkov, P.; Robbins, S.; Cherukuri, R.; Patel, A.; Gupta, R.; et al. Family History of Cancer in Benign Brain Tumor Subtypes versus Gliomas. Front. Oncol. 2012, 2, 19. [Google Scholar] [CrossRef]
- Benson, V.S.; Pirie, K.; Green, J.; Casabonne, D.; Beral, V. Million Women Study Collaborators Lifestyle Factors and Primary Glioma and Meningioma Tumours in the Million Women Study Cohort. Br. J. Cancer 2008, 99, 185–190. [Google Scholar] [CrossRef]
- Schwartzbaum, J.; Jonsson, F.; Ahlbom, A.; Preston-Martin, S.; Lönn, S.; Söderberg, K.C.; Feychting, M. Cohort Studies of Association between Self-Reported Allergic Conditions, Immune-Related Diagnoses and Glioma and Meningioma Risk. Int. J. Cancer 2003, 106, 423–428. [Google Scholar] [CrossRef]
- Schlehofer, B.; Blettner, M.; Preston-Martin, S.; Niehoff, D.; Wahrendorf, J.; Arslan, A.; Ahlbom, A.; Choi, W.N.; Giles, G.G.; Howe, G.R.; et al. Role of Medical History in Brain Tumour Development. Results from the International Adult Brain Tumour Study. Int. J. Cancer 1999, 82, 155–160. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Adel Fahmideh, M.; Cote, D.J.; Muskens, I.S.; Schraw, J.M.; Scheurer, M.E.; Bondy, M.L. Risk Factors for Childhood and Adult Primary Brain Tumors. Neuro Oncol. 2019, 21, 1357–1375. [Google Scholar] [CrossRef]
- Schwartzbaum, J.; Jonsson, F.; Ahlbom, A.; Preston-Martin, S.; Malmer, B.; Lönn, S.; Söderberg, K.; Feychting, M. Prior Hospitalization for Epilepsy, Diabetes, and Stroke and Subsequent Glioma and Meningioma Risk. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Kuroi, Y.; Matsumoto, K.; Shibuya, M.; Kasuya, H. Progesterone Receptor Is Responsible for Benign Biology of Skull Base Meningioma. World Neurosurg. 2018, 118, e918–e924. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, F.; Montagnani, S.; Gallicchio, B. Estrogen and Progesterone Receptors in Meningiomas. Surg. Neurol. 1986, 26, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, G.; Aoyagi, M.; Wakimoto, H.; Tamaki, M.; Ohno, K.; Hirakawa, K. Immunohistochemical Detection of Progesterone Receptors and the Correlation with Ki-67 Labeling Indices in Paraffin-Embedded Sections of Meningiomas. Neurosurgery 1995, 37, 478–482; discussion 483. [Google Scholar] [CrossRef]
- Hsu, D.W.; Efird, J.T.; Hedley-Whyte, E.T. Progesterone and Estrogen Receptors in Meningiomas: Prognostic Considerations. J. Neurosurg. 1997, 86, 113–120. [Google Scholar] [CrossRef]
- Blankenstein, M.A.; Verheijen, F.M.; Jacobs, J.M.; Donker, T.H.; van Duijnhoven, M.W.; Thijssen, J.H. Occurrence, Regulation, and Significance of Progesterone Receptors in Human Meningioma. Steroids 2000, 65, 795–800. [Google Scholar] [CrossRef]
- Wolfsberger, S.; Doostkam, S.; Boecher-Schwarz, H.-G.; Roessler, K.; van Trotsenburg, M.; Hainfellner, J.A.; Knosp, E. Progesterone-Receptor Index in Meningiomas: Correlation with Clinico-Pathological Parameters and Review of the Literature. Neurosurg. Rev. 2004, 27, 238–245. [Google Scholar] [CrossRef]
- Pletzer, B.; Winkler-Crepaz, K.; Maria Hillerer, K. Progesterone and Contraceptive Progestin Actions on the Brain: A Systematic Review of Animal Studies and Comparison to Human Neuroimaging Studies. Front. Neuroendocrinol. 2023, 69, 101060. [Google Scholar] [CrossRef]
- Peyre, M.; Gaillard, S.; de Marcellus, C.; Giry, M.; Bielle, F.; Villa, C.; Boch, A.L.; Loiseau, H.; Baussart, B.; Cazabat, L.; et al. Progestin-associated shift of meningioma mutational landscape. Ann Oncol. 2018, 29, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Samuels, Y.; Diaz, L.A., Jr.; Schmidt-Kittler, O.; Cummins, J.M.; Delong, L.; Cheong, I.; Rago, C.; Huso, D.L.; Lengauer, C.; Kinzler, K.W.; et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005, 7, 561–573. [Google Scholar] [CrossRef] [PubMed]
- El-Habr, E.A.; Levidou, G.; Trigka, E.A.; Sakalidou, J.; Piperi, C.; Chatziandreou, I.; Spyropoulou, A.; Soldatos, R.; Tomara, G.; Petraki, K.; et al. Complex interactions between the components of the PI3K/AKT/mTOR pathway, and with components of MAPK, JAK/STAT and Notch-1 pathways, indicate their involvement in meningioma development. Virchows Arch. 2014, 465, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.L.; Cantley, L.C. PI3K pathway alterations in cancer: Variations on a theme. Oncogene 2008, 27, 5497–5510. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anzalone, C.L.; Glasgow, A.; Habermann, E.; Grossard, B.R.; Van Gompel, J.J.; Carlson, M.L. Geographical Differences in Intracranial Meningioma Management: Examining 65,973 Patients across the United States. J. Neurol. Surg. B Skull Base 2019, 80, 547–554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaiser Family Foundation. DMPA Contraception Injection: Use and Coverage. Available online: https://www.kff.org/womens-health-policy/fact-sheet/dmpa-contraceptive-injection-use-and-coverage/ (accessed on 26 August 2024).
| Topography | Histology (in Order of Descending Frequency) | |
|---|---|---|
| Cerebral meningioma cases | C70.0, C70.9 | 953 (100.0%) |
| Skin tumor controls * | C44.1, C44.2, C44.3, C44.4, C44.5, C44.6, C44.7, C44.9 | 872 (58.4%), 874 (37.7%), 877 (0.9%), 883 (0.7%), 824 (0.4%), 841 (0.4%), 970 (0.4%), 807 (0.2%), 820 (0.2%), 840 (0.2%), 878 (0.2%), 880 (0.2%), 912 (0.2%), 959 (0.2%) |
| Non-meningioma brain tumor controls * | C71.0, C71.1, C71.2, C71.3, C71.4, C71.5, C71.6, C71.7, C71.8, C71.9 | 944 (42.2%), 940 (23.5%), 938 (9.6%), 945 (8.0%), 950 (2.8%), 947 (2.4%), 968 (2.4%), 916 (2.0%), 939 (2.0%), 956 (2.0%), 942 (1.6%), 896 (0.4%), 912 (0.4%), 935 (0.4%), 936 (0.4%) |
| Breast tumor controls * | C50.0, C50.1, C50.2, C50.3, C50.4, C50.5, C50.6, C50.8, C50.9 | 850 (72.4%), 852 (21.0%), 821 (1.7%), 801 (0.9%), 820 (0.8%), 848 (0.8%), 814 (0.5%), 902 (0.4%), 823 (0.2%), 857 (0.2%), 853 (0.2%), 851 (0.1%), 854 (0.1%), 912 (0.1%), 800 (0.1%), 805 (0.1%), 825 (0.1%), 834 (0.1%), 880 (0.1%), 975 (0.1%) |
| Tumor Control Type (ICD-O-3 Topography Code) | |||||
|---|---|---|---|---|---|
| Cases (N = 241) | Controls (95% CI) * | Brain (C71) (95% CI) * | Breast (C50) (95% CI) * | Skin (C44) (95% CI) * | |
| Mean Age, Years | 59.6 | 59.3 (59.1–59.4) | 58.3 (58.1–58.5) | 59.7 (59.4–59.9) | 59.5 (59.3–59.8) |
| Race, % | |||||
| White | 58.6 | 78.8 (76.6–81.1) | 77.6 (74.9–80.2) | 63.7 (58.2–69.2) | 95.3 (93.5–97.0) |
| Non-White | 41.4 | 21.2 (18.9–23.4) | 22.4 (19.9–25.1) | 36.3 (30.8–41.8) | 4.7 (3.0–6.5) |
| Rural–Urban Commuting Area, % | |||||
| Metropolitan | 73.6 | 77.6(75.4–79.9) | 73.7 (71.0–76.6) | 81.4 (76.7–85.8) | 76.6 (73.1–80.1) |
| Micropolitan | 15.1 | 12.0 (10.2–13.7) | 12.7 (10.1–15.0) | 9.2 (5.9–12.6) | 14.3 (11.2–17.3) |
| Small Metro/Rural | 11.3 | 10.4 (8.7–12.1) | 13.6 (11.5–15.9) | 9.4 (5.9–12.9) | 9.1 (6.6–11.3) |
| Insurance type, % | |||||
| Medicaid/Medicare | 12.8 | 10.4 (8.6–12.3) | 10.0 (8.2–11.9) | 13.4 (9.2–17.6) | 7.7 (5.6–9.9) |
| Commercial | 71.4 | 78.6 (76.3–80.8) | 75.6 (73.0–78.5) | 75.7 (70.9–80.6) | 83.7 (80.6–86.9) |
| Other | 8.6 | 6.6 (5.2–8.0) | 7.4 (5.7–8.9) | 7.4 (4.6–10.5) | 5.0 (3.1–6.9) |
| Uninsured | 7.3 | 4.5 (3.4–5.6) | 7.0 (5.2–8.4) | 3.5 (1.7–5.9) | 3.5 (2.2–5.2) |
| Elixhauser Comorbidities | |||||
| Mean Unweighted Score | 2.3 | 1.7 (1.6–1.8) | 2.9 (2.7–3.0) | 1.4 (1.2–1.7) | 1.2 (1.1–1.3) |
| Category, % | |||||
| 0 Comorbidities | 36.4 | 43.2 (40.6–45.9) | 28.2 (25.4–30.8) | 49.8 (44.0–55.5) | 47.6 (43.4–52.0) |
| 1 Comorbidity | 15.9 | 24.9 (22.5–27.1) | 15.4 (13.2–17.7) | 24.7 (19.9–29.7) | 32.2 (28.2–35.9) |
| 2 Comorbidities | 18.0 | 11.8 (10.0–13.5) | 17.0 (14.8–19.1) | 10.7 (6.7–14.6) | 9.1 (6.8–11.6) |
| 3 Comorbidities | 11.3 | 6.9 (5.6–8.2) | 12.0 (9.8–14.1) | 5.6 (2.9–8.7) | 4.4 (2.6–6.1) |
| ≥4 Comorbidities | 18.4 | 13.2 (11.7–14.7) | 27.3 (25.6–30.1) | 9.2 (5.9–12.9) | 6.7 (4.7–8.6) |
| Exposure, % | dMPA Exposure vs. Comparator Exposure, OR (95% CI) | ||||
|---|---|---|---|---|---|
| Controls (95% CI) | Cases | Crude | Age- and Race- Adjusted | Fully Adjusted | |
| ANY EXPOSURE | |||||
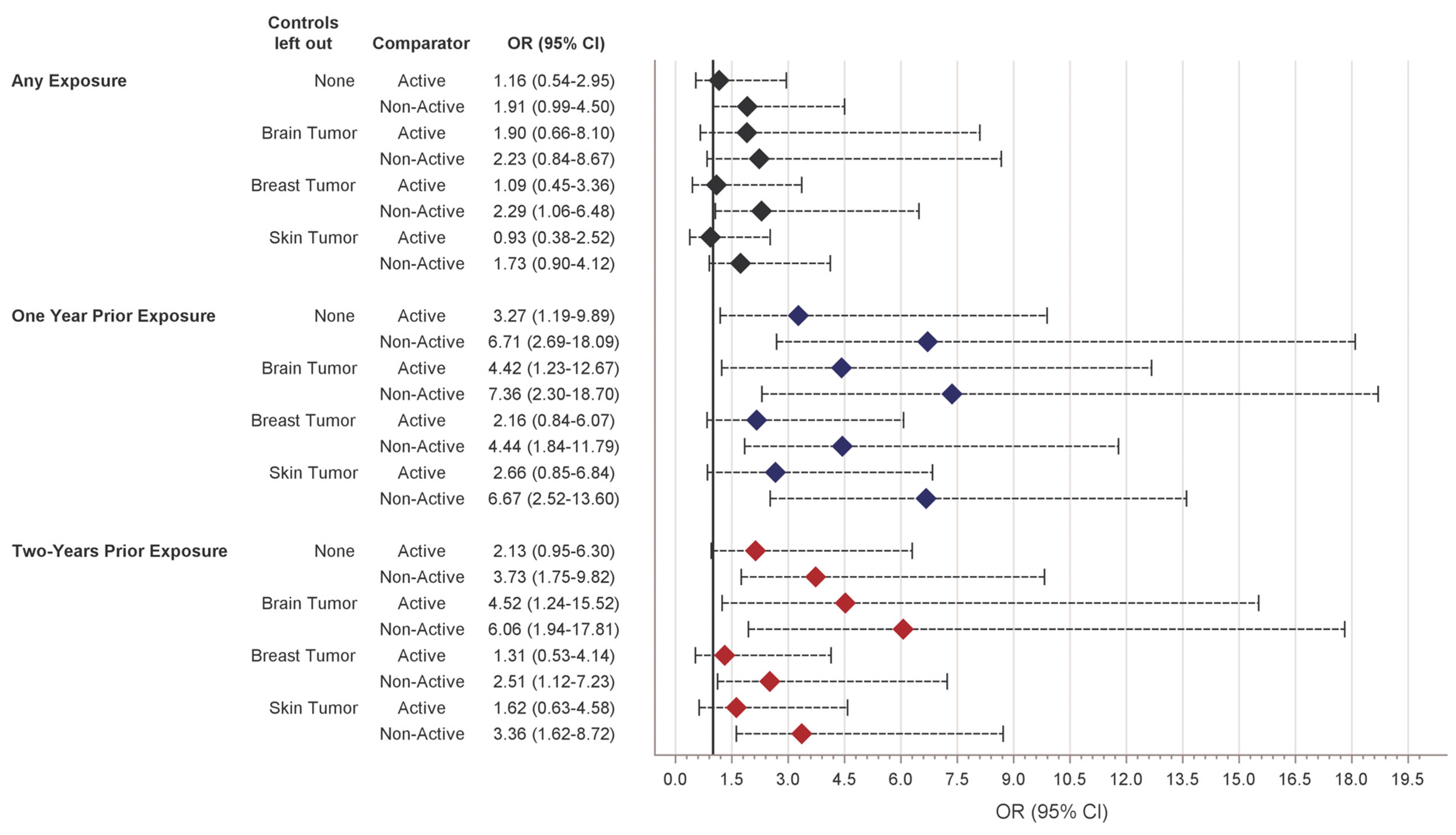
| dMPA | 1.1 (0.6–1.7) | 2.9 | - | - | - |
| Levonorgestrel/norethindrone | 5.4 (4.2–6.8) | 6.7 | 2.08 (1.17–4.26) | 1.25 (0.61–2.96) | 1.16 (0.54–2.95) |
| Non-active | 93.4 (92.0–95.0) | 90.4 | 2.75 (1.69–5.56) | 1.95 (1.07–4.26) | 1.91 (0.99–4.50) |
| ONE YEAR PRIOR EXPOSURE | |||||
| dMPA | 0.4 (0.2–0.8) | 2.9 | - | - | - |
| Levonorgestrel/norethindrone | 3.8 (2.8–4.9) | 5.4 | 5.52 (2.36–14.09) | 3.51 (1.37–9.28) | 3.27 (1.19–9.89) |
| Non-active | 95.8 (94.7–96.9) | 91.6 | 8.56 (3.91–19.01) | 6.73 (2.88–16.07) | 6.71 (2.69–18.09) |
| TWO YEARS PRIOR EXPOSURE | |||||
| dMPA | 0.7 (0.3–1.1) | 2.9 | - | - | - |
| Levonorgestrel/norethindrone | 4.3 (3.2–5.5) | 5.4 | 3.58 (1.88–8.00) | 2.27 (1.03–5.76) | 2.13 (0.95–6.30) |
| Non-active | 95.0 (93.8–96.2) | 91.6 | 4.83 (2.72–9.45) | 3.79 (1.93–8.92) | 3.73 (1.75–9.82) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griffin, R.; Arend, R. A Matched Case-Control Study Examining the Association Between Exposure to Depot Medroxyprogesterone Acetate and Cerebral Meningioma Using an Active Comparator. Curr. Oncol. 2025, 32, 401. https://doi.org/10.3390/curroncol32070401
Griffin R, Arend R. A Matched Case-Control Study Examining the Association Between Exposure to Depot Medroxyprogesterone Acetate and Cerebral Meningioma Using an Active Comparator. Current Oncology. 2025; 32(7):401. https://doi.org/10.3390/curroncol32070401
Chicago/Turabian StyleGriffin, Russell, and Rebecca Arend. 2025. "A Matched Case-Control Study Examining the Association Between Exposure to Depot Medroxyprogesterone Acetate and Cerebral Meningioma Using an Active Comparator" Current Oncology 32, no. 7: 401. https://doi.org/10.3390/curroncol32070401
APA StyleGriffin, R., & Arend, R. (2025). A Matched Case-Control Study Examining the Association Between Exposure to Depot Medroxyprogesterone Acetate and Cerebral Meningioma Using an Active Comparator. Current Oncology, 32(7), 401. https://doi.org/10.3390/curroncol32070401





