Optimizing Adjuvant Care in Early Breast Cancer: Multidisciplinary Strategies and Innovative Models from Canadian Centers
Simple Summary
Abstract
1. Introduction
Objective
2. Nurse and NP-Led Care Models
2.1. Overview
2.2. What Are Some Examples of Nurse- and NP-Led Care Models?
2.3. What Are the Benefits and Challenges of Nurse- and NP-Led Care Models?
2.4. What Are Some Recommendations for Practice?
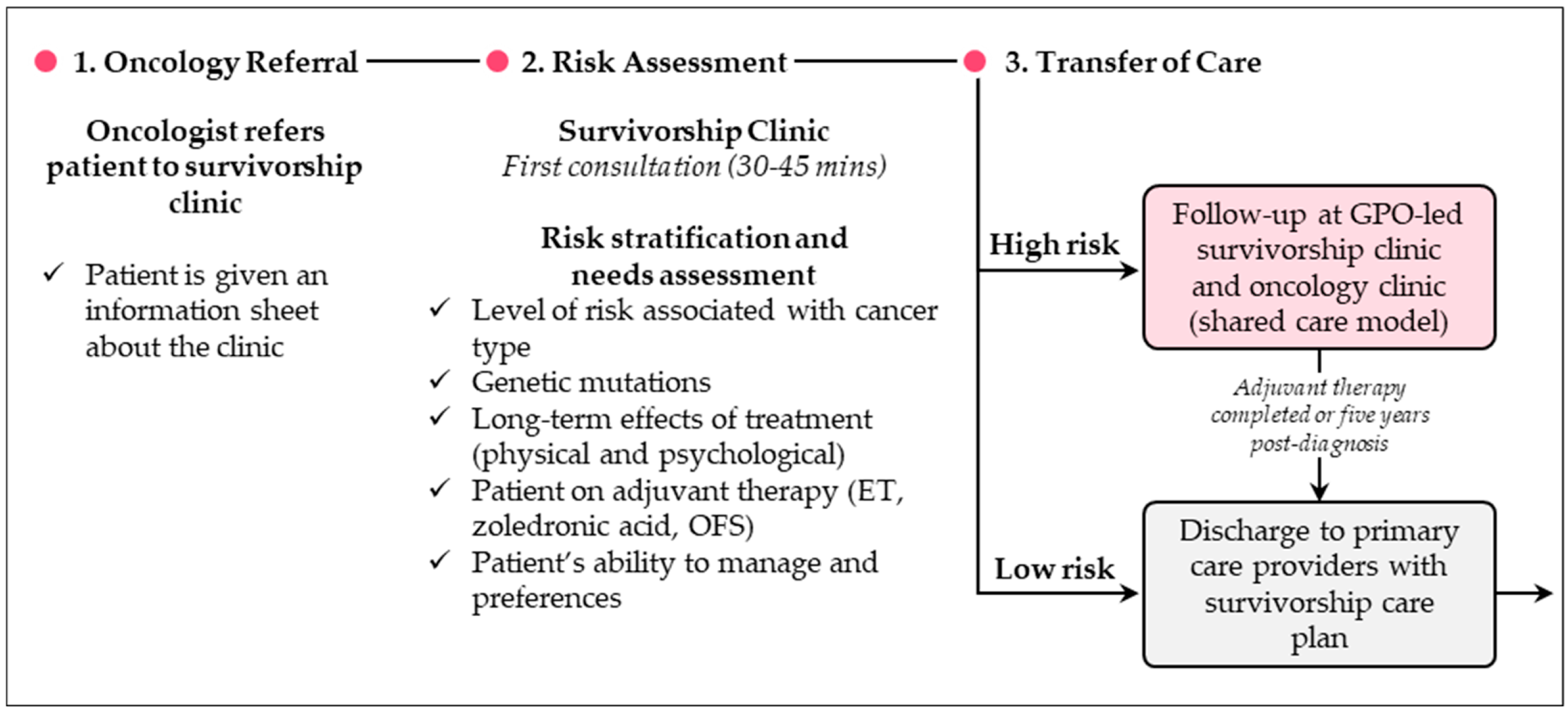
3. GPO-Led Care Models
3.1. Overview
3.2. What Are Some Examples of GPO-Led Care Models?
3.3. What Are the Benefits and Challenges of GPO-Led Care Models?
3.4. What Are Some Recommendations for Practice?
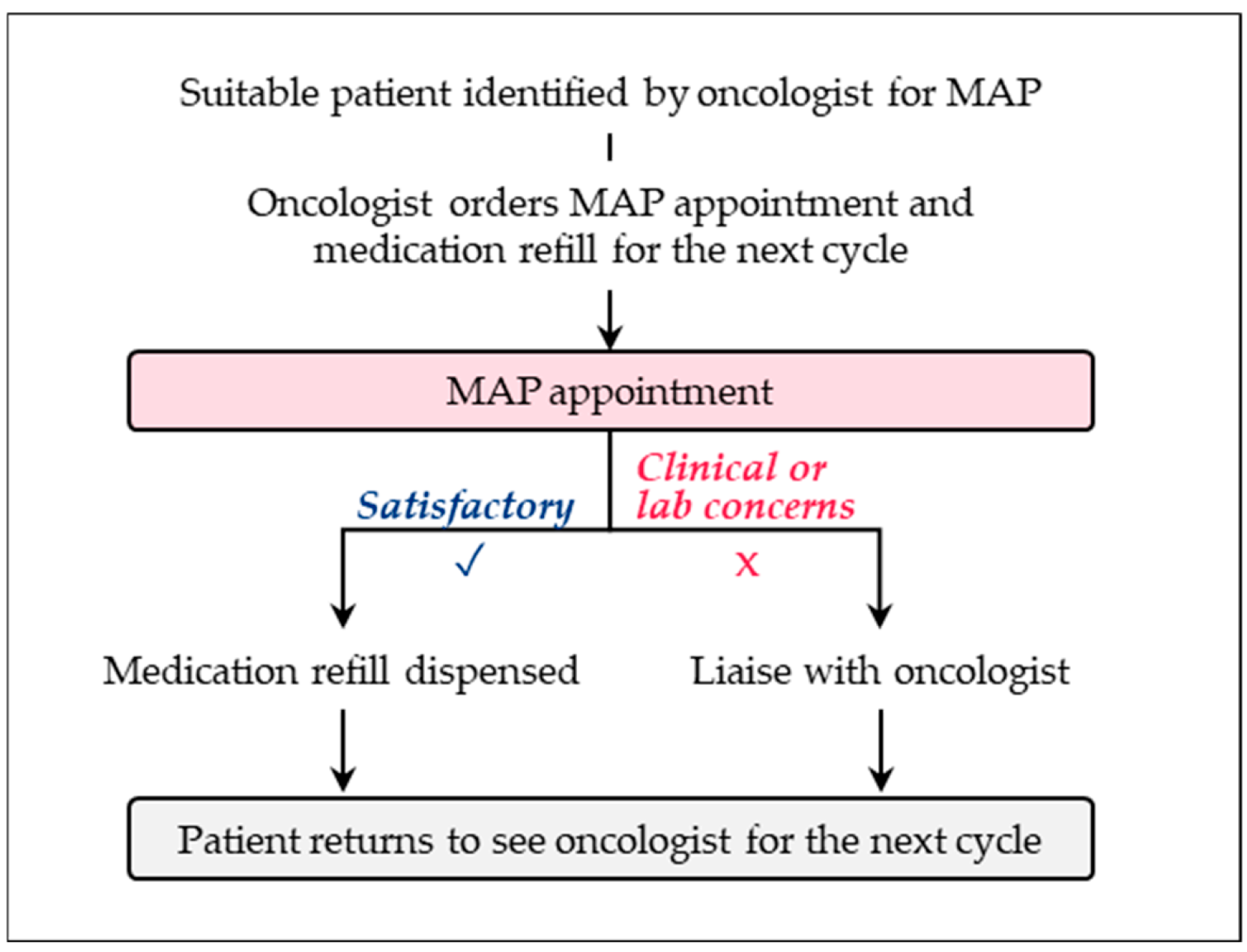
4. Pharmacist-Led Care Models
4.1. Overview
4.2. What Are Some Examples of Pharmacist-Led Care Models?
4.3. What Are the Benefits and Challenges of Pharmacist-Led Models?
4.4. What Are Some Recommendations for Practice?
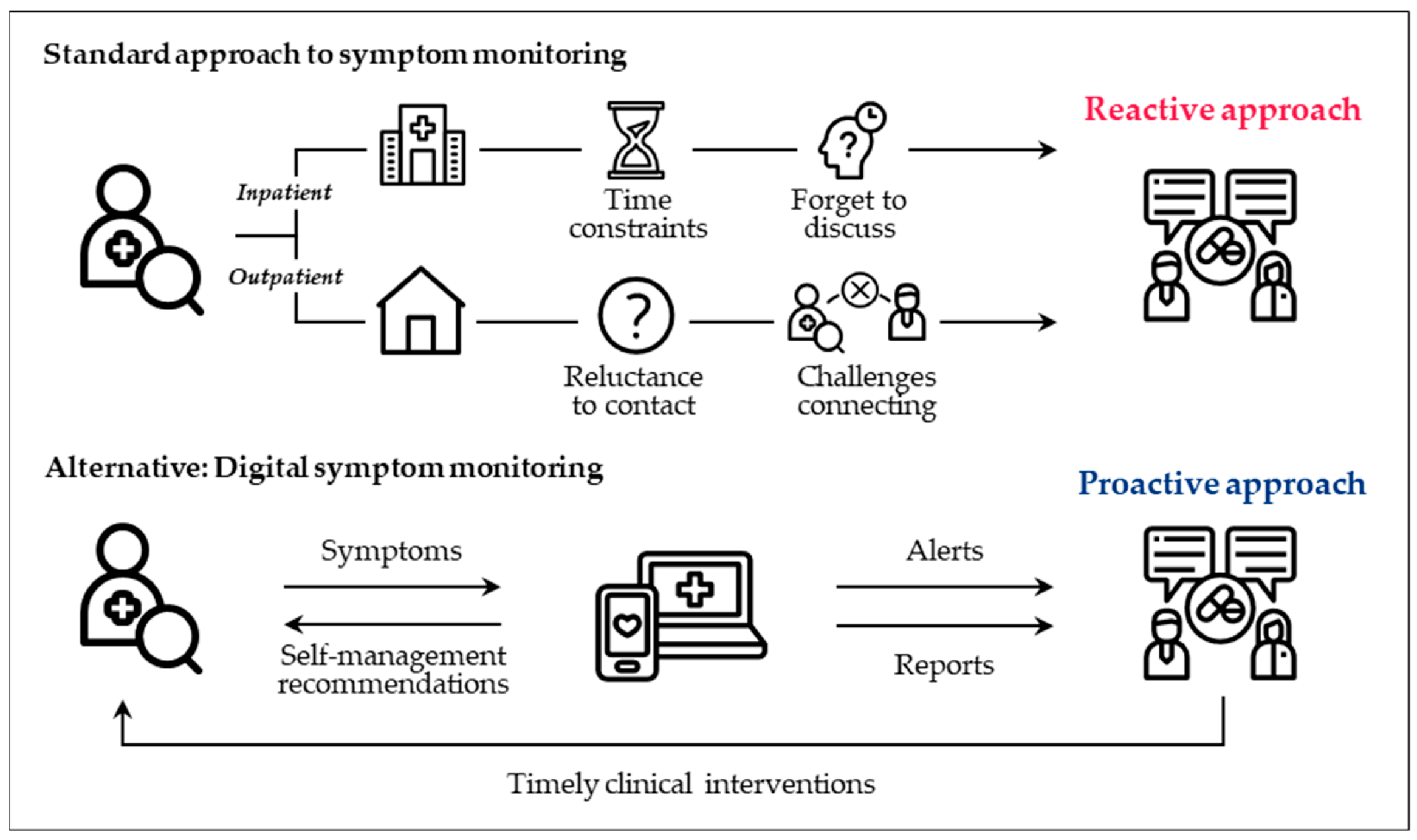
5. Digital Health Tools
5.1. Overview
5.2. What Are Some Implementation Examples of Digital Health Tools?
5.3. What Are the Benefits and Challenges of Digital Health Tools?
5.4. What Are Some Recommendations for Practice?
6. Discussion
6.1. How Should Patients Be Transitioned to an Innovative Care Model?
6.2. What Should Be the Role of the Medical Oncologist?
6.3. What Are Some Recommendations for Monitoring and Managing Adverse Events?
6.4. How Do We Ensure Quality of Care?
6.5. What Are Some Future Directions for Innovative Models of Care?
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHS | Alberta Health Services |
| ASCO | American Society of Clinical Oncology |
| BCTH | Bloods closer to home |
| cpKPI | Clinical pharmacy key performance indicator |
| DRP | Drug-related problem |
| EBC | Early breast cancer |
| EMR | Electronic medical record |
| ER | Emergency room |
| GPO | General practitioner in oncology |
| HER2– | Human epidermal growth factor receptor 2-negative |
| HR+ | Hormone receptor-positive |
| HRQoL | Health-related quality of life |
| ITT | Intent-to-treat |
| MAP | Medication Assessment by Pharmacists |
| NCODA | National Community Oncology Dispensing Association |
| NP | Nurse practitioner |
| OAM | Oral anticancer medication |
| OFS | Ovarian function suppression |
| PDSA | Plan-do-study-act |
| PEPPA | Participatory, evidence-based, patient-focused process for advanced practice nursing |
| PRO | Patient-reported outcome |
| SARO-MAVO | Suivi Actif des Résultats rapportés par le patient en Oncologie—Médicaments Anti-néoplasiques administrés par Voie Orale |
| SCP | Survivorship care plan |
| SURC | Symptom and urgent review clinic |
References
- Howlader, N.; Altekruse, S.F.; Li, C.I.; Chen, V.W.; Clarke, C.A.; Ries, L.A.G.; Cronin, K.A. US Incidence of Breast Cancer Subtypes Defined by Joint Hormone Receptor and HER2 Status. J. Natl. Cancer Inst. 2014, 106, dju055. [Google Scholar] [CrossRef]
- Iqbal, J.; Ginsburg, O.; Rochon, P.A.; Sun, P.; Narod, S.A. Differences in Breast Cancer Stage at Diagnosis and Cancer-Specific Survival by Race and Ethnicity in the United States. JAMA 2015, 313, 165–173. [Google Scholar] [CrossRef]
- Brenner, D.R.; Gillis, J.; Demers, A.A.; Ellison, L.F.; Billette, J.-M.; Zhang, S.X.; Liu, J.L.; Woods, R.R.; Finley, C.; Ma, N.F.; et al. Projected Estimates of Cancer in Canada in 2024. Cent. Nerv. Syst. 2024, 196, E615–E623. [Google Scholar] [CrossRef]
- Johnston, S.R.D.; Harbeck, N.; Hegg, R.; Toi, M.; Martin, M.; Shao, Z.M.; Zhang, Q.Y.; Martinez Rodriguez, J.L.; Campone, M.; Hamilton, E.; et al. Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2−, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J. Clin. Oncol. 2020, 38, 3987–3998. [Google Scholar] [CrossRef]
- Slamon, D.; Lipatov, O.; Nowecki, Z.; McAndrew, N.; Kukielka-Budny, B.; Stroyakovskiy, D.; Yardley, D.A.; Huang, C.-S.; Fasching, P.A.; Crown, J.; et al. Ribociclib plus Endocrine Therapy in Early Breast Cancer. N. Engl. J. Med. 2024, 390, 1080–1091. [Google Scholar] [CrossRef]
- Tarantino, P.; Jin, Q.; Mittendorf, E.A.; King, T.A.; Curigliano, G.; Tolaney, S.M. Clinical and Pathological Features of Breast Cancer Patients Eligible for Adjuvant Abemaciclib. Ann. Oncol. 2022, 33, 845–847. [Google Scholar] [CrossRef]
- Caswell-Jin, J.L.; Freedman, R.A.; Hassett, M.J.; Tang, H.; Garrett-Mayer, E.; Somerfield, M.R.; Giordano, S.H. Optimal Adjuvant Chemotherapy and Targeted Therapy for Early Breast Cancer—CDK4/6 Inhibitors: ASCO Rapid Guideline Update Clinical Insights. JCO Oncol. Pract. 2024, 27, OP-24-00663. [Google Scholar] [CrossRef]
- Tarantino, P.; Rugo, H.S.; Curigliano, G.; O’Shaughnessy, J.; Janni, W.; Jhaveri, K.; Mouabbi, J.A.; Brufsky, A.M.; Hamilton, E.P.; ORegan, R.; et al. 245P Characteristics of Real-World (RW) NATALEE and monarchE Eligible Populations: A US Electronic Health Records (EHR) Database Analysis. Ann. Oncol. 2024, 35, S317–S318. [Google Scholar] [CrossRef]
- Garattini, S.K.; Valent, F.; Minisini, A.; Riosa, C.; Favaretti, C.; Regattin, L.; Fasola, G. Analysis of Workload Generated in the Two Years Following First Consultation by Each New Cancer Patient: Studying the Past to Plan the Future of Cancer Care. BMC Health Serv. Res. 2022, 22, 1184. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. CA. Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Lawton, K.; Royals, K.; Carson-Chahhoud, K.V.; Campbell, F.; Smith, B.J. Nurse-Led versus Doctor-Led Care for Bronchiectasis. Cochrane Database Syst. Rev. 2018, 2018, CD004359. [Google Scholar] [CrossRef]
- Oatley, M.; Fry, M. A Nurse Practitioner-Led Model of Care Improves Access, Early Assessment and Integration of Oncology Services: An Evaluation Study. Support. Care Cancer 2020, 28, 5023–5029. [Google Scholar] [CrossRef] [PubMed]
- Bryant-Lukosius, D.; Cosby, R.; Bakker, D.; Earle, C.; Fitzgerald, B.; Burkovski, V. Effective Use of Advanced Practice Nurses in the Delivery of Adult Cancer Services in Ontario. In Program in Evidence-Based Care Guideline No.: 16-4; Cancer Care Ontario: Toronto, ON, Canada, 2015. [Google Scholar]
- Jefford, M.; Howell, D.; Li, Q.; Lisy, K.; Maher, J.; Alfano, C.M.; Rynderman, M.; Emery, J. Improved Models of Care for Cancer Survivors. Lancet 2022, 399, 1551–1560. [Google Scholar] [CrossRef]
- LeBar, K. Care Models Preventing Readmission. Semin. Oncol. Nurs. 2020, 36, 151021. [Google Scholar] [CrossRef]
- Jefford, M.; Emery, J.D.; James Martin, A.; De Abreu Lourenco, R.; Lisy, K.; Grunfeld, E.; Mohamed, M.A.; King, D.; Tebbutt, N.C.; Lee, M.; et al. SCORE: A Randomised Controlled Trial Evaluating Shared Care (General Practitioner and Oncologist) Follow-up Compared to Usual Oncologist Follow-up for Survivors of Colorectal Cancer. eClinicalMedicine 2023, 66, 102346. [Google Scholar] [CrossRef]
- Trabjerg, T.B.; Jensen, L.H.; Søndergaard, J.; Sisler, J.J.; Hansen, D.G. Improving Continuity by Bringing the Cancer Patient, General Practitioner and Oncologist Together in a Shared Video-Based Consultation—Protocol for a Randomised Controlled Trial. BMC Fam. Pract. 2019, 20, 86. [Google Scholar] [CrossRef]
- Chaput, G.; Lilly, E. Oncologists and General Practitioners in Oncology: Allies in Cancer Care Delivery. Curr. Oncol. 2020, 27, 6–7. [Google Scholar] [CrossRef]
- Pape, Z.A.; Hale, G.; Joseph, T.; Moreau, C.; Wolowich, W.R. Impact of Pharmacist-Led Heart Failure Tool Kits on Patient-Reported Self-Care Behaviors in a Primary Care–Based Accountable Care Organization. J. Am. Pharm. Assoc. 2019, 59, 891–895.e3. [Google Scholar] [CrossRef]
- Ngo, N.U.T.; Tangpraphaphorn, S.; Kahaku, D.; Canamar, C.P.; Young, A. Clinical Pharmacist Transition of Care Model Improves Hospital System Practice by Reducing Readmissions. J. Heal. Qual. 2023, 45, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Strumia, M.; Fargeas, J.-B.; Marcellaud, E.; Del, M.; Dintilhac, A.; Remenieras, L.; Dmytruck, N.; Moreau, S.; Jaccard, A.; Jost, J. Development of a Decision Tree for the Pharmacy-Led Consultation of Elderly Patients with Haematological Malignancies. J. Oncol. Pharm. Pract. 2023, 29, 685–694. [Google Scholar] [CrossRef]
- Valgus, J.; Jarr, S.; Schwartz, R.; Rice, M.; Bernard, S.A. Pharmacist-Led, Interdisciplinary Model for Delivery of Supportive Care in the Ambulatory Cancer Clinic Setting. J. Oncol. Pract. 2010, 6, e1–e4. [Google Scholar] [CrossRef]
- Cossette, B.; Ricard, G.; Poirier, R.; Gosselin, S.; Langlois, M.-F.; Imbeault, P.; Breton, M.; Couturier, Y.; Sirois, C.; Lessard-Beaudoin, M.; et al. Pharmacist-Led Transitions of Care between Hospitals, Primary Care Clinics, and Community Pharmacies. J. Am. Geriatr. Soc. 2022, 70, 766–776. [Google Scholar] [CrossRef]
- Segal, E.M.; Bates, J.; Fleszar, S.L.; Holle, L.M.; Kennerly-Shah, J.; Rockey, M.; Jeffers, K.D. Demonstrating the Value of the Oncology Pharmacist within the Healthcare Team. J. Oncol. Pharm. Pract. 2019, 25, 1945–1967. [Google Scholar] [CrossRef]
- Randolph, L.A.; Walker, C.K.; Nguyen, A.T.; Zachariah, S.R. Impact of Pharmacist Interventions on Cost Avoidance in an Ambulatory Cancer Center. J. Oncol. Pharm. Pract. 2018, 24, 3–8. [Google Scholar] [CrossRef]
- Shih, S.T.F.; Mellerick, A.; Akers, G.; Whitfield, K.; Moodie, M. Economic Assessment of a New Model of Care to Support Patients With Cancer Experiencing Cancer- and Treatment-Related Toxicities. JCO Oncol. Pract. 2020, 16, e884–e892. [Google Scholar] [CrossRef]
- Chan, R.J.; Crawford-Williams, F.; Crichton, M.; Joseph, R.; Hart, N.H.; Milley, K.; Druce, P.; Zhang, J.; Jefford, M.; Lisy, K.; et al. Effectiveness and Implementation of Models of Cancer Survivorship Care: An Overview of Systematic Reviews. J. Cancer Surviv. 2023, 17, 197–221. [Google Scholar] [CrossRef]
- Bartakova, J.; Zúñiga, F.; Guerbaai, R.-A.; Basinska, K.; Brunkert, T.; Simon, M.; Denhaerynck, K.; De Geest, S.; Wellens, N.I.H.; Serdaly, C.; et al. Health Economic Evaluation of a Nurse-Led Care Model from the Nursing Home Perspective Focusing on Residents’ Hospitalisations. BMC Geriatr. 2022, 22, 496. [Google Scholar] [CrossRef]
- Lai, X.B.; Ching, S.S.Y.; Wong, F.K.Y.; Leung, C.W.Y.; Lee, L.H.; Wong, J.S.Y.; Lo, Y.F. A Nurse-Led Care Program for Breast Cancer Patients in a Chemotherapy Day Center: A Randomized Controlled Trial. Cancer Nurs. 2019, 42, 20–34. [Google Scholar] [CrossRef]
- Dufton, P.H.; Tarasenko, E.; Midgley, K.; Lee, K.; Kelly, R.; Rodrigues, J.; Yates, P.; Arulananda, S.; Parakh, S. Implementation of a Nurse-Led, Multidisciplinary Model of Care for Older Adults with Cancer: A Process Evaluation Protocol. BMJ Open 2024, 14, e077005. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.S. An Oncology NP-MD Partnership: Challenges and Rewards. J. Adv. Pract. Oncol. 2018, 9, 430–433. [Google Scholar] [PubMed]
- Cooper, J.M.; Loeb, S.J.; Smith, C.A. The Primary Care Nurse Practitioner and Cancer Survivorship Care. J. Am. Acad. Nurse Pract. 2010, 22, 394–402. [Google Scholar] [CrossRef]
- Peacock, M.; Hernandez, S. A Concept Analysis of Nurse Practitioner Autonomy. J. Am. Assoc. Nurse Pract. 2020, 32, 113–119. [Google Scholar] [CrossRef]
- MacLeod, A.; Branch, A.; Cassidy, J.; McDonald, A.; Mohammed, N.; MacDonald, L. A Nurse-/Pharmacy-Led Capecitabine Clinic for Colorectal Cancer: Results of a Prospective Audit and Retrospective Survey of Patient Experiences. Eur. J. Oncol. Nurs. Off. J. Eur. Oncol. Nurs. Soc. 2007, 11, 247–254. [Google Scholar] [CrossRef]
- Graze, L.; Brady-Copertino, C.; Varner, A.; Stiver, W.S. The Development of a Nursing Assessment and Symptom Management Clinic. Clin. J. Oncol. Nurs. 2014, 18, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Saltbæk, L.; Bidstrup, P.E.; Karlsen, R.V.; Høeg, B.L.; Horsboel, T.A.; Belmonte, F.; Andersen, E.A.W.; Zoffmann, V.; Friberg, A.S.; Svendsen, M.N.; et al. Nurse-Led Individualized Follow-Up Versus Regular Physician-Led Visits After Early Breast Cancer (MyHealth): A Phase III Randomized, Controlled Trial. J. Clin. Oncol. 2024, 42, 2038–2049. [Google Scholar] [CrossRef]
- Gyldenvang, H.H.; Christiansen, M.G.; Jarden, M.; Piil, K. Experiences and Perspectives of Patients and Clinicians in Nurse-Led Clinics in an Oncological Setting: A Sequential Multi-Methods Study. Eur. J. Oncol. Nurs. 2022, 61, 102203. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Malik, G.; Murray, C. Nurses’ Experiences and Perceptions of Running Nurse-led Clinics: A Scoping Review. Int. J. Nurs. Pract. 2024, 30, e13285. [Google Scholar] [CrossRef]
- Grunfeld, E.; Levine, M.N.; Julian, J.A.; Coyle, D.; Szechtman, B.; Mirsky, D.; Verma, S.; Dent, S.; Sawka, C.; Pritchard, K.I.; et al. Randomized Trial of Long-Term Follow-up for Early-Stage Breast Cancer: A Comparison of Family Physician versus Specialist Care. J. Clin. Oncol. 2006, 24, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Vanhuyse, M.; Bedard, P.L.; Sheiner, J.; Fitzgerald, B.; Clemons, M. Transfer of Follow-up Care to Family Physicians for Early-Stage Breast Cancer. Clin. Oncol. 2007, 19, 172–176. [Google Scholar] [CrossRef]
- Thind, A.; Liu, Y.; Maly, R.C. Patient Satisfaction with Breast Cancer Follow-up Care Provided by Family Physicians. J. Am. Board Fam. Med. 2011, 24, 710–716. [Google Scholar] [CrossRef]
- Renton, J.P.; Twelves, C.J.; Yuille, F.A.P. Follow-up in Women with Breast Cancer: The Patients’ Perspective. Breast Edinb. Scotl. 2002, 11, 257–261. [Google Scholar] [CrossRef]
- Kerrigan, D.; Waters, P.; Ryan, M.; Irfan, M.; Hanaghan, J.; Khan, W.; Kerin, M.J.; Barry, K. Follow-up Arrangements for Breast Cancer Patients; Is It Appropriate to Transfer Surveillance to General Practitioners? Ir. Med. J. 2014, 107, 273–275. [Google Scholar] [PubMed]
- Alkhaifi, M.; Zhang, E.; Peera, M.; Jerzak, K.; Czarnota, G.; Eisen, A.; Roberts, A.; Carmona-Gonzalez, C.A.; Pezo, R.; Gandhi, S. Risk Factors for Treatment Toxicity and High Side Effect Burden Among Breast Cancer Survivors: A Retrospective Chart Review. Cancers 2025, 17, 328. [Google Scholar] [CrossRef]
- Peera, M. To Close the Gap of Survivorship Education: A Series of Breast Cancer Webinars in Collaboration with Wellspring. Obstet. Gynecol. Reprod. Sci. 2024, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Passey, D.G.; Healy, R.; Qualls, J.; Halwani, A.; Sauer, B.C. Pharmacist-Led Collaborative Medication Management Programs for Oral Antineoplastic Therapies: A Systematic Literature Review. J. Am. Pharm. Assoc. 2021, 61, e7–e18. [Google Scholar] [CrossRef] [PubMed]
- Marineau, A.; St-Pierre, C.; Lessard-Hurtubise, R.; David, M.-È.; Adam, J.-P.; Chabot, I. Cyclin-Dependent Kinase 4/6 Inhibitor Treatment Use in Women Treated for Advanced Breast Cancer: Integrating ASCO/NCODA Patient-Centered Standards in a Community Pharmacy. J. Oncol. Pharm. Pract. 2023, 29, 1144–1153. [Google Scholar] [CrossRef]
- Muluneh, B.; Schneider, M.; Faso, A.; Amerine, L.; Daniels, R.; Crisp, B.; Valgus, J.; Savage, S. Improved Adherence Rates and Clinical Outcomes of an Integrated, Closed-Loop, Pharmacist-Led Oral Chemotherapy Management Program. J. Oncol. Pract. 2018, 14, e324–e334. [Google Scholar] [CrossRef]
- Conliffe, B.; Figg, L.; Moffett, P.; Lauterwasser, L.; Parsons, L.B. Impact of a Formal Pharmacist-Run Oral Antineoplastic Monitoring Program: A Pilot Study in an Adult Genitourinary Oncology Clinic. J. Oncol. Pharm. Pract. 2019, 25, 777–786. [Google Scholar] [CrossRef]
- Brown, N.L.; Tivey, A.; Frank, S.; Phua, S.C.; Johnson, J.E.; Armstrong, A.; Wilson, C.; Raby, S.; Low, S.; Hulmes, Y.; et al. Development and Evaluation of a Remote Monitoring Regional Adjuvant Abemaciclib Service for Patients With High-Risk Early Breast Cancer. Clin. Breast Cancer 2024, 25, 368–379.e8. [Google Scholar] [CrossRef]
- Edwards, S.; Wood, L.; McCarthy, J.S.; Collins, A.; Hartery, L.; Abbott, R.; Whelan, M. Pharmacist-Led Monitoring Program for Patients on Sunitinib for Metastatic Renal-Cell Carcinoma: A Canadian Experience. J. Clin. Oncol. 2014, 32, 479. [Google Scholar] [CrossRef]
- Tibensky, B.; Hutton, L.; Wentzell, J.; LeBlanc, M.; Edwards, S.; McFarlane, T. Clinical Pharmacy Services in Ambulatory Oncology: An Environmental Scan of the Canadian Practice Landscape. Can. J. Hosp. Pharm. 2022, 75, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Peragine, C.; Thawer, A.; Michalopoulos, M.; Shloush, J.; Doherty, M.; Menjak, I.; Cheng, S.; Wasson, K.; Morar, R.; Sayed, Z.; et al. Multidisciplinary Lung Cancer Care Pathway for EGFR-Positive Advanced Non-Small Cell Lung Cancer Patients at the Sunnybrook Odette Cancer Centre: A Process Map. Available online: https://www.metrodis.org/uploads/document_library/8b47289575a339b9d0c218704253c806.pdf (accessed on 1 December 2024).
- Cheema, P.K.; Thawer, A.; Leake, J.; Cheng, S.Y.; Khanna, S.; Charles Victor, J. Multi-Disciplinary Proactive Follow-up Algorithm for Patients with Advanced NSCLC Receiving Afatinib. Support. Care Cancer 2019, 27, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Rico, V.; Thawer, A.; DeAngelis, C.; Peragine, C. 27A Patient Satisfaction and Experience with Oral Anticancer Medication Pharmacy Services at the Sunnybrook Odette Cancer Centre: A Cross-Sectional Survey Study. J. Oncol. Pharm. Pract. 2021, 27, 1–12. [Google Scholar] [CrossRef]
- Goh, E.; Labelle, S.; Chan, A. Implementation and Evaluation of a Shared Care Model between Oncologists and Pharmacists for Breast Cancer Patients at a Canadian Regional Ambulatory Cancer Centre. J. Oncol. Pharm. Pract. 2024, 30, 622–627. [Google Scholar] [CrossRef]
- Goh, E.; Labelle, S.; Chan, A. BC Cancer, Surrey, BC, Canada. 2025; manuscript in preparation. [Google Scholar]
- Edwards, S.J.; Abbott, R.; Edwards, J.; LeBlanc, M.; Dranitsaris, G.; Donnan, J.; Laing, K.; Whelan, M.A.; MacKinnon, N.J. Outcomes Assessment of a Pharmacist-Directed Seamless Care Program in an Ambulatory Oncology Clinic. J. Pharm. Pract. 2014, 27, 46–52. [Google Scholar] [CrossRef]
- Edwards, S.; Abbott, R.; Dranitsaris, G. Patient Monitoring Programs in Oncology Pharmacy Practice: A Survey of Oncology Pharmacists in Atlantic Canada. J. Oncol. Pharm. Pract. 2018, 25, 891–895. [Google Scholar] [CrossRef]
- Minard, L.; Phonchareon, N.; Scott, S.; Daniels, A.; Harding, C. 17A Development and Implementation of a New Pharmacy Oncology Clinic in Halifax. J. Oncol. Pharm. Pract. 2023, 29, S1–S16. [Google Scholar] [CrossRef]
- Minard, L.; Phonchareon, N.; Scott, S.; Toulany, N.; Daniels, A. 217A Development and Implementation of a Pharmacist-Led Gynecology Oncology Poly (ADP-Ribose) Polymerase Inhibitor Clinic in Nova Scotia Health, Central Zone. J. Oncol. Pharm. Pract. 2024, 30, S1–S16. [Google Scholar] [CrossRef]
- Interprofessional Programs Top Project 2025. Available online: https://hope-awards.com/interprofessional-programs-top-project-2025/ (accessed on 4 April 2025).
- Underhill, H.; LeBlanc, M.; Macfarlane, R.; Hutton, L. Using Quality Improvement Frameworks to Develop, Implement, and Evaluate a Novel Ambulatory Oncology Pharmacy Practice Model: A Descriptive Example. Am. J. Health. Syst. Pharm. 2024, 81, 1297–1304. [Google Scholar] [CrossRef]
- Chan, A.; Labelle, S.; Goh, E. Abstract P1-18-23: Implementation and Evaluation of a Shared Care Model between Oncologists and Pharmacists for Advanced Breast Cancer Patients on Cyclin-Dependent Kinase (CDK) 4/6 Inhibitors. Cancer Res. 2022, 82, P1-18-23. [Google Scholar] [CrossRef]
- Arriola, E.; Jaal, J.; Edvardsen, A.; Silvoniemi, M.; Araújo, A.; Vikström, A.; Zairi, E.; Rodriguez-Mues, M.C.; Roccato, M.; Schneider, S.; et al. Feasibility and User Experience of Digital Patient Monitoring for Real-World Patients With Lung or Breast Cancer. The Oncologist 2024, 29, e561–e569. [Google Scholar] [CrossRef] [PubMed]
- Oldhoff-Nuijsink, C.; Derksen, M.E.; Engelsma, T.; Peute, L.W.P.; Fransen, M.P. Digital Tools to Support Informed Decision Making among Screening Invitees in a Vulnerable Position for Population-Based Cancer Screening: A Scoping Review. Int. J. Med. Inf. 2024, 192, 105625. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, F.; D’Amico, S.; Zazzetti, E.; Gaudio, M.; Benvenuti, C.; Saltalamacchia, G.; Gerosa, R.; Gentile, D.; Lasagna, A.; Pedrazzoli, P.; et al. Digital Innovations in Breast Cancer Care: Exploring the Potential and Challenges of Digital Therapeutics and Clinical Decision Support Systems. Digit. Health 2024, 10, 20552076241288821. [Google Scholar] [CrossRef]
- Fromme, E.K.; Eilers, K.M.; Mori, M.; Hsieh, Y.-C.; Beer, T.M. How Accurate Is Clinician Reporting of Chemotherapy Adverse Effects? A Comparison with Patient-Reported Symptoms from the Quality-of-Life Questionnaire C30. J. Clin. Oncol. 2004, 22, 3485–3490. [Google Scholar] [CrossRef]
- Laugsand, E.A.; Sprangers, M.A.G.; Bjordal, K.; Skorpen, F.; Kaasa, S.; Klepstad, P. Health Care Providers Underestimate Symptom Intensities of Cancer Patients: A Multicenter European Study. Health Qual. Life Outcomes 2010, 8, 104. [Google Scholar] [CrossRef]
- Basch, E.; Iasonos, A.; McDonough, T.; Barz, A.; Culkin, A.; Kris, M.G.; Scher, H.I.; Schrag, D. Patient versus Clinician Symptom Reporting Using the National Cancer Institute Common Terminology Criteria for Adverse Events: Results of a Questionnaire-Based Study. Lancet Oncol. 2006, 7, 903–909. [Google Scholar] [CrossRef]
- Basch, E.; Schrag, D.; Jansen, J.; Henson, S.; Ginos, B.; Stover, A.M.; Carr, P.; Spears, P.A.; Jonsson, M.; Deal, A.M.; et al. Symptom Monitoring with Electronic Patient-Reported Outcomes during Cancer Treatment: Final Results of the PRO-TECT Cluster-Randomized Trial. Nat. Med. 2025, 31, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Denis, F.; Basch, E.; Septans, A.-L.; Bennouna, J.; Urban, T.; Dueck, A.C.; Letellier, C. Two-Year Survival Comparing Web-Based Symptom Monitoring vs Routine Surveillance Following Treatment for Lung Cancer. JAMA 2019, 321, 306–307. [Google Scholar] [CrossRef]
- Basch, E.; Leahy, A.B.; Dueck, A.C. Benefits of Digital Symptom Monitoring With Patient-Reported Outcomes During Adjuvant Cancer Treatment. J. Clin. Oncol. 2021, 39, 701–703. [Google Scholar] [CrossRef]
- Basch, E.; Deal, A.M.; Dueck, A.C.; Scher, H.I.; Kris, M.G.; Hudis, C.; Schrag, D. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA 2017, 318, 197. [Google Scholar] [CrossRef]
- Absolom, K.; Warrington, L.; Hudson, E.; Hewison, J.; Morris, C.; Holch, P.; Carter, R.; Gibson, A.; Holmes, M.; Clayton, B.; et al. Phase III Randomized Controlled Trial of eRAPID: eHealth Intervention During Chemotherapy. J. Clin. Oncol. 2021, 39, 734–747. [Google Scholar] [CrossRef] [PubMed]
- Prix d’excellence Innovation 2024|Association Des Pharmaciens Des Établissements de Santé Du Québec. Available online: https://www.apesquebec.org/qui-sommes-nous/prix-excellence/innovation/2024 (accessed on 11 February 2025).
- Tremblay, D.; Joly-Mischlich, T.; Dufour, A.; Battista, M.-C.; Berbiche, D.; Côté, J.; Décelles, M.; Forget, C.; Guérin, B.; Larivière, M.; et al. Telehomecare Monitoring for Patients Receiving Anticancer Oral Therapy: Protocol for a Mixed Methods Evaluability Study. JMIR Res. Protoc. 2025, 14, e63099. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.; Miller, A. Transitional Care Management Visits to Improve Coordination of Care. Am. J. Manag. Care 2021, 27, e130–e134. [Google Scholar] [CrossRef]
- Killmurray, C. Creating Solutions for a “Continual State of Transition” in Cancer Care. Peers Perspect. Oncol. 2024, 2, 68–71. [Google Scholar]
- Passwater, C. Bridging the Gap: Achieving Excellence in Oncology Transitional Care. J. Oncol. Navig. Surviv. 2021, 12, 1012–1019. [Google Scholar]
- Tremblay, D.; Latreille, J.; Bilodeau, K.; Samson, A.; Roy, L.; L’Italien, M.-F.; Mimeault, C. Improving the Transition From Oncology to Primary Care Teams: A Case for Shared Leadership. J. Oncol. Pract. 2016, 12, 1012–1019. [Google Scholar] [CrossRef]
- Naylor, M.; Keating, S.A. Transitional Care: Moving Patients from One Care Setting to Another. Am. J. Nurs. 2008, 108, 58–63. [Google Scholar] [CrossRef]
- Earl, T.; Katapodis, N.; Schneiderman, S. Care Transitions. In Making Healthcare Safer III: A Critical Analysis of Existing and Emerging Patient Safety Practices [Internet]; Agency for Healthcare Research and Quality: North Bethesda, MD, USA, 2020. [Google Scholar]
- Kripalani, S.; Chen, G.; Ciampa, P.; Theobald, C.; Cao, A.; McBride, M.; Dittus, R.S.; Speroff, T. A Transition Care Coordinator Model Reduces Hospital Readmissions and Costs. Contemp. Clin. Trials 2019, 81, 55–61. [Google Scholar] [CrossRef]
- Nam, S.; Gilbert, J.; Sussman, J.; Forbes, L.; Zwicker, V.; Doering, P.; Beglaryan, H. The Impact of a Well Breast Cancer Patient Transition Model of Care on Oncologists’ Practice. J. Clin. Oncol. 2018, 36, 39. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Higashiyama, N.; Umemiya, M.; Inayama, Y.; Koike, A.; Ueda, A.; Mizuno, R.; Taki, M.; Yamanoi, K.; Murakami, R.; et al. Electronic Patient-Reported Outcomes as Digital Therapeutics for Patients with Cancer: A Narrative Review of Current Practices and Future Directions. Int. J. Clin. Oncol. 2025, 30, 1–16. [Google Scholar] [CrossRef]
- Aremu, T.O.; Oluwole, O.E.; Adeyinka, K.O.; Schommer, J.C. Medication Adherence and Compliance: Recipe for Improving Patient Outcomes. Pharmacy 2022, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Perry, O.; Edwards, S. ISOPP CAPhO Symposium 2025 Abstracts. J. Oncol. Pharm. Pract. 2025, 31, 3–53. [Google Scholar] [CrossRef]
- Mason, M.; Cho, Y.; Rayo, J.; Gong, Y.; Harris, M.; Jiang, Y. Technologies for Medication Adherence Monitoring and Technology Assessment Criteria: Narrative Review. JMIR Mhealth Uhealth 2022, 10, e35157. [Google Scholar] [CrossRef] [PubMed]
- Building the Next Generation of Oncology Pas. Available online: https://www.onclive.com/view/building-the-next-generation-of-oncology-pas (accessed on 2 March 2025).
- Sheringham, J.; King, A.; Plackett, R.; Khan, A.; Cornes, M.; Kassianos, A.P. Physician Associate/Assistant Contributions to Cancer Diagnosis in Primary Care: A Rapid Systematic Review. BMC Health Serv. Res. 2021, 21, 644. [Google Scholar] [CrossRef]
- McCabe, M.S.; Pickard, T.A. Planning for the Future: The Role of Nurse Practitioners and Physician Assistants in Survivorship Care. Am. Soc. Clin. Oncol. Educ. Book 2012, 32, e56–e61. [Google Scholar] [CrossRef]
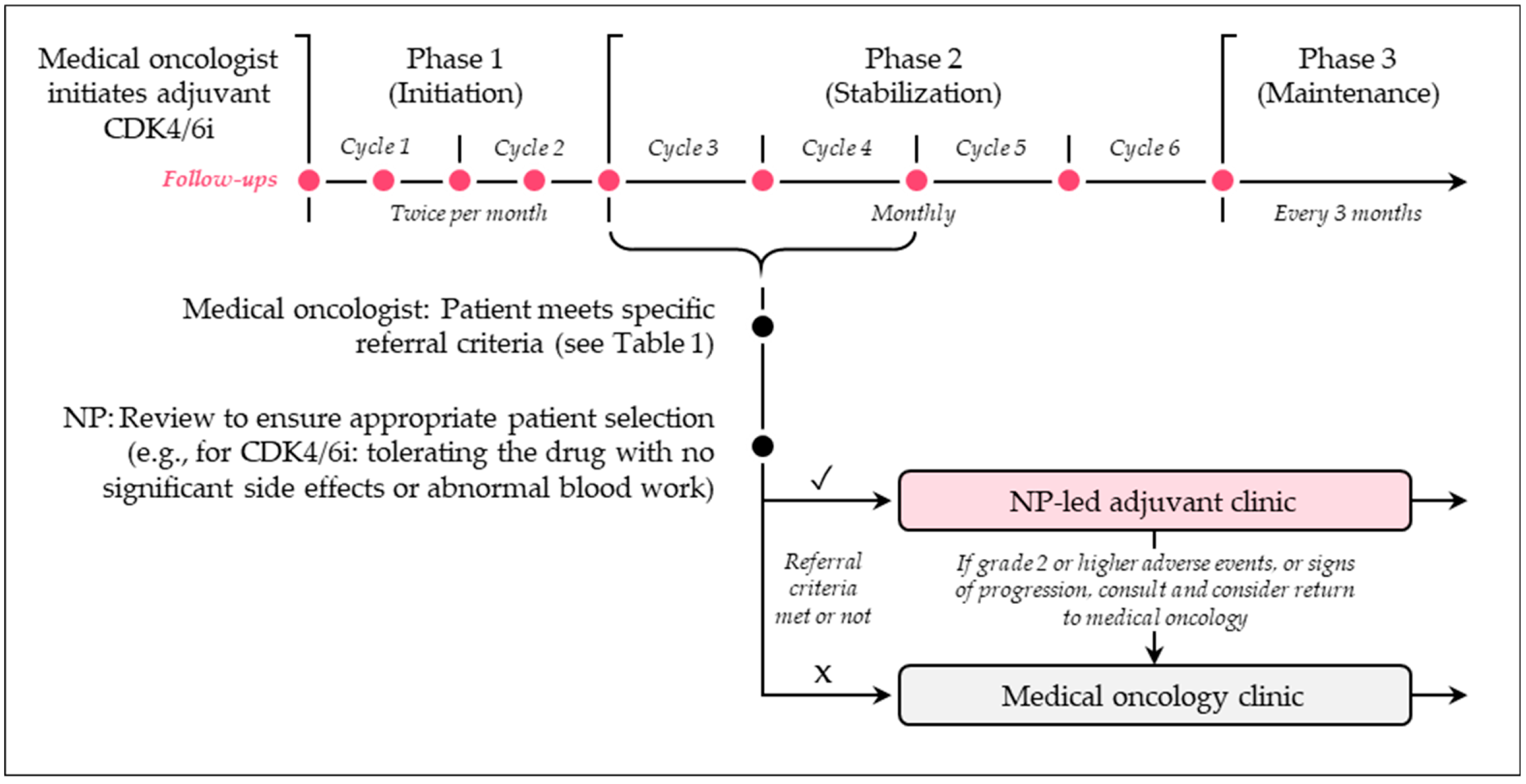
| Referral Criteria | Yes |
|---|---|
| 1. History of invasive breast cancer | ✓ |
| 2. ECOG PS 0-1 and tolerating treatment | ✓ |
| 3. Not on a clinical trial that requires follow-up by Medical Oncology | ✓ |
| 4. Must be receiving one of the following treatments: | ✓ |
| Adjuvant trastuzumab or T-DM1 | |
| Adjuvant capecitabine | |
| Adjuvant olaparib | |
| Adjuvant weekly paclitaxel (+/− trastuzumab) or docetaxel and cyclophosphamide after completion of 1 cycle and tolerating well with no significant adverse events | |
| Adjuvant CDK4/6 inhibitor, after completion of 2 cycles and tolerating well with no significant adverse events | |
| Adjuvant zoledronic acid +/− ET | |
| Adjuvant ET without primary-care provider and patient cannot be discharged |
| Category | Recommendations |
|---|---|
| Referral Pathways and Risk Criteria | - Establish clear, standardized referral pathways between oncologists and innovative care model providers (e.g., GPO, NP). - Define patient eligibility and risk stratification criteria. - Use EMR systems for seamless communication and documentation. - Employ structured transfer-of-care letters. |
| Patient Education | - Provide comprehensive educational materials. - Direct patients to resources like cancer navigation services and organizations (e.g., Wellspring). - Encourage self-care and prompt symptom reporting. |
| Clinic Structure and Follow-Up | - Standardize clinic structure and follow-up schedules. - Focus appointments on adherence, toxicity management, and recurrence surveillance. - Use virtual visits as needed. - Monitor for complications and make specialist referrals as necessary. |
| Workflow and Resource Optimization | - Optimize workflow and resource allocation. - Ensure institutional funding for clinic space, resources, and staffing. - Maintain continuity of care with consistent nursing staff. - Implement scalable patient volume management. |
| Quality Assessment | - Implement quality assessment measures. - Collect PROs for satisfaction, adherence, QoL, and symptom management. - Monitor provider satisfaction. - Track health resource utilization for cost-effectiveness. |
| Category | Recommendations |
|---|---|
| Scope and Training | - Clearly outline pharmacists’ roles within oncology teams. - Provide comprehensive training in oncology pharmacology, toxicity management, and patient communication. |
| Proactive Care Interventions | - Implement proactive telephone assessments. - Ensure consistent patient education to improve care quality and reduce reactive visits. |
| Collaboration | - Enhance teamwork with oncologists, nurses, and other healthcare professionals. - Secure clinic space near the multidisciplinary clinic setting. - Ensure seamless care transitions and comprehensive patient support. - Alternate follow-ups between pharmacists and physicians. - Coordinate with a physician to order tests if not within scope of practice. |
| Infrastructure and Support | - Invest in clinic space, digital tools, and streamlined workflows. - Ensure adequate clerical support. - Integrate telehealth, EMR, and patient portals for better accessibility. |
| Quality Assessment | - Use standardized metrics like adherence rates and patient satisfaction. - Identify quality improvements and demonstrate clinical value. |
| Expanded Scope | - Broaden pharmacist-led models to community settings with secured funding. - Expand prescribing authority for supportive care medications and OAMs. |
| Recommendation | Description |
|---|---|
| Cultural Shift | - Promote a healthcare culture that emphasizes proactive symptom management alongside patient empowerment. |
| Dedicated Resources | - Secure organizational backing to provide the necessary support and resources for long-term success. - Allocate financial and human resources to create a role for a clinical champion in symptom monitoring. |
| Technology Implementation | - Implement symptom reporting questionnaires that capture adverse-event grades to ensure appropriate and timely clinical interventions. - Allow individual centers to tailor the monitoring model to their unique clinical environments. - Ensure patients have access to user-friendly technology for seamless system engagement. |
| Continuous Evaluation | - Conduct ongoing research to refine and optimize the monitoring system for evolving needs. |
| Care Model | Funding | Referral | Services a | Strengths | Limitations |
|---|---|---|---|---|---|
| Nurse/NP-led | - Provider: Institution or health authority - Resources: b Institution (e.g., for training, clinic space, administrative staff) | - Oncologist prescribes and initiates therapy - Some models transition patients early (first cycle), others when stable (e.g., no grade ≥ 2 AEs) | - Education - F/u care - Toxicity management - NPs: Dose adjustments - Prescriptions (refills) Survivorship care | - Wide scope of practice (e.g., medication dose adjustments, prescribe refills) - Longitudinal support for adherence, especially with endocrine agents | - Shortage of trained NPs - Limitations on prescribing for nurses - Limitations on scope of practice for nurses |
| GPO-led | - Provider: Institution, health authority or provincial budget - Resources: b Institution | - Oncologist prescribes and initiates therapy - Some models transition patients early (first cycle), others when patients are stable (e.g., no grade ≥ 2 AEs) | - Education - F/u care - Toxicity management - Dose adjustments - Prescriptions (refills) - Survivorship care | - Wide scope of practice (e.g., medication dose adjustments, prescribe refills) - Effective bridge to primary care - May be cost-effective if funded by province | - Shortage of trained GPOs |
| Pharmacist-led | - Provider: Institution or health authority; in some centers, revenue from dispensing medications - Resources: b Institution or revenue from pharmacy | - Oncologist prescribes and initiates therapy - Patient consults with pharmacist at treatment onset - Some models transition patients early (first cycle), others when stable (e.g., no grade ≥ 2 AEs) | E ducation - F/u care - Medication reviews - Toxicity management - Adherence checks - Reimbursement navigation | - Experience with OAMs - Strong DDI detection capacity - Expertise in polypharmacy contexts | - Staff shortages - Limitations on prescribing - Limitations on conducting physical exams and ordering diagnostic imaging - Variable referral processes |
| Digital health tools | - Institution (e.g., for digital platforms, tech support, staff to monitor alerts) and/or grants (e.g., for pilot projects) | - Patient opt-in | - Symptom monitoring - Self-management tools | - Early detection of symptoms - Early clinical interventions - Reduced ER visits - Aggregate data | - Patient opt-in - Limited access for some patients (e.g., elderly, restricted digital literacy, socioeconomic reasons) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, A.; Nixon, N.; Al-Khaifi, M.; Bestavros, A.; Blyth, C.; Cheung, W.Y.; Hamm, C.; Joly-Mischlich, T.; Manna, M.; McFarlane, T.; et al. Optimizing Adjuvant Care in Early Breast Cancer: Multidisciplinary Strategies and Innovative Models from Canadian Centers. Curr. Oncol. 2025, 32, 402. https://doi.org/10.3390/curroncol32070402
Chan A, Nixon N, Al-Khaifi M, Bestavros A, Blyth C, Cheung WY, Hamm C, Joly-Mischlich T, Manna M, McFarlane T, et al. Optimizing Adjuvant Care in Early Breast Cancer: Multidisciplinary Strategies and Innovative Models from Canadian Centers. Current Oncology. 2025; 32(7):402. https://doi.org/10.3390/curroncol32070402
Chicago/Turabian StyleChan, Angela, Nancy Nixon, Muna Al-Khaifi, Alain Bestavros, Christine Blyth, Winson Y. Cheung, Caroline Hamm, Thomas Joly-Mischlich, Mita Manna, Tom McFarlane, and et al. 2025. "Optimizing Adjuvant Care in Early Breast Cancer: Multidisciplinary Strategies and Innovative Models from Canadian Centers" Current Oncology 32, no. 7: 402. https://doi.org/10.3390/curroncol32070402
APA StyleChan, A., Nixon, N., Al-Khaifi, M., Bestavros, A., Blyth, C., Cheung, W. Y., Hamm, C., Joly-Mischlich, T., Manna, M., McFarlane, T., Minard, L. V., Naujokaitis, S., Peragine, C., Railton, C., & Edwards, S. (2025). Optimizing Adjuvant Care in Early Breast Cancer: Multidisciplinary Strategies and Innovative Models from Canadian Centers. Current Oncology, 32(7), 402. https://doi.org/10.3390/curroncol32070402







