Radiation Quality-Dependent Progressive Increase in Oxidative DNA Damage and Intestinal Tumorigenesis in Apc1638N/+ Mice
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
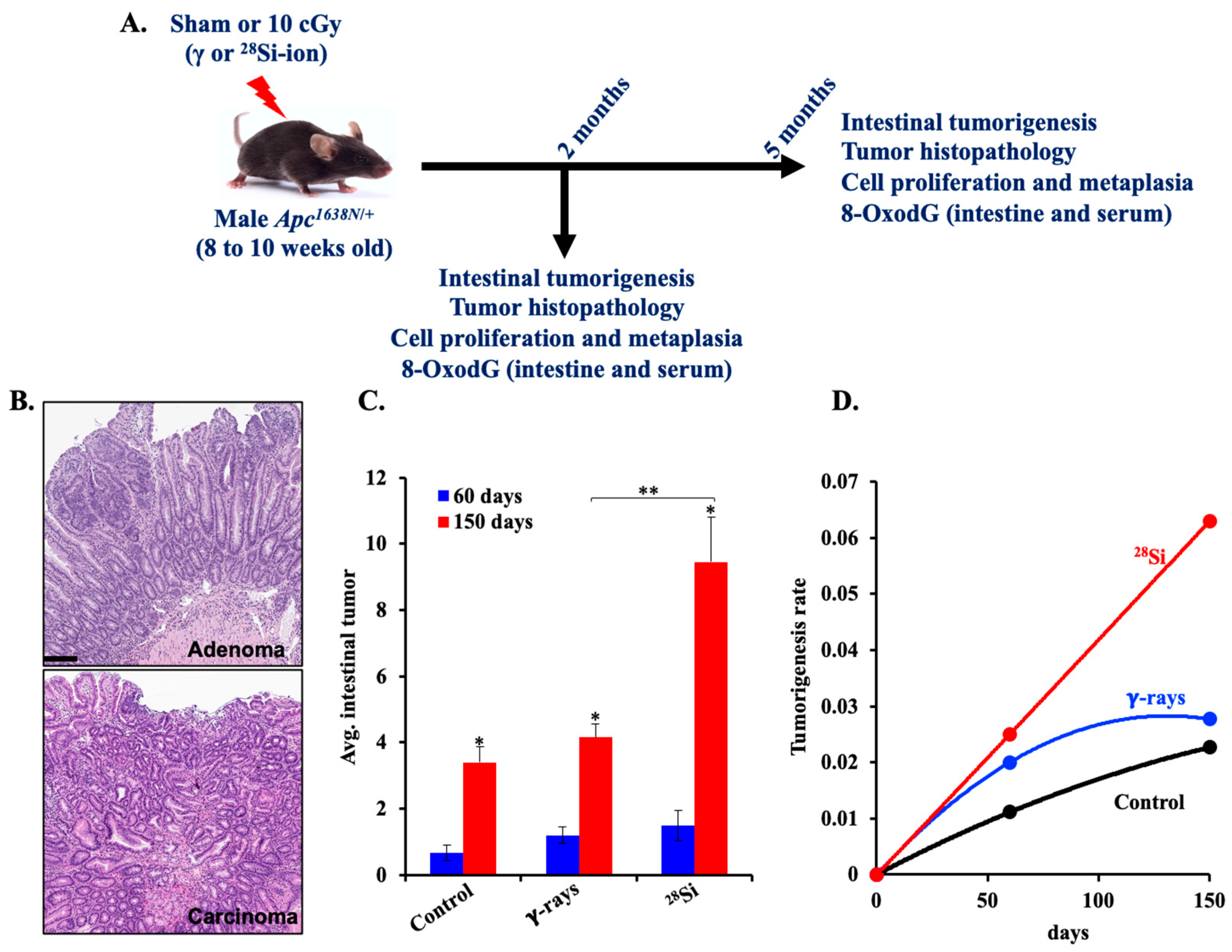
2.1. Animal Breeding, Housing, and Irradiations
2.2. Euthanasia, Biospecimen Collection, and Tumorigenesis Analysis
2.3. Serum 8-OxodG Quantitation by ELISA
2.4. Immunohistochemical Staining of Intestinal Tissue Section
2.5. Imaging and Data Analysis
2.6. Statistical Analysis
3. Results
3.1. Radiation Quality-Dependent Progression of Intestinal Tumorigenesis in Apc1638N/+ Mice
3.2. 28Si-Ion Exposure Induced Progressive Accumulations of 8-OxodG in Apc1638N/+ Mouse Serum
3.3. 28Si-Ion Exposure Induced Progressive Accumulations of 8-OxodG in Apc1638N/+ Mouse Intestine
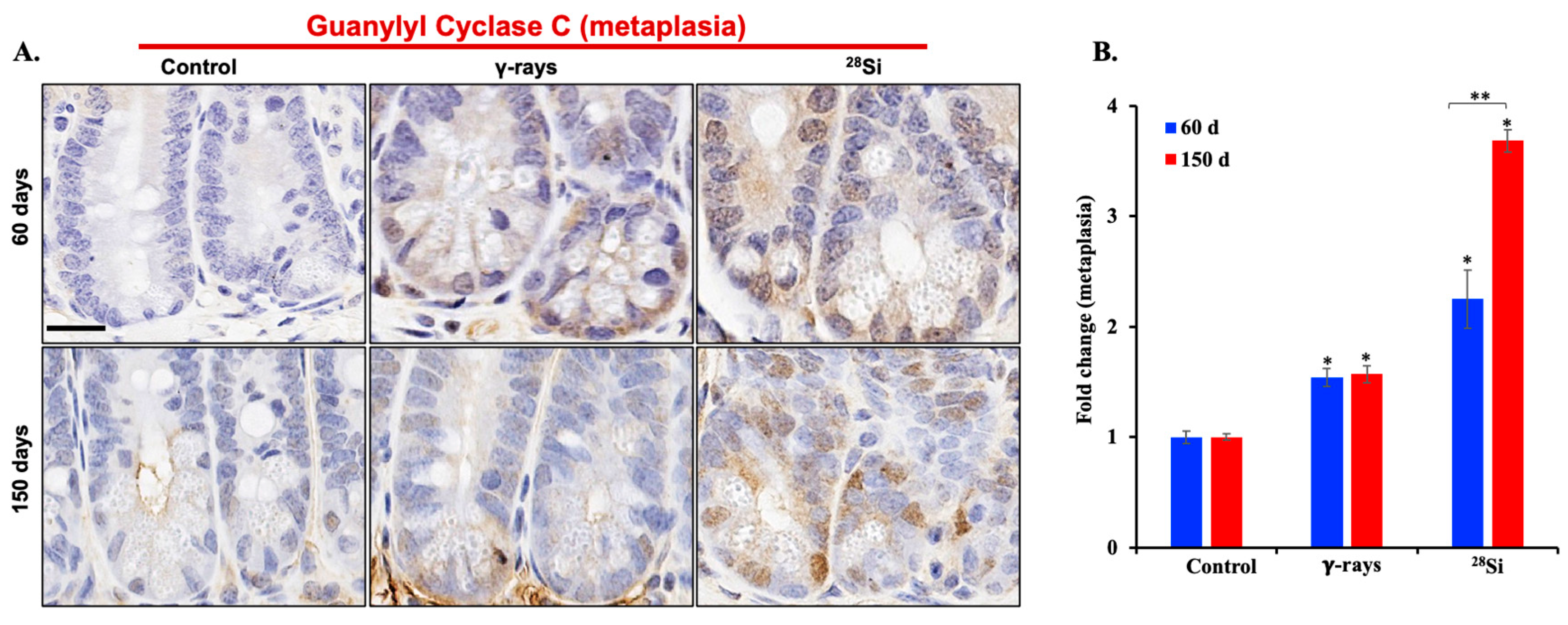
3.4. 28Si-Ion Exposure Induced Cell Proliferation and Metaplasia in Apc1638N/+ Mouse Intestine
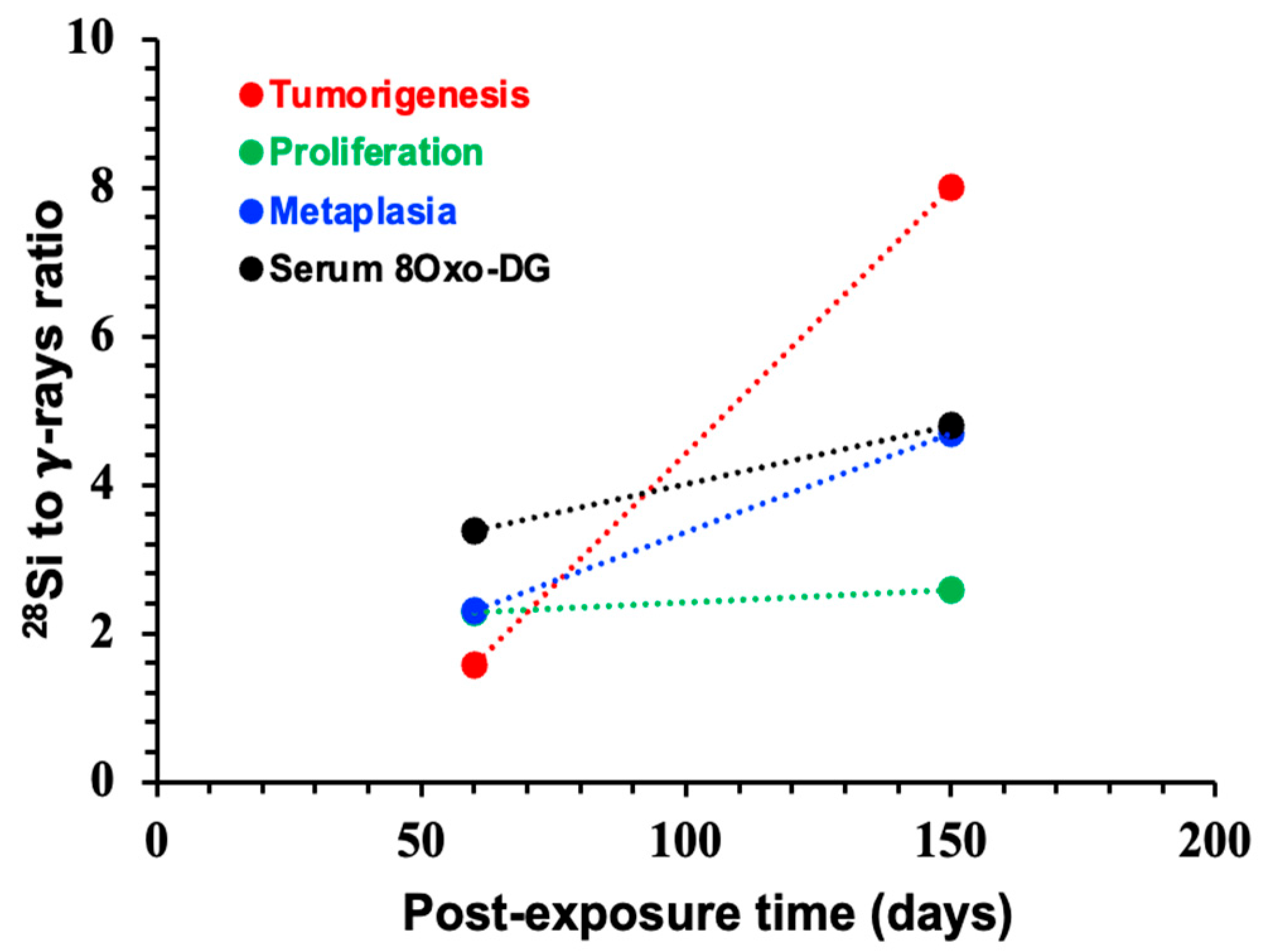
3.5. Systemic Increase in 8-OxodG Positively Correlates with Tumorigenesis-Associated Endpoints
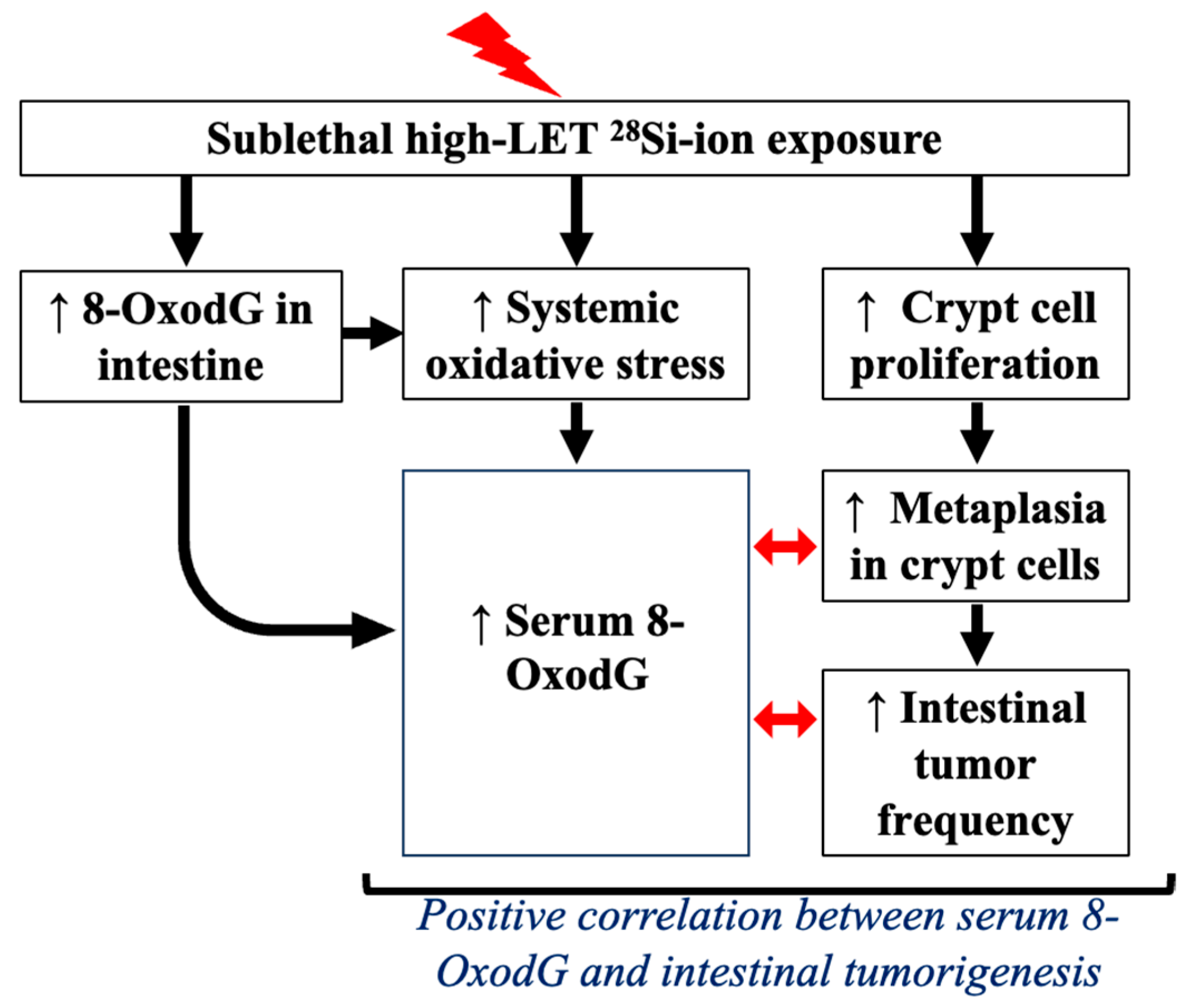
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Apc | Adenomatous polyposis coli gene |
| ANOVA | Analysis of Variance |
| CRC | Colorectal cancer |
| cGy | Centigray |
| DAB | 3,3′-diaminobenzidine |
| DDR | DNA damage response |
| GUCY2C | Guanylyl Cyclase C |
| IR | Ionizing radiation |
| LET | Linear energy transfer |
| NTEs | Non-targeted effects |
| OGG1 | 8-oxoguanine DNA glycosylase |
| RBE | Relative biological effectiveness |
| 8-OxodG | 8-oxo-7,8-dihydro-2′-deoxyguanosine |
References
- Guo, Z.; Zhou, G.; Hu, W. Carcinogenesis induced by space radiation: A systematic review. Neoplasia 2022, 32, 100828. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A.; To, K.; Cacao, E. Predictions of space radiation fatality risk for exploration missions. Life Sci. Space Res. 2017, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huff, J.L.; Poignant, F.; Rahmanian, S.; Khan, N.; Blakely, E.A.; Britten, R.A.; Chang, P.; Fornace, A.J.; Hada, M.; Kronenberg, A.; et al. Galactic cosmic ray simulation at the NASA space radiation laboratory—Progress, challenges and recommendations on mixed-field effects. Life Sci. Space Res. 2023, 36, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Ikeda, H.; Yoshida, Y. Role of High-Linear Energy Transfer Radiobiology in Space Radiation Exposure Risks. Int. J. Part. Ther. 2018, 5, 151–159. [Google Scholar] [CrossRef]
- Johnson, D.; Chen, Y.; Ahmad, S. Dose and linear energy transfer distributions of primary and secondary particles in carbon ion radiation therapy: A Monte Carlo simulation study in water. J. Med. Phys. 2015, 40, 214–219. [Google Scholar]
- Chen, G.T.; Castro, J.R.; Quivey, J.M. Heavy charged particle radiotherapy. Annu. Rev. Biophys. Bioeng. 1981, 10, 499–529. [Google Scholar] [CrossRef]
- Pan, V.A.; Parisi, A.; Bolst, D.; Williams, J.; Inaniwa, T.; Jackson, M.; Ahern, V.; Rosenfeld, A.B.; Tran, L.T. Comparative study of a microdosimetric biological weighting function for RBE10 modeling in particle therapy with a solid state SOI microdosimeter. Phys. Med. Biol. 2025, 70, 015020. [Google Scholar] [CrossRef]
- Suman, S.; Kumar, S.; Moon, B.H.; Strawn, S.J.; Thakor, H.; Fan, Z.; Shay, J.W.; Fornace, A.J.; Datta, K. Relative Biological Effectiveness of Energetic Heavy Ions for Intestinal Tumorigenesis Shows Male Preponderance and Radiation Type and Energy Dependence in APC(1638N/+) Mice. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 131–138. [Google Scholar] [CrossRef]
- Paganetti, H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys. Med. Biol. 2014, 59, R419–R472. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y. Heavier ions with a different linear energy transfer spectrum kill more cells due to similar interference with the Ku-dependent DNA repair pathway. Radiat. Res. 2014, 182, 458–461. [Google Scholar] [CrossRef]
- Hunter, N.; Muirhead, C.R. Review of relative biological effectiveness dependence on linear energy transfer for low-LET radiations. J. Radiol. Prot. 2009, 29, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Averbeck, D. Low-Dose Non-Targeted Effects and Mitochondrial Control. Int. J. Mol. Sci. 2023, 24, 11460. [Google Scholar] [CrossRef] [PubMed]
- Pouget, J.P.; Georgakilas, A.G.; Ravanat, J.L. Targeted and Off-Target (Bystander and Abscopal) Effects of Radiation Therapy: Redox Mechanisms and Risk/Benefit Analysis. Antioxid. Redox Signal 2018, 29, 1447–1487. [Google Scholar] [CrossRef]
- Shuryak, I.; Fornace, A.J.; Datta, K.; Suman, S.; Kumar, S.; Sachs, R.K.; Brenner, D.J. Scaling Human Cancer Risks from Low LET to High LET when Dose-Effect Relationships are Complex. Radiat. Res. 2017, 187, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, L.; Gopinathan, C. Ionizing radiation-induced cancer: Perplexities of the bystander effect. Ecancermedicalscience 2023, 17, 1579. [Google Scholar] [CrossRef]
- Campa, A.; Balduzzi, M.; Dini, V.; Esposito, G.; Tabocchini, M.A. The complex interactions between radiation induced non-targeted effects and cancer. Cancer Lett. 2015, 356, 126–136. [Google Scholar] [CrossRef]
- Kadhim, M.; Salomaa, S.; Wright, E.; Hildebrandt, G.; Belyakov, O.V.; Prise, K.M.; Little, M.P. Non-targeted effects of ionising radiation--implications for low dose risk. Mutat. Res. 2013, 752, 84–98. [Google Scholar] [CrossRef]
- Lu, H.; Yan, H.; Li, X.; Xing, Y.; Ye, Y.; Jiang, S.; Ma, L.; Ping, J.; Zuo, H.; Hao, Y.; et al. Single-cell map of dynamic cellular microenvironment of radiation-induced intestinal injury. Commun. Biol. 2023, 6, 1248. [Google Scholar] [CrossRef]
- McBride, W.H.; Schaue, D. Radiation-induced tissue damage and response. J. Pathol. 2020, 250, 647–655. [Google Scholar] [CrossRef]
- Suman, S.; Jaruga, P.; Dizdaroglu, M.; Fornace, A.J.; Datta, K. Heavy ion space radiation triggers ongoing DNA base damage by downregulating DNA repair pathways. Life Sci. Space Res. 2020, 27, 27–32. [Google Scholar] [CrossRef]
- Kumar, K.; Moon, B.H.; Kumar, S.; Angdisen, J.; Kallakury, B.V.S.; Fornace, A.J.; Suman, S. Senolytic agent ABT-263 mitigates low- and high-LET radiation-induced gastrointestinal cancer development in Apc1638N/+ mice. Aging 2025, 17, 97–115. [Google Scholar] [CrossRef]
- Prevost, V.; Sichel, F.; Pottier, I.; Leduc, A.; Lagadu, S.; Laurent, C. Production of early and late nuclear DNA damage and extracellular 8-oxodG in normal human skin fibroblasts after carbon ion irradiation compared to X-rays. Toxicol. Vitr. 2018, 52, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Hahm, J.Y.; Park, J.; Jang, E.S.; Chi, S.W. 8-Oxoguanine: From oxidative damage to epigenetic and epitranscriptional modification. Exp. Mol. Med. 2022, 54, 1626–1642. [Google Scholar] [CrossRef]
- Gorini, F.; Scala, G.; Cooke, M.S.; Majello, B.; Amente, S. Towards a comprehensive view of 8-oxo-7,8-dihydro-2′-deoxyguanosine: Highlighting the intertwined roles of DNA damage and epigenetics in genomic instability. DNA Repair. 2021, 97, 103027. [Google Scholar] [CrossRef]
- Fleming, A.M.; Burrows, C.J. 8-Oxo-7,8-dihydroguanine, friend and foe: Epigenetic-like regulator versus initiator of mutagenesis. DNA Repair. 2017, 56, 75–83. [Google Scholar] [CrossRef]
- Caspi, A.; Entezari, A.A.; Crutcher, M.; Snook, A.E.; Waldman, S.A. Guanylyl cyclase C as a diagnostic and therapeutic target in colorectal cancer. Per Med. 2022, 19, 457–472. [Google Scholar] [CrossRef]
- Danaee, H.; Kalebic, T.; Wyant, T.; Fassan, M.; Mescoli, C.; Gao, F.; Trepicchio, W.L.; Rugge, M. Consistent expression of guanylyl cyclase-C in primary and metastatic gastrointestinal cancers. PLoS ONE 2017, 12, e0189953. [Google Scholar] [CrossRef]
- Birbe, R.; Palazzo, J.P.; Walters, R.; Weinberg, D.; Schulz, S.; Waldman, S.A. Guanylyl cyclase C is a marker of intestinal metaplasia, dysplasia, and adenocarcinoma of the gastrointestinal tract. Hum. Pathol. 2005, 36, 170–179. [Google Scholar] [CrossRef]
- Oliver, A.J.; Huang, N.; Bartolome-Casado, R.; Li, R.; Koplev, S.; Nilsen, H.R.; Moy, M.; Cakir, B.; Polanski, K.; Gudiño, V.; et al. Single-cell integration reveals metaplasia in inflammatory gut diseases. Nature 2024, 635, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Smits, R.; van der Houven van Oordt, W.; Luz, A.; Zurcher, C.; Jagmohan-Changur, S.; Breukel, C.; Khan, P.M.; Fodde, R. Apc1638N: A mouse model for familial adenomatous polyposis-associated desmoid tumors and cutaneous cysts. Gastroenterology 1998, 114, 275–283. [Google Scholar] [CrossRef]
- van der Houven van Oordt, C.W.; Smits, R.; Schouten, T.G.; Houwing-Duistermaat, J.J.; Williamson, S.L.; Luz, A.; Meera Khan, P.; van der Eb, A.J.; Breuer, M.L.; Fodde, R. The genetic background modifies the spontaneous and X-ray-induced tumor spectrum in the Apc1638N mouse model. Genes. Chromosomes Cancer 1999, 24, 191–198. [Google Scholar] [CrossRef]
- Fodde, R.; Edelmann, W.; Yang, K.; van Leeuwen, C.; Carlson, C.; Renault, B.; Breukel, C.; Alt, E.; Lipkin, M.; Khan, P.M. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc. Natl. Acad. Sci. USA 1994, 91, 8969–8973. [Google Scholar] [CrossRef]
- Montalto, F.I.; De Amicis, F. Cyclin D1 in Cancer: A Molecular Connection for Cell Cycle Control, Adhesion and Invasion in Tumor and Stroma. Cells 2020, 9, 2648. [Google Scholar] [CrossRef]
- Trani, D.; Datta, K.; Doiron, K.; Kallakury, B.; Fornace, A.J. Enhanced intestinal tumor multiplicity and grade in vivo after HZE exposure: Mouse models for space radiation risk estimates. Radiat. Environ. Biophys. 2010, 49, 389–396. [Google Scholar] [CrossRef]
- Carusillo, A.; Mussolino, C. DNA Damage: From Threat to Treatment. Cells 2020, 9, 1665. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xue, Y.; Ba, X.; Wang, R. The Role of 8-oxoG Repair Systems in Tumorigenesis and Cancer Therapy. Cells 2022, 11, 3798. [Google Scholar] [CrossRef] [PubMed]
- Bøhn, S.K.; Smeland, S.; Sakhi, A.K.; Thoresen, M.; Russnes, K.M.; Tausjø, J.; Svilaas, A.; Svilaas, T.; Blomhoff, R. Post-radiotherapy plasma total glutathione is associated to outcome in patients with head and neck squamous cell carcinoma. Cancer Lett. 2006, 238, 240–247. [Google Scholar] [CrossRef]
- Kumar, K.; Fornace, A.J.; Suman, S. 8-OxodG: A Potential Biomarker for Chronic Oxidative Stress Induced by High-LET Radiation. DNA 2024, 4, 221–238. [Google Scholar] [CrossRef]
- Mansouri, B.; Moradi, A.; Saba, F. Blood oxidative stress parameters in hospital workers occupationally exposed to low doses of ionizing radiation: A systematic review and meta-analysis. Heliyon 2024, 10, e39989. [Google Scholar] [CrossRef]
- Datta, K.; Suman, S.; Kallakury, B.V.; Fornace, A.J. Heavy ion radiation exposure triggered higher intestinal tumor frequency and greater β-catenin activation than γ radiation in APC(Min/+) mice. PLoS ONE 2013, 8, e59295. [Google Scholar] [CrossRef]
- Suman, S.; Moon, B.H.; Datta, K.; Kallakury, B.V.S.; Fornace, A.J. Heavy-ion radiation-induced colitis and colorectal carcinogenesis in Il10-/- mice display co-activation of β-catenin and NF-κB signaling. PLoS ONE 2022, 17, e0279771. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A.; Schimmerling, W.; Wilson, J.W.; Peterson, L.E.; Badhwar, G.D.; Saganti, P.B.; Dicello, J.F. Space radiation cancer risks and uncertainties for Mars missions. Radiat. Res. 2001, 156, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Cucinotta, F.A. Heavy ion carcinogenesis and human space exploration. Nat. Rev. Cancer 2008, 8, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Alwood, J.S.; Tran, L.H.; Schreurs, A.S.; Shirazi-Fard, Y.; Kumar, A.; Hilton, D.; Tahimic, C.G.T.; Globus, R.K. Dose- and Ion-Dependent Effects in the Oxidative Stress Response to Space-Like Radiation Exposure in the Skeletal System. Int. J. Mol. Sci. 2017, 18, 2117. [Google Scholar] [CrossRef]
- Oyefeso, F.A.; Goldberg, G.; Opoku, N.Y.P.S.; Vazquez, M.; Bertucci, A.; Chen, Z.; Wang, C.; Muotri, A.R.; Pecaut, M.J. Effects of acute low-moderate dose ionizing radiation to human brain organoids. PLoS ONE 2023, 18, e0282958. [Google Scholar] [CrossRef]
- Tseng, B.P.; Giedzinski, E.; Izadi, A.; Suarez, T.; Lan, M.L.; Tran, K.K.; Acharya, M.M.; Nelson, G.A.; Raber, J.; Parihar, V.K.; et al. Functional consequences of radiation-induced oxidative stress in cultured neural stem cells and the brain exposed to charged particle irradiation. Antioxid. Redox Signal 2014, 20, 1410–1422. [Google Scholar] [CrossRef]
- Cheng, K.C.; Cahill, D.S.; Kasai, H.; Nishimura, S.; Loeb, L.A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G-T and A-C substitutions. J. Biol. Chem. 1992, 267, 166–172. [Google Scholar] [CrossRef]
- Shibutani, S.; Takeshita, M.; Grollman, A.P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature 1991, 349, 431–434. [Google Scholar] [CrossRef]
- Shibata, H.; Toyama, K.; Shioya, H.; Ito, M.; Hirota, M.; Hasegawa, S.; Matsumoto, H.; Takano, H.; Akiyama, T.; Toyoshima, K.; et al. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science 1997, 278, 120–123. [Google Scholar] [CrossRef]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Fan, P.C.; Zhang, Y.; Wang, Y.; Wei, W.; Zhou, Y.X.; Xie, Y.; Wang, X.; Qi, Y.Z.; Chang, L.; Jia, Z.P.; et al. Quantitative proteomics reveals mitochondrial respiratory chain as a dominant target for carbon ion radiation: Delayed reactive oxygen species generation caused DNA damage. Free Radic. Biol. Med. 2019, 130, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, J.; Sun, C.; Li, G.; Li, S.; Zhang, L.; Di, C.; Gan, L.; Wang, Y.; Zhou, R.; et al. Ameliorating mitochondrial dysfunction restores carbon ion-induced cognitive deficits via co-activation of NRF2 and PINK1 signaling pathway. Redox Biol. 2018, 17, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Lișcu, H.D.; Verga, N.; Atasiei, D.I.; Badiu, D.C.; Dumitru, A.V.; Ultimescu, F.; Pavel, C.; Stefan, R.E.; Manole, D.C.; Ionescu, A.I. Biomarkers in Colorectal Cancer: Actual and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 11535. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked. Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Suman, S.; Fornace, A.J. Countermeasure development against space radiation-induced gastrointestinal carcinogenesis: Current and future perspectives. Life Sci. Space Res. 2022, 35, 53–59. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Sun, C.; Chen, X.; Han, L.; Wang, T.; Liu, J.; Chen, X.; Zhao, D. Effect of Pterostilbene, a Natural Derivative of Resveratrol, in the Treatment of Colorectal Cancer through Top1/Tdp1-Mediated DNA Repair Pathway. Cancers 2021, 13, 4002. [Google Scholar] [CrossRef]
- Tao, L.; Fan, X.; Sun, J.; Zhang, Z. Metformin prevented high glucose-induced endothelial reactive oxygen species via OGG1 in an AMPKα-Lin-28 dependent pathway. Life Sci. 2021, 268, 119015. [Google Scholar] [CrossRef]
- Khan, N.; Jajeh, F.; Eberhardt, E.L.; Miller, D.D.; Albrecht, D.M.; Van Doorn, R.; Hruby, M.D.; Maresh, M.E.; Clipson, L.; Mukhtar, H.; et al. Fisetin and 5-fluorouracil: Effective combination for PIK3CA-mutant colorectal cancer. Int. J. Cancer 2019, 145, 3022–3032. [Google Scholar] [CrossRef]
- Leu, J.D.; Wang, B.S.; Chiu, S.J.; Chang, C.Y.; Chen, C.C.; Chen, F.D.; Avirmed, S.; Lee, Y.J. Combining fisetin and ionizing radiation suppresses the growth of mammalian colorectal cancers in xenograft tumor models. Oncol. Lett. 2016, 12, 4975–4982. [Google Scholar] [CrossRef]
- Surjana, D.; Halliday, G.M.; Damian, D.L. Role of nicotinamide in DNA damage, mutagenesis, and DNA repair. J. Nucleic Acids 2010, 2010, 157591. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, K.; Kumar, S.; Angdisen, J.; Datta, K.; Fornace, A.J., Jr.; Suman, S. Radiation Quality-Dependent Progressive Increase in Oxidative DNA Damage and Intestinal Tumorigenesis in Apc1638N/+ Mice. Curr. Oncol. 2025, 32, 382. https://doi.org/10.3390/curroncol32070382
Kumar K, Kumar S, Angdisen J, Datta K, Fornace AJ Jr., Suman S. Radiation Quality-Dependent Progressive Increase in Oxidative DNA Damage and Intestinal Tumorigenesis in Apc1638N/+ Mice. Current Oncology. 2025; 32(7):382. https://doi.org/10.3390/curroncol32070382
Chicago/Turabian StyleKumar, Kamendra, Santosh Kumar, Jerry Angdisen, Kamal Datta, Albert J. Fornace, Jr., and Shubhankar Suman. 2025. "Radiation Quality-Dependent Progressive Increase in Oxidative DNA Damage and Intestinal Tumorigenesis in Apc1638N/+ Mice" Current Oncology 32, no. 7: 382. https://doi.org/10.3390/curroncol32070382
APA StyleKumar, K., Kumar, S., Angdisen, J., Datta, K., Fornace, A. J., Jr., & Suman, S. (2025). Radiation Quality-Dependent Progressive Increase in Oxidative DNA Damage and Intestinal Tumorigenesis in Apc1638N/+ Mice. Current Oncology, 32(7), 382. https://doi.org/10.3390/curroncol32070382






