A Comparison of Radiation and Alkylator-Based Conditioning Therapy Regimens for Allogeneic Stem Cell Transplantation in Acute Myeloid Leukemia: A Clinician’s Perspective
Simple Summary
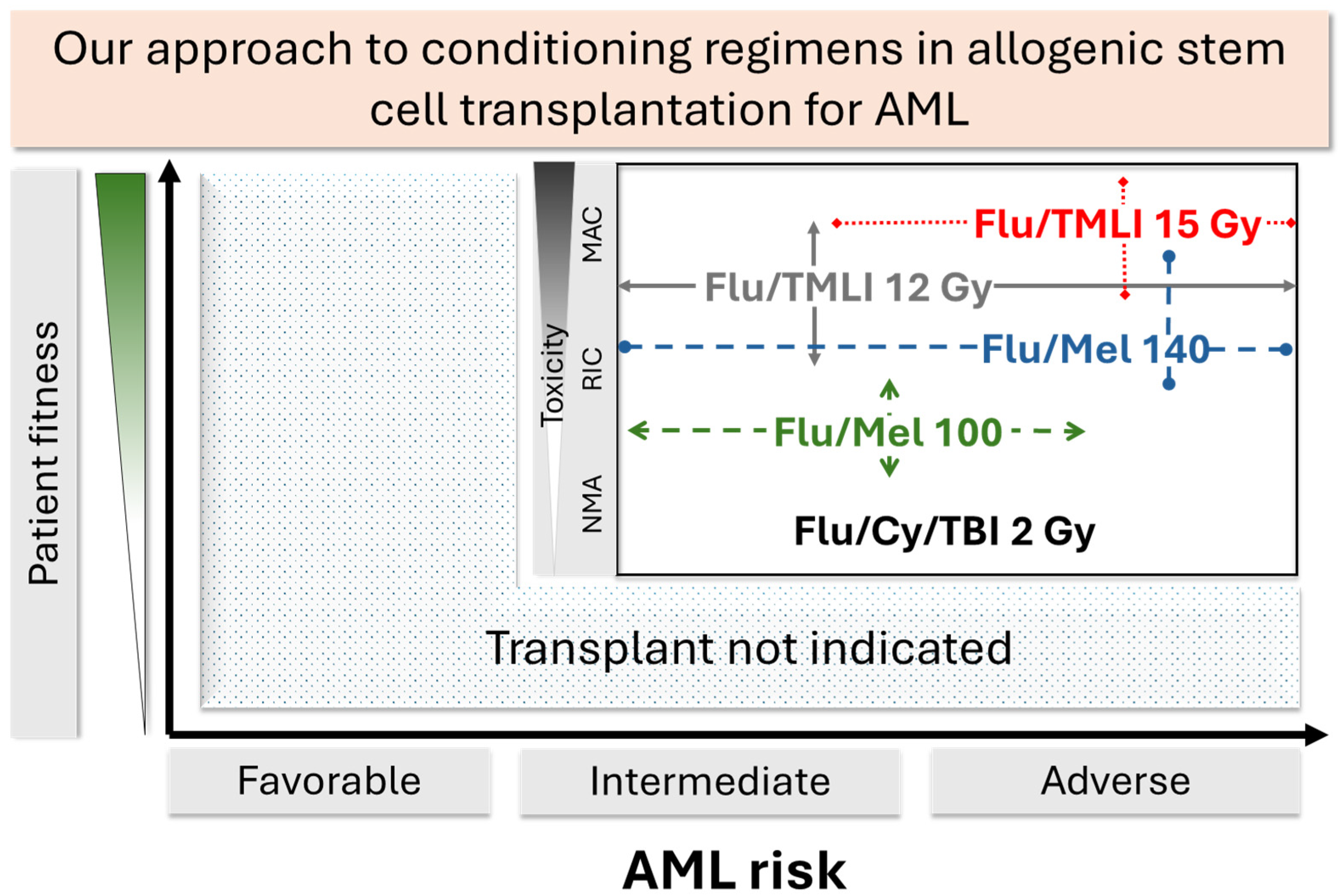
Abstract
1. Introduction
2. Conditioning Regimens by Intensity
2.1. Myeloablative Conditioning
2.1.1. Radiation-Based Myeloablative Conditioning
2.1.2. Chemotherapy-Based Myeloablative Conditioning
2.1.3. To Radiate or Not to Radiate
2.2. Reduced-Intensity Conditioning
3. Conditioning for Patients with Active or Refractory Disease
4. Conditioning Regimens for Second Allogeneic Stem Cell Transplants
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AML | Acute myeloid leukemia |
| Bu/Cy | Busulfan, cyclophosphamide |
| Bu/Flu | Busulfan, fludarabine |
| CIBMTR | Center for International Blood and Marrow Transplant Research |
| CR | Complete remission |
| Cy/TBI | Cytarabine, total body irradiation |
| FLAMSA | Fludarabine, cytarabine, amsacrine |
| Flu/Mel | Fludarabine, melphalan |
| HR | Hazard ratio |
| HSCT | Hematopoietic stem cell transplantation |
| LFS | Leukemia-free survival |
| MAC | Myeloablative conditioning |
| MDS | Myelodysplastic syndrome |
| NMA | Nonmyeloablative |
| NRM | Non-relapse mortality |
| OS | Overall survival |
| RFS | Relapse-free survival |
| RIC | Reduced intensity conditioning |
| TBI | Total body irradiation |
| TDM | Therapeutic drug monitoring |
| TMI | Total marrow irradiation |
| TMLI | Total marrow and lymphoid irradiation |
| TRM | Transplant-related mortality |
| VOD | Veno-occlusive disease |
References
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, F.R. Indications for allogeneic hematopoietic cell transplantation for acute myeloid leukemia in the genomic era. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, e327–e333. [Google Scholar] [CrossRef] [PubMed]
- Vriesendorp, H.M. Aims of conditioning. Exp. Hematol. 2003, 31, 844–854. [Google Scholar] [CrossRef]
- Scott, B.L.; Pasquini, M.C.; Fei, M.; Fraser, R.; Wu, J.; Devine, S.M.; Porter, D.L.; Maziarz, R.T.; Warlick, E.; Fernandez, H.F.; et al. Myeloablative versus Reduced-Intensity Conditioning for Hematopoietic Cell Transplantation in Acute Myelogenous Leukemia and Myelodysplastic Syndromes-Long-Term Follow-Up of the BMT CTN 0901 Clinical Trial. Transplant. Cell. Ther. 2021, 27, 483.e1–483.e6. [Google Scholar] [CrossRef]
- Hourigan, C.S.; Dillon, L.W.; Gui, G.; Logan, B.R.; Fei, M.; Ghannam, J.; Li, Y.; Licon, A.; Alyea, E.P.; Bashey; et al. Impact of Conditioning Intensity of Allogeneic Transplantation for Acute Myeloid Leukemia With Genomic Evidence of Residual Disease. J. Clin. Oncol. 2020, 38, 1273–1283. [Google Scholar] [CrossRef]
- Bacigalupo, A.; Ballen, K.; Rizzo, D.; Giralt, S.; Lazarus, H.; Ho, V.; Apperley, J.; Slavin, S.; Pasquini, M.; Sandmaier, B.M.; et al. Defining the intensity of conditioning regimens: Working definitions. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transpl. 2009, 15, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupo, A. Hematopoietic stem cell transplants after reduced intensity conditioning regimen (RI-HSCT): Report of a workshop of the European group for Blood and Marrow Transplantation (EBMT). Bone Marrow Transpl. 2000, 25, 803–805. [Google Scholar] [CrossRef]
- Spyridonidis, A.; Labopin, M.; Savani, B.N.; Niittyvuopio, R.; Blaise, D.; Craddock, C.; Socié, G.; Platzbecker, U.; Beelen, D.; Milpied, N.; et al. Redefining and measuring transplant conditioning intensity in current era: A study in acute myeloid leukemia patients. Bone Marrow Transpl. 2020, 55, 1114–1125. [Google Scholar] [CrossRef]
- Spyridonidis, A.; Labopin, M.; Gedde-Dahl, T.; Ganser, A.; Stelljes, M.; Craddock, C.; Wagner-Drouet, E.M.; Versluis, J.; Schroeder, T.; Blau, I.W.; et al. Validation of the transplant conditioning intensity (TCI) index for allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2024, 59, 217–223. [Google Scholar] [CrossRef]
- Lhuillier, C.; Rudqvist, N.P.; Yamazaki, T.; Zhang, T.; Charpentier, M.; Galluzzi, L.; Dephoure, N.; Clement, C.C.; Santambrogio, L.; Zhou, X.K.; et al. Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. J. Clin. Invest. 2021, 131, e138740. [Google Scholar] [CrossRef]
- Wong, J.Y.C.; Filippi, A.R.; Dabaja, B.S.; Yahalom, J.; Specht, L. Total Body Irradiation: Guidelines from the International Lymphoma Radiation Oncology Group (ILROG). Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.D.; Clift, R.A.; Hersman, J.; Sanders, J.E.; Stewart, P.; Buckner, C.D.; Fefer, A.; McGuffin, R.; Smith, J.W.; Storb, R. Marrow transplantation for acute nonlymphoblastic leukemic in first remission using fractionated or single-dose irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1982, 8, 817–821. [Google Scholar] [CrossRef]
- Wong, J.Y.; Forman, S.; Somlo, G.; Rosenthal, J.; Liu, A.; Schultheiss, T.; Radany, E.; Palmer, J.; Stein, A. Dose escalation of total marrow irradiation with concurrent chemotherapy in patients with advanced acute leukemia undergoing allogeneic hematopoietic cell transplantation. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 148–156. [Google Scholar] [CrossRef]
- Riddell, S.; Appelbaum, F.R.; Buckner, C.D.; Stewart, P.; Clift, R.; Sanders, J.; Storb, R.; Sullivan, K.; Thomas, E.D. High-dose cytarabine and total body irradiation with or without cyclophosphamide as a preparative regimen for marrow transplantation for acute leukemia. J. Clin. Oncol. 1988, 6, 576–582. [Google Scholar] [CrossRef]
- Arslan, S.; Desai, A.; Yang, D.; Mokhtari, S.; Tiemann, K.; Otoukesh, S.; Samara, Y.; Blackmon, A.; Agrawal, V.; Pourhassan, H.; et al. Total Body Irradiation and Fludarabine with Post-Transplantation Cyclophosphamide for Mismatched Related or Unrelated Donor Hematopoietic Cell Transplantation. Transplant. Cell. Ther. 2024, 30, 1013.e1–1013.e12. [Google Scholar] [CrossRef]
- Luo, C.; Wu, G.; Huang, X.; Ding, Y.; Huang, Y.; Song, Q.; Hou, Y.; Chen, J.; Li, X.; Xu, S. Myeloablative conditioning regimens in adult patients with acute myeloid leukemia undergoing allogeneic hematopoietic stem cell transplantation in complete remission: A systematic review and network meta-analysis. Bone Marrow Transpl. 2023, 58, 175–185. [Google Scholar] [CrossRef]
- Sabloff, M.; Chhabra, S.; Wang, T.; Fretham, C.; Kekre, N.; Abraham, A.; Adekola, K.; Auletta, J.J.; Barker, C.; Beitinjaneh, A.M.; et al. Comparison of High Doses of Total Body Irradiation in Myeloablative Conditioning before Hematopoietic Cell Transplantation. Biol. Blood Marrow Transpl. 2019, 25, 2398–2407. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.K.; Das, R.K.; Thomadsen, B.; Henderson, D. CT-based analysis of dose homogeneity in total body irradiation using lateral beam. J. Appl. Clin. Med. Phys. 2004, 5, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.H.; Gogineni, K.; Subhedar, P.D.; Lin, J.Y.; McCullough, L.E. Obesity and cancer treatment efficacy: Existing challenges and opportunities. Cancer 2019, 125, 1588–1592. [Google Scholar] [CrossRef]
- Wong, J.Y.C.; Liu, A.; Han, C.; Dandapani, S.; Schultheiss, T.; Palmer, J.; Yang, D.; Somlo, G.; Salhotra, A.; Hui, S.; et al. Total marrow irradiation (TMI): Addressing an unmet need in hematopoietic cell transplantation—a single institution experience review. Front. Oncol. 2022, 12, 1003908. [Google Scholar] [CrossRef]
- Al Malki, M.M.; Palmer, J.; Tsai, N.C.; Mokhtari, S.; Hui, S.; Tsai, W.; Aldoss, I.; Ali, H.; Aribi, A.; Cao, T.; et al. Total marrow and lymphoid irradiation as conditioning in haploidentical transplant with posttransplant cyclophosphamide. Blood Adv. 2022, 6, 4098–4106. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Palmer, J.; Tsai, N.C.; Al Malki, M.M.; Aldoss, I.; Ali, H.; Aribi, A.; Farol, L.; Karanes, C.; Khaled, S.; et al. Phase I Trial of Total Marrow and Lymphoid Irradiation Transplantation Conditioning in Patients with Relapsed/Refractory Acute Leukemia. Biol. Blood Marrow Transpl. 2017, 23, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.S.; Al Malki, M.M.; Yang, D.; Palmer, J.M.; Tsai, N.C.; Aldoss, I.; Ali, H.; Aribi, A.; Artz, A.; Dandapani, S.; et al. Total Marrow and Lymphoid Irradiation with Post-Transplantation Cyclophosphamide for Patients with AML in Remission. Transplant. Cell. Ther. 2022, 28, 368.e1–368.e7. [Google Scholar] [CrossRef]
- Zorutti, F.; Saldi, S.; Ruggeri, L.; Viglione, V.; Fulcheri, C.; Sembenico, R.; Caridi, M.; Castaldo, A.; Mancusi, A.; Zucchetti, C.; et al. Safety of 20Gy Total Marrow Irradiation Based Conditioning Regimen for Hematopoietic Cell Transplantation with T Cell Adoptive Immunotherapy. Blood 2024, 144, 269. [Google Scholar] [CrossRef]
- Gyurkocza, B.; Sandmaier, B.M. Conditioning regimens for hematopoietic cell transplantation: One size does not fit all. Blood 2014, 124, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.W.; Tutschka, P.J.; Brookmeyer, R.; Saral, R.; Beschorner, W.E.; Bias, W.B.; Braine, H.G.; Burns, W.H.; Elfenbein, G.J.; Kaizer, H. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N. Engl. J. Med. 1983, 309, 1347–1353. [Google Scholar] [CrossRef]
- Tutschka, P.J.; Copelan, E.A.; Klein, J.P. Bone marrow transplantation for leukemia following a new busulfan and cyclophosphamide regimen. Blood 1987, 70, 1382–1388. [Google Scholar] [CrossRef][Green Version]
- de Lima, M.; Couriel, D.; Thall, P.F.; Wang, X.; Madden, T.; Jones, R.; Shpall, E.J.; Shahjahan, M.; Pierre, B.; Giralt, S.; et al. Once-daily intravenous busulfan and fludarabine: Clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood 2004, 104, 857–864. [Google Scholar] [CrossRef]
- Ben-Barouch, S.; Cohen, O.; Vidal, L.; Avivi, I.; Ram, R. Busulfan fludarabine vs. busulfan cyclophosphamide as a preparative regimen before allogeneic hematopoietic cell transplantation: Systematic review and meta-analysis. Bone Marrow Transpl. 2016, 51, 232–240. [Google Scholar] [CrossRef]
- Lee, J.H.; Joo, Y.D.; Kim, H.; Ryoo, H.M.; Kim, M.K.; Lee, G.W.; Lee, J.H.; Lee, W.S.; Park, J.H.; Bae, S.H.; et al. Randomized trial of myeloablative conditioning regimens: Busulfan plus cyclophosphamide versus busulfan plus fludarabine. J. Clin. Oncol. 2013, 31, 701–709. [Google Scholar] [CrossRef]
- Andersson, B.S.; Thall, P.F.; Valdez, B.C.; Milton, D.R.; Al-Atrash, G.; Chen, J.; Gulbis, A.; Chu, D.; Martinez, C.; Parmar, S.; et al. Fludarabine with pharmacokinetically guided IV busulfan is superior to fixed-dose delivery in pretransplant conditioning of AML/MDS patients. Bone Marrow Transpl. 2017, 52, 580–587. [Google Scholar] [CrossRef]
- Slattery, J.T.; Risler, L.J. Therapeutic monitoring of busulfan in hematopoietic stem cell transplantation. Ther. Drug Monit. 1998, 20, 543–549. [Google Scholar] [CrossRef]
- Palmer, J.; McCune, J.S.; Perales, M.A.; Marks, D.; Bubalo, J.; Mohty, M.; Wingard, J.R.; Paci, A.; Hassan, M.; Bredeson, C.; et al. Personalizing Busulfan-Based Conditioning: Considerations from the American Society for Blood and Marrow Transplantation Practice Guidelines Committee. Biol. Blood Marrow Transpl. 2016, 22, 1915–1925. [Google Scholar] [CrossRef] [PubMed]
- Popat, U.; Lontos, K.; Bassett, R.; Kawedia, J.; Valdez, B.C.; Gulbis, A.; Alousi, A.M.; Al-Atrash, G.; Bashir, Q.; Hosing, C.M.; et al. P1270: Myeloablative Fractionated Busulfan-Based Conditioning Regimen in Patients with AML and MDS: Results of a Randomized Clinical Trial Comparing 2 Fractionation Schedules. HemaSphere 2023, 7, e069901e. [Google Scholar] [CrossRef]
- Popat, U.R.; Pasvolsky, O.; Bassett, R., Jr.; Mehta, R.S.; Olson, A.; Chen, J.; Alousi, A.M.; Al-Atrash, G.; Bashir, Q.; Gulbis, A.M.; et al. Myeloablative fractionated busulfan for allogeneic stem cell transplant in older patients or patients with comorbidities. Blood Adv. 2023, 7, 6196–6205. [Google Scholar] [CrossRef]
- Gupta, T.; Kannan, S.; Dantkale, V.; Laskar, S. Cyclophosphamide plus total body irradiation compared with busulfan plus cyclophosphamide as a conditioning regimen prior to hematopoietic stem cell transplantation in patients with leukemia: A systematic review and meta-analysis. Hematol. Oncol. Stem Cell Ther. 2011, 4, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Hartman, A.R.; Williams, S.F.; Dillon, J.J. Survival, disease-free survival and adverse effects of conditioning for allogeneic bone marrow transplantation with busulfan/cyclophosphamide vs. total body irradiation: A meta-analysis. Bone Marrow Transpl. 1998, 22, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lu, X.; Tang, L.; Yan, H.; Chen, W.; Shi, W.; Zhong, Z.; You, Y.; Xia, L.; Hu, Y.; et al. Comparing the outcomes between TMLI and non-TMLI conditioning regimens for adult high-risk acute lymphoblastic leukemia patients undergoing allogeneic hematopoietic stem cell transplantation: A single-center experience. Leuk. Lymphoma 2020, 61, 2567–2859. [Google Scholar] [CrossRef]
- Slavin, S.; Nagler, A.; Naparstek, E.; Kapelushnik, Y.; Aker, M.; Cividalli, G.; Varadi, G.; Kirschbaum, M.; Ackerstein, A.; Samuel, S.; et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 1998, 91, 756–763. [Google Scholar] [CrossRef]
- Oran, B.; Giralt, S.; Saliba, R.; Hosing, C.; Popat, U.; Khouri, I.; Couriel, D.; Qazilbash, M.; Anderlini, P.; Kebriaei, P.; et al. Allogeneic hematopoietic stem cell transplantation for the treatment of high-risk acute myelogenous leukemia and myelodysplastic syndrome using reduced-intensity conditioning with fludarabine and melphalan. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transpl. 2007, 13, 454–462. [Google Scholar] [CrossRef]
- Zhou, Z.; Nath, R.; Cerny, J.; Wang, H.L.; Zhang, M.J.; Abdel-Azim, H.; Agrawal, V.; Ahmed, G.; Al-Homsi, A.S.; Aljurf, M.; et al. Reduced intensity conditioning for acute myeloid leukemia using melphalan- vs. busulfan-based regimens: A CIBMTR report. Blood Adv. 2020, 4, 3180–3190. [Google Scholar] [CrossRef] [PubMed]
- Ciurea, S.O.; Kongtim, P.; Varma, A.; Rondon, G.; Chen, J.; Srour, S.; Bashir, Q.; Alousi, A.; Mehta, R.; Oran, B.; et al. Is there an optimal conditioning for older patients with AML receiving allogeneic hematopoietic cell transplantation? Blood 2020, 135, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Bornhäuser, M.; Kienast, J.; Trenschel, R.; Burchert, A.; Hegenbart, U.; Stadler, M.; Baurmann, H.; Schäfer-Eckart, K.; Holler, E.; Kröger, N.; et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: A prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012, 13, 1035–1044. [Google Scholar] [CrossRef]
- Scott, B.L.; Pasquini, M.C.; Logan, B.R.; Wu, J.; Devine, S.M.; Porter, D.L.; Maziarz, R.T.; Warlick, E.D.; Fernandez, H.F.; Alyea, E.P.; et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J. Clin. Oncol. 2017, 35, 1154–1161. [Google Scholar] [CrossRef]
- Kröger, N.; Iacobelli, S.; Franke, G.N.; Platzbecker, U.; Uddin, R.; Hübel, K.; Scheid, C.; Weber, T.; Robin, M.; Stelljes, M.; et al. Dose-Reduced Versus Standard Conditioning Followed by Allogeneic Stem-Cell Transplantation for Patients With Myelodysplastic Syndrome: A Prospective Randomized Phase III Study of the EBMT (RICMAC Trial). J. Clin. Oncol. 2017, 35, 2157–2164. [Google Scholar] [CrossRef]
- Mohty, M.; de Lavallade, H.; Ladaique, P.; Faucher, C.; Vey, N.; Coso, D.; Stoppa, A.M.; Gastaut, J.A.; Blaise, D. The role of reduced intensity conditioning allogeneic stem cell transplantation in patients with acute myeloid leukemia: A donor vs. no donor comparison. Leukemia 2005, 19, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Beelen, D.W.; Trenschel, R.; Stelljes, M.; Groth, C.; Masszi, T.; Reményi, P.; Wagner-Drouet, E.M.; Hauptrock, B.; Dreger, P.; Luft, T.; et al. Treosulfan or busulfan plus fludarabine as conditioning treatment before allogeneic haemopoietic stem cell transplantation for older patients with acute myeloid leukaemia or myelodysplastic syndrome (MC-FludT.14/L): A randomised, non-inferiority, phase 3 trial. The Lancet. Haematology 2020, 7, e28–e39. [Google Scholar] [CrossRef] [PubMed]
- Gyurkocza, B.; Nath, R.; Seropian, S.; Choe, H.; Litzow, M.R.; Abboud, C.; Koshy, N.; Stiff, P.; Tomlinson, B.; Abhyankar, S.; et al. Randomized Phase III SIERRA Trial of 131I-Apamistamab Before Allogeneic Hematopoietic Cell Transplantation Versus Conventional Care for Relapsed/Refractory AML. J. Clin. Oncol. 2025, 43, 201–213. [Google Scholar] [CrossRef]
- Wong, J.Y.C.; Monzr, A.M.; Salhotra, A.; Dandapani, S.V.; Wang, Y.; Palmer, J.; Han, C.; Liu, A.; Radany, E.H.; Aldoss, I.; et al. Phase II Trial of TMLI 20 Gy in Combination with Cyclophosphamide and Etoposide in Patients with Poor-Risk Acute Leukemia. Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, S90–S91. [Google Scholar] [CrossRef]
- Stelljes, M.; Middeke, J.M.; Bug, G.; Wagner-Drouet, E.M.; Müller, L.P.; Schmid, C.; Krause, S.W.; Bethge, W.; Jost, E.; Platzbecker, U.; et al. Remission induction versus immediate allogeneic haematopoietic stem cell transplantation for patients with relapsed or poor responsive acute myeloid leukaemia (ASAP): A randomised, open-label, phase 3, non-inferiority trial. Lancet. Haematol. 2024, 11, e324–e335. [Google Scholar] [CrossRef]
- Schmälter, A.K.; Ngoya, M.; Galimard, J.E.; Bazarbachi, A.; Finke, J.; Kröger, N.; Bornhäuser, M.; Stelljes, M.; Stölzel, F.; Tischer, J.; et al. Continuously improving outcome over time after second allogeneic stem cell transplantation in relapsed acute myeloid leukemia: An EBMT registry analysis of 1540 patients. Blood Cancer J. 2024, 14, 76. [Google Scholar] [CrossRef] [PubMed]

| Organs | Percent of the Prescribed Target Dose |
|---|---|
| Bowel | 70.91% |
| Brain | 71.00% |
| Esophagus | 48.24% |
| Eyes | 56.78% |
| Lens | 56.27% |
| Heart | 53.06% |
| Kidneys | 62.33% |
| Larynx | 56.85% |
| Liver | 60.00% |
| Lungs | 58.83% |
| Oral Cavity | 29.25% |
| Parotids | 71.37% |
| Rectum | 87.64% |
| Thyroid | 89.65% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinos Velarde, A.; Alvarenga Thiebaud, J.; Madanat, Y.; Toor, A. A Comparison of Radiation and Alkylator-Based Conditioning Therapy Regimens for Allogeneic Stem Cell Transplantation in Acute Myeloid Leukemia: A Clinician’s Perspective. Curr. Oncol. 2025, 32, 381. https://doi.org/10.3390/curroncol32070381
Marinos Velarde A, Alvarenga Thiebaud J, Madanat Y, Toor A. A Comparison of Radiation and Alkylator-Based Conditioning Therapy Regimens for Allogeneic Stem Cell Transplantation in Acute Myeloid Leukemia: A Clinician’s Perspective. Current Oncology. 2025; 32(7):381. https://doi.org/10.3390/curroncol32070381
Chicago/Turabian StyleMarinos Velarde, Alejandro, Julio Alvarenga Thiebaud, Yazan Madanat, and Amir Toor. 2025. "A Comparison of Radiation and Alkylator-Based Conditioning Therapy Regimens for Allogeneic Stem Cell Transplantation in Acute Myeloid Leukemia: A Clinician’s Perspective" Current Oncology 32, no. 7: 381. https://doi.org/10.3390/curroncol32070381
APA StyleMarinos Velarde, A., Alvarenga Thiebaud, J., Madanat, Y., & Toor, A. (2025). A Comparison of Radiation and Alkylator-Based Conditioning Therapy Regimens for Allogeneic Stem Cell Transplantation in Acute Myeloid Leukemia: A Clinician’s Perspective. Current Oncology, 32(7), 381. https://doi.org/10.3390/curroncol32070381





