Perivascular Epithelioid Cell Tumor (PEComa) of the Sigmoid Colon: Case Report and Literature Review
Abstract
1. Introduction
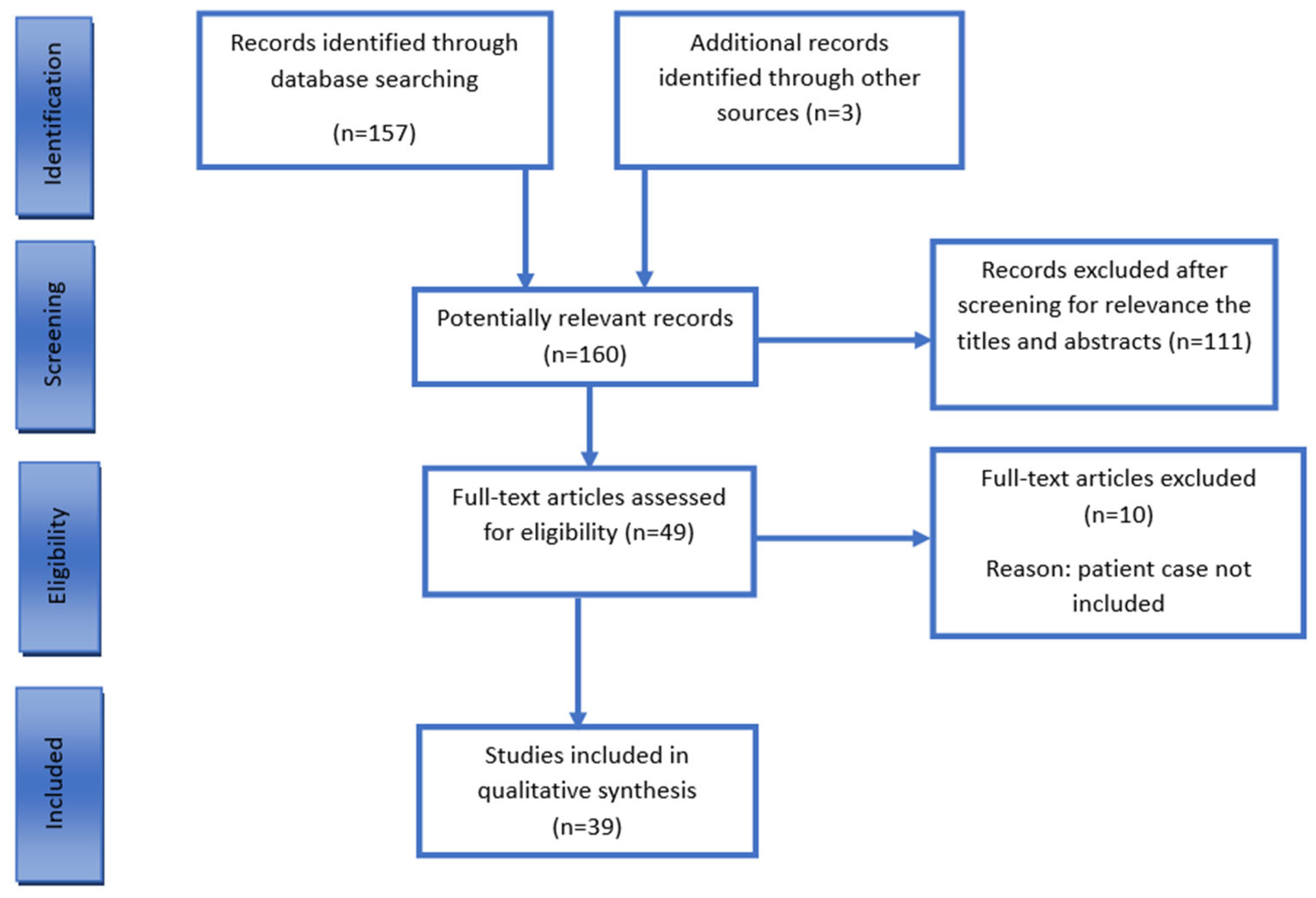
2. Methods
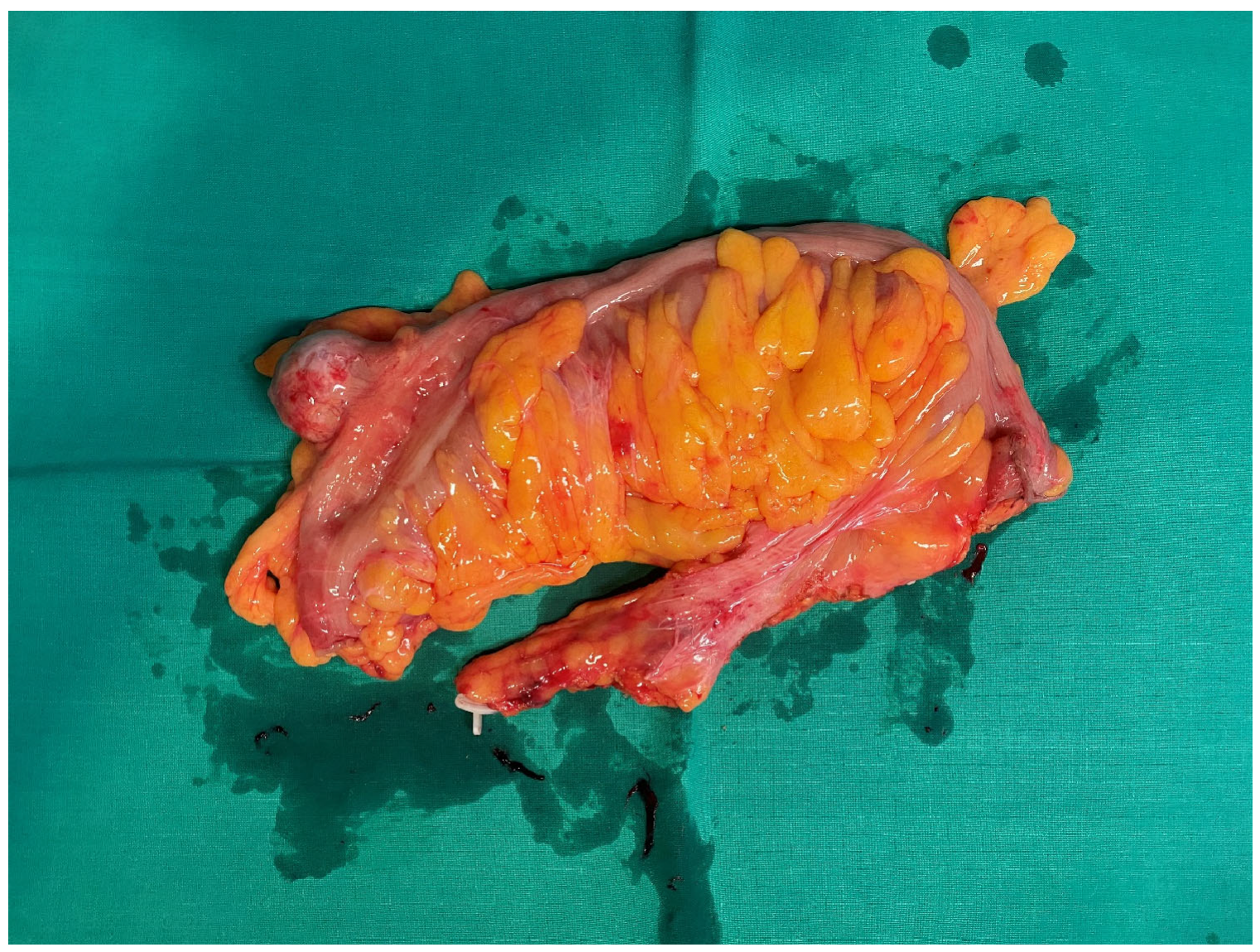
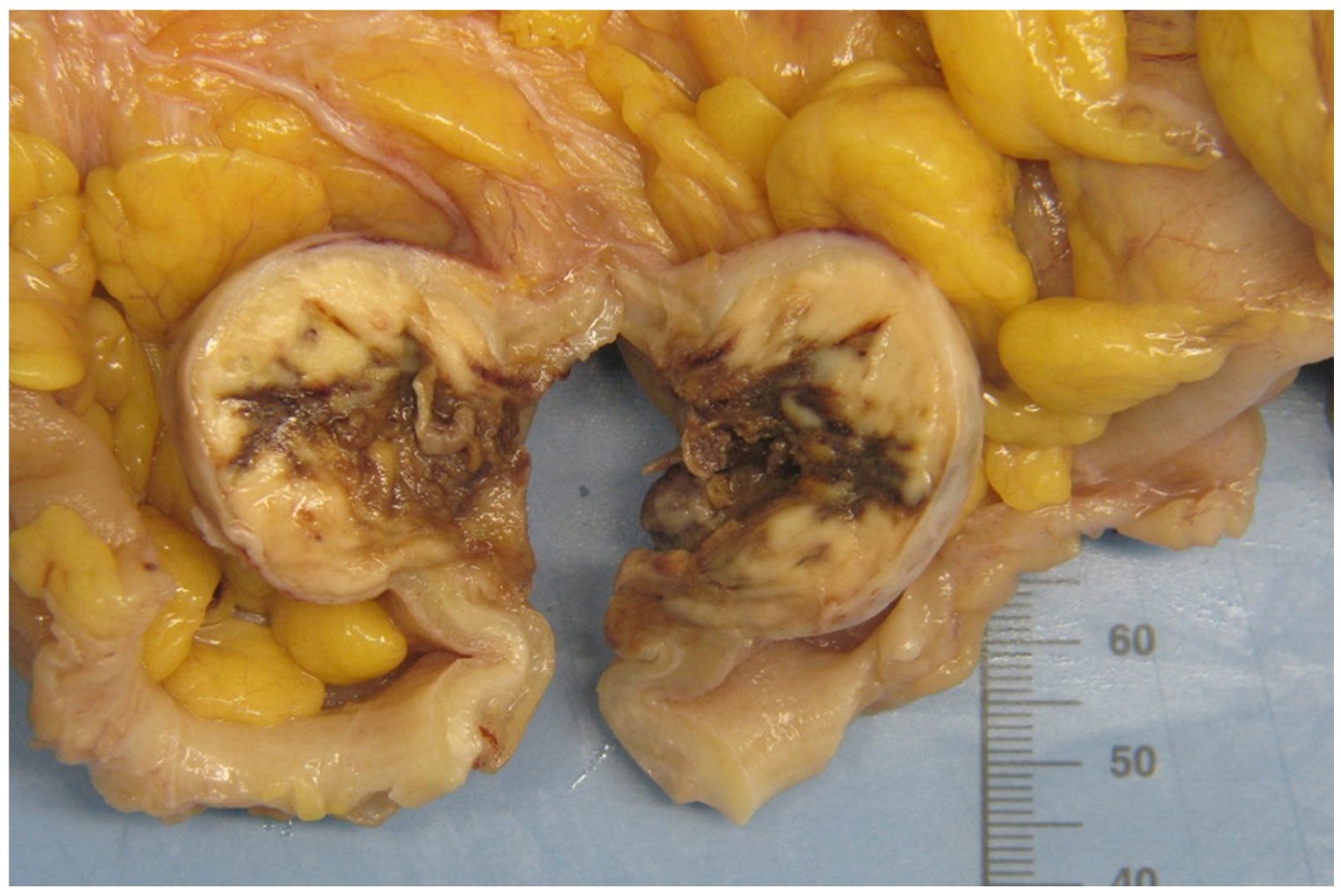
2.1. Clinical Case
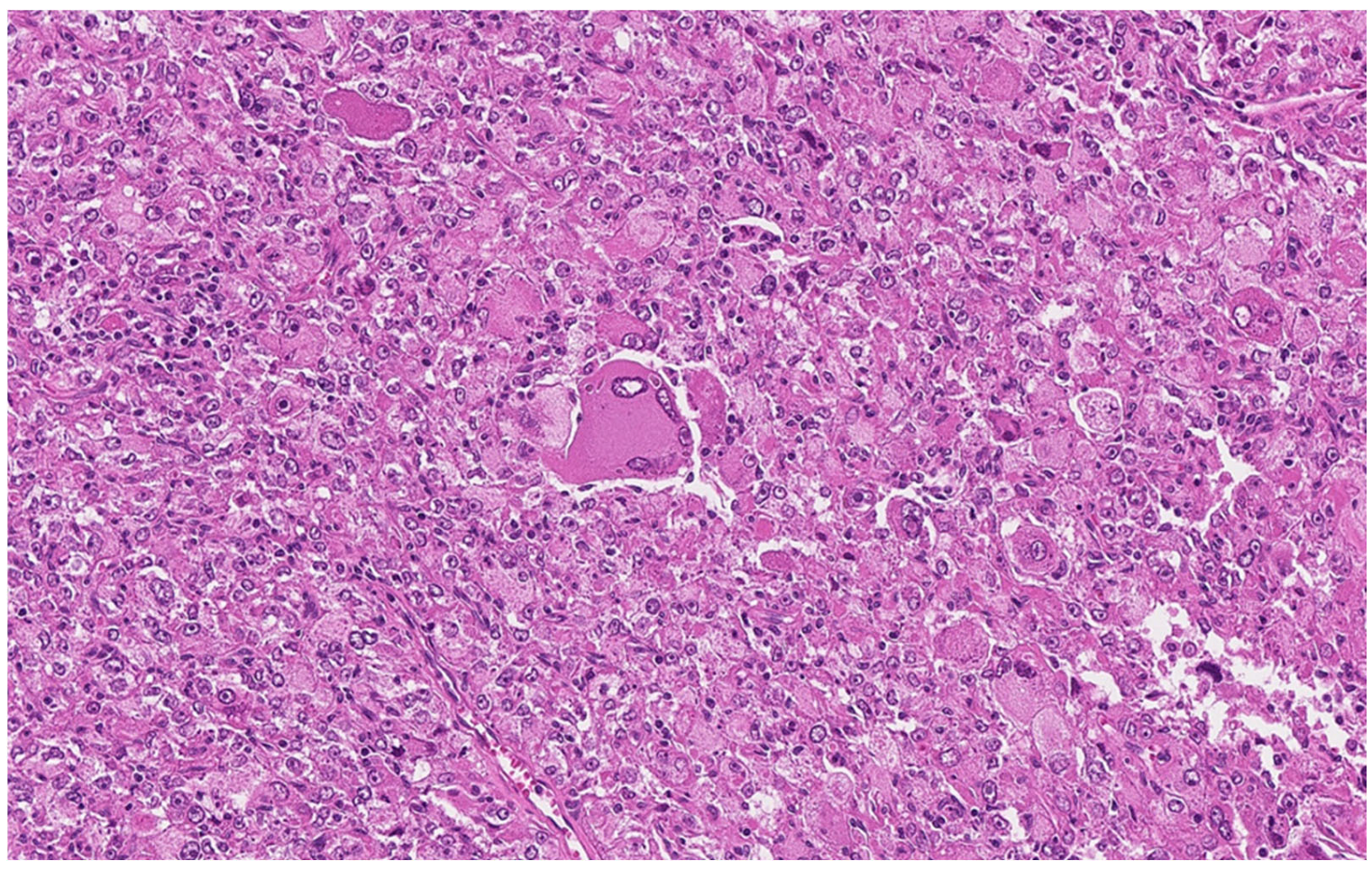
2.2. Pathologic Examination
2.3. Results of the Literature Review
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonetti, F.; Pea, M.; Martignoni, G.; Zamboni, G. PEC and sugar. Am. J. Surg. Pathol. 1992, 16, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.A.; Hornick, J.L.; Fletcher, C.D.M. PEComa of the Gastrointestinal Tract: Clinicopathologic Study of 35 Cases with Evaluation of Prognostic Parameters. Am. J. Surg. Pathol. 2013, 37, 1769–1782. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Zheng, W.W.; Jia, L.; Wang, X.L.; Zhou, J.H.; Hao, J.R.; Liu, Z.; Gao, F.-Y. Pediatric case of colonic perivascular epithelioid cell tumor complicated with intussusception and anal incarceration: A case report. World J. Gastrointest. Oncol. 2022, 14, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.C.; Kuo, C.Y.; Huang, C.W.; Shih, H.H.; Lin, C.H.; Wang, J.Y. Unusual paediatric sigmoid perivascular epithelioid cell tumour with regional lymph node metastasis treated using gemcitabine and docetaxel: A case report and literature review. J. Int. Med. Res. 2021, 49, 030006052110415. [Google Scholar] [CrossRef]
- Im, S.; Yoo, C.; Jung, J.H.; Choi, H.J.; Yoo, J.; Kang, C.S. Primary perivascular epithelioid cell tumor in the rectum: A case report and review of the literature. Pathol.-Res. Pract. 2013, 209, 244–248. [Google Scholar] [CrossRef]
- Maran-Gonzalez, A.; Baldet, P.; Costes, V. PECome polypoïde du rectum: À propos d’un cas et revue de la littérature. Ann. Pathol. 2011, 31, 102–107. [Google Scholar] [CrossRef]
- Gross, E.; Vernea, F.; Weintraub, M.; Koplewitz, B.Z. Perivascular epithelioid cell tumor of the ascending colon mesentery in a child: Case report and review of the literature. J. Pediatr. Surg. 2010, 45, 830–833. [Google Scholar] [CrossRef]
- Freeman, H.J. Perivascular epithelioid cell neoplasm of the colon. World J. Gastrointest. Oncol. 2010, 2, 205. [Google Scholar] [CrossRef]
- Park, S.J.; Han, D.K.; Baek, H.J.; Chung, S.Y.; Nam, J.H.; Kook, H.; Hwang, T.J. Perivascular epithelioid cell tumor (PEComa) of the ascending colon: The implication of IFN-α2b treatment. Korean J. Pediatr. 2010, 53, 975. [Google Scholar] [CrossRef]
- Ryan, P.; Nguyen, V.H.; Gholoum, S.; Carpineta, L.; Abish, S.; Ahmed, N.N.; Laberge, J.-M.; Riddell, R.H. Polypoid PEComa in the Rectum of a 15-year-old Girl: Case Report and Review of PEComa in the Gastrointestinal Tract. Am. J. Surg. Pathol. 2009, 33, 475–482. [Google Scholar] [CrossRef]
- Tanaka, M.; Kato, K.; Gomi, K.; Matsumoto, M.; Kudo, H.; Shinkai, M.; Ohama, Y.; Kigasawa, H.; Tanaka, Y. Perivascular Epithelioid Cell Tumor with SFPQ/PSF-TFE3 Gene Fusion in a Patient With Advanced Neuroblastoma. Am. J. Surg. Pathol. 2009, 33, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhang, S.; Ba, Y.; Li, K.; Gao, G.; Li, Y.; Zhang, Y.; Liu, C.; Shi, N. Case Report: Perivascular epithelioid tumors of the gastrointestinal tract. Front. Oncol. 2023, 12, 1026825. [Google Scholar] [CrossRef] [PubMed]
- Hornick, J.L.; Fletcher, C.D.M. PEComa: What do we know so far? Histopathology 2006, 48, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Sobiborowicz, A.; Czarnecka, A.M.; Szumera-Ciećkiewicz, A.; Rutkowski, P.; Świtaj, T. Diagnosis and treatment of malignant PEComa tumours. Oncol. Clin. Pract. 2020, 16, 22–33. [Google Scholar] [CrossRef]
- Bleeker, J.S.; Quevedo, J.F.; Folpe, A.L. “Malignant” Perivascular Epithelioid Cell Neoplasm: Risk Stratification and Treatment Strategies. Sarcoma 2012, 2012, 541626. [Google Scholar] [CrossRef]
- Uhlenhopp, D.J.; West, J.; Heckart, J.; Campbell, R.; Elhaddad, A. Rapidly enlarging malignant abdominal PEComa with hepatic metastasis: A promising initial response to sirolimus following surgical excision of primary tumor. Oxf. Med. Case Rep. 2020, 2020, omaa013. [Google Scholar] [CrossRef]
- Prasad, M.L.; Keating, J.P.; Teoh, H.H.; McCarthy, S.W.; Battifora, H.; Wasef, E.; Rosai, J. Pleomorphic Angiomyolipoma of Digestive Tract: AHeretofore Unrecognized Entity. Int. J. Surg. Pathol. 2000, 8, 67–72. [Google Scholar] [CrossRef]
- Tazelaar, H.D.; Batts, K.P.; Srigley, J.R. Primary Extrapulmonary Sugar Tumor (PEST): A Report of Four Cases. Mod. Pathol. 2001, 14, 615–622. [Google Scholar] [CrossRef]
- Birkhaeuser, F.; Ackermann, C.; Flueckiger, T.; Guenin, M.O.; Kern, B.; Tondelli, P.; Peterli, R. First Description of a PEComa (Perivascular Epithelioid Cell Tumor) of the Colon: Report of a Case and Review of the Literature. Dis. Colon. Rectum. 2004, 47, 1734–1737. [Google Scholar] [CrossRef]
- Genevay, M.; Mc Kee, T.; Zimmer, G.; Cathomas, G.; Guillou, L. Digestive PEComas: A solution when the diagnosis Fails to “fit”. Ann. Diagn. Pathol. 2004, 8, 367–372. [Google Scholar] [CrossRef]
- Evert, M.; Wardelmann, E.; Nestler, G.; Schulz, H.; Roessner, A.; Röcken, C. Abdominopelvic perivascular epithelioid cell sarcoma (malignant PEComa) mimicking gastrointestinal stromal tumour of the rectum. Histopathology 2005, 46, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Oda, Y.; Yao, T.; Oiwa, T.; Kobayashi, C.; Tamiya, S.; Kawaguchi, K.; Hino, O.; Tsuneyoshi, M. Malignant perivascular epithelioid cell tumor of the colon: Report of a case with molecular analysis. Pathol. Int. 2006, 56, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Gi Chung, M.; Hae Jung, D.; Oh, J.H. Perivascular Epithelioid Cell Tumor (Pecoma) in the Transverse Colon of an Adolescent: A Case Report. Tumori J. 2007, 93, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Pisharody, U.; Craver, R.D.; Brown, R.F.; Gardner, R.; Schmidt-Sommerfeld, E. Metastatic Perivascular Epithelioid Cell Tumor of the Colon in a Child. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 598–601. [Google Scholar] [CrossRef]
- Righi, A.; Dimosthenous, K.; Rosai, J. PEComa: Another Member of the MiT Tumor Family? Int. J. Surg. Pathol. 2008, 16, 16–20. [Google Scholar] [CrossRef]
- Qu, G.M.; Hu, J.C.; Cai, L.; Lang, Z.Q. Perivascular epithelioid cell tumor of the cecum: A case report and review of literatures. Chin. Med. J. 2009, 122, 1713–1715. [Google Scholar]
- Shi, H.Y.; Wei, L.X.; Sun, L.; Guo, A.T. Clinicopathologic Analysis of 4 Perivascular Epithelioid Cell Tumors (PEComas) of the Gastrointestinal Tract. Int. J. Surg. Pathol. 2010, 18, 243–247. [Google Scholar] [CrossRef]
- Lee, M.; Cho, K.J.; Yu, C.; Park, Y.; Kim, J.C.; Kim, J.; Yu, E.; Kim, M.J. Perivascular epithelioid cell tumor of the sigmoid colon with transcription factor E3 expression. Ann. Diagn. Pathol. 2012, 16, 306–311. [Google Scholar] [CrossRef]
- Scheppach, W. PEComa of the colon resistant to sirolimus but responsive to doxorubicin/ifosfamide. World J. Gastroenterol. 2013, 19, 1657. [Google Scholar] [CrossRef]
- Korytnaya, E.; Liu, J.; Camelo-Piragua, S.; Sullivan, S.; Auchus, R.J.; Barkan, A. Ectopic Prolactin Secretion From a Perivascular Epithelioid Cell Tumor (PEComa). J. Clin. Endocrinol. Metab. 2014, 99, 3960–3964. [Google Scholar] [CrossRef]
- Kanazawa, A.; Fujii, S.; Godai, T.I.; Ishibe, A.; Oshima, T.; Fukushima, T.; Ota, M.; Yukawa, N.; Rino, Y.; Imada, T.; et al. Perivascular epithelioid cell tumor of the rectum: Report of a case and review of the literature. World J. Surg. Oncol. 2014, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Balta, S.; Wallace, S. Perivascular epithelioid cell tumour of the rectum: Uncommon tumour in unusual location. Pathology 2015, 47, S60. [Google Scholar] [CrossRef]
- Iwamoto, R.; Kataoka, T.R.; Furuhata, A.; Ono, K.; Hirota, S.; Kawada, K.; Sakai, Y.; Haga, H. Perivascular epithelioid cell tumor of the descending colon mimicking a gastrointestinal stromal tumor: A case report. World J. Surg. Oncol. 2016, 14, 285. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Deng, M.; Gao, J.; Tao, K. A recurrent perivascular epithelioid cell tumor of sigmoid colon with pancreatic metastasis: An extremely rare case report and review of the literature. Int. J. Color. Dis. 2016, 31, 1237–1240. [Google Scholar] [CrossRef]
- Batereau, C.; Knösel, T.; Angele, M.; Dürr, H.R.; D’Anastasi, M.; Kampmann, E.; Ismann, B.; Bücklein, V.; Lindner, L.H. Neoadjuvant or adjuvant sirolimus for malignant metastatic or locally advanced perivascular epithelioid cell tumors: Two case reports. Anticancer Drugs 2016, 27, 254–258. [Google Scholar] [CrossRef]
- Kolin, D.L.; Duan, K.; Ngan, B.; Gerstle, J.T.; Krzyzanowska, M.K.; Somers, G.R.; Mete, O. Expanding the Spectrum of Colonic Manifestations in Tuberous Sclerosis: L-Cell Neuroendocrine Tumor Arising in the Background of Rectal PEComa. Endocr. Pathol. 2018, 29, 21–26. [Google Scholar] [CrossRef]
- Neuhaus, L.; Probst, A.; Messmann, H.; Siebert, L.; Vlasenko, D.; Agaimy, A.; Ting, S. Invagination as Manifestation of a Perivascular Epithelioid Cell Neoplasm (PEComa) of the Colon. Am. J. Gastroenterol. 2018, 113, 1115. [Google Scholar] [CrossRef]
- Lin, K.H.; Chang, N.J.; Liou, L.R.; Su, M.S.; Tsao, M.J.; Huang, M.L. Successful management of perivascular epithelioid cell tumor of the rectum with recurrent liver metastases: A case report. Medicine 2018, 97, e11679. [Google Scholar] [CrossRef]
- Iwa, N.; Yutani, C.; Kobayashi, T.K. Presence of eosinophilic intracytoplasmic inclusions diagnosed by fine needle aspiration cytology in perivascular epithelioid cell tumor (PEComa) arising from the cecum. Diagn. Cytopathol. 2019, 47, 359–361. [Google Scholar] [CrossRef]
- Bennett, J.; Laury, R.; Dai, H.; Walde, C.; Kasi, A. A Curious Case of Colonic Perivascular Epithelioid Cell Tumor: A Unique Diagnosis With Variable Presentations. Cureus 2020, 12, e11164. [Google Scholar] [CrossRef]
- Fuse, Y.; Mori, S.; Sato, S.; Kato, D.; Shibazaki, T.; Nakada, T.; Yabe, M.; Matsudaira, H.; Hirano, J.; Ohtsuka, T. A successful case of complete surgical resection via left upper and right lower lobectomy for bilateral lung metastases of a perivascular epithelioid cell tumor in the colon: A case report. Surg. Case Rep. 2021, 7, 233. [Google Scholar] [CrossRef] [PubMed]
- Razak, O.A.; Varela, C.; Nassr, M.M.A.; Jang, M.; Han, Y.D. A case of caecal “PECOMA”: An uncommon entity. Int. J. Surg. Case Rep. 2022, 90, 106689. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, P.; Zhang, X.; Zhang, J. Rare perivascular epithelial cell tumor of the colon 18F-FDG PET/CT imaging: A case report. Medicine 2023, 102, e33802. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, N.; Hirata, D.; Iwatate, M.; Hattori, S.; Fujita, M.; Sano, W.; Fujimori, T.; Sano, Y. A diminutive perivascular epithelioid cell tumor in the colon. DEN Open 2025, 5, e390. [Google Scholar] [CrossRef]
- Wu, J.H.; Zhou, J.L.; Cui, Y.; Jing, Q.P.; Shang, L.; Zhang, J.Z. Malignant perivascular epithelioid cell tumor of the retroperitoneum. Int. J. Clin. Exp. Pathol. 2013, 6, 2251–2256. [Google Scholar]
- Sobiborowicz, A.; Świtaj, T.; Teterycz, P.; Spałek, M.J.; Szumera-Ciećkiewicz, A.; Wągrodzki, M.; Zdzienicki, M.; Czarnecka, A.M.; Rutkowski, P. Feasibility and Long-Term Efficacy of PEComa Treatment—20 Years of Experience. J. Clin. Med. 2021, 10, 2200. [Google Scholar] [CrossRef]
- Chen, Z.; Han, S.; Wu, J.; Xiong, M.; Huang, Y.; Chen, J.; Yuan, Y.; Peng, J.; Song, W. A systematic review: Perivascular epithelioid cell tumor of gastrointestinal tract. Medicine 2016, 95, e3890. [Google Scholar] [CrossRef]
- Okamoto, S.; Komura, M.; Terao, Y.; Kurisaki-Arakawa, A.; Hayashi, T.; Saito, T.; Togo, S.; Shiokawa, A.; Mitani, K.; Kobayashi, E. Pneumothorax caused by cystic and nodular lung metastases from a malignant uterine perivascular epithelioid cell tumor (PEComa). Respir. Med. Case Rep. 2017, 22, 77–82. [Google Scholar] [CrossRef]
- Wagner, A.J.; Malinowska-Kolodziej, I.; Morgan, J.A.; Qin, W.; Fletcher, C.D.; Vena, N.; Ligon, A.H.; Antonescu, C.R.; Ramaiya, N.H.; Demetri, G.D. Clinical Activity of mTORInhibition with Sirolimus in Malignant Perivascular Epithelioid Cell Tumors: Targeting the Pathogenic Activation of mTORC1 in Tumors. J. Clin. Oncol. 2010, 28, 835–840. [Google Scholar] [CrossRef]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Huang, J.; Wu, S.; Wu, C.L.; Manning, B.D. Signaling Events Downstream of Mammalian Target of Rapamycin Complex 2 Are Attenuated in Cells and Tumors Deficient for the Tuberous Sclerosis Complex Tumor Suppressors. Cancer Res. 2009, 69, 6107–6114. [Google Scholar] [CrossRef]
- Ali, E.S.; Mitra, K.; Akter, S.; Ramproshad, S.; Mondal, B.; Khan, I.N.; Islam, M.T.; Sharifi-Rad, J.; Calina, D.; Cho, W.C. Recent advances and limitations of mTOR inhibitors in the treatment of cancer. Cancer Cell Int. 2022, 22, 284. [Google Scholar] [CrossRef]
| Markers | Staining Intensity | Positive Cells (%) |
|---|---|---|
| Cathepsin K | ++ | 100% |
| CD68 | +/++ | 5% |
| MiTF | +++ | 10% |
| HMB45 | ++ | 2% |
| Vimentin | +/+++ | 30% |
| TFE3 | +/++ | 30% |
| Smooth muscle actin | + | 5% |
| CyclinD1 | +/++ | 40% |
| Ki-67 | + | 10% |
| Desmin | ++/+++ | 60% |
| MelanA/HMB | + | <1% |
| Pancytokeratin (AE1/AE3) | − | − |
| Caldesmon | − | − |
| Myogenin | − | − |
| MyoD1 | − | − |
| DOG1 | − | − |
| CD117 | − | − |
| S100 | − | − |
| SOX-11 | − | − |
| ALK1 | − | − |
| MUM1 | − | − |
| Authors | Year | Gender | Age | Symptoms | Tumor Size (CT or Colonoscopy) | Localization | Metastasis | Initial Treatment | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Prasad et al. [17] | 2000 | Female | 22 | None (incidental) | 3 cm | Cecum | No | Surgery | NED at 6 mo |
| Tazelaar et al. [18] | 2001 | Female | 9 | Tumor prolapse per anus | 3 cm | Rectum | No | Surgery | NED at 14 mo |
| Tazelaar et al. [18] | 2001 | Female | 40 | Profuse rectal bleeding | 3 cm | Rectum | No | Surgery | NED at 6 mo. |
| Birkhaeuser et al. [19] | 2004 | Female | 35 | Occult blood in the feces | 3–4 cm | Cecum | No | Surgery | NED at 9 mo |
| Genevay et al. [20] | 2004 | Female | 36 | Anemia and rectorrhagia | 3.5 cm | Cecum | No | Surgery | NA |
| Genevay et al. [20] | 2004 | Female | 35 | Pararectal mass | - | Rectum | No | Surgery | NA |
| Evert et al. [21] | 2005 | Female | 56 | Rectal obstruction, gluteal pain, recurrent diarrhea, weight loss | 8 × 5 cm | Rectum | Lung metastasis | Surgery | NA |
| Yamamoto et al. [22] | 2006 | Female | 43 | Abdominal pain | 8 cm | Descending colon | No | Surgery | Peritoneal dissemination of tumor at 20 mo DOD at 36 mo |
| Baek et al. [23] | 2007 | Female | 16 | Rectal bleeding | 2.5 × 2.0 cm | Proximal transverse colon | No | Endoscopic resection | NED at 24 mo |
| Pisharody et al. [24] | 2008 | Male | 11 | Rectal bleeding | 3 cm | Sigmoid colon | Lymph node metastasis | Surgery | NED at 5 mo |
| Righi et al. [25] | 2008 | Male | 11 | Rectal bleeding | 3.5 cm | Descending/sigmoid colon | No | Surgery | NA |
| Qu et al. [26] | 2009 | Female | 43 | - | 2 cm | Cecum | No | Surgery | NED at 25 mo |
| Ryan et al. [10] | 2009 | Female | 15 | Rectal bleeding | 4 cm | Rectum | Lymph node metastasis | Surgery and adjuvant chemotherapy | NED |
| Tanaka et al. [11] | 2009 | Female | 14 | Physical examination | 4 cm | Sigmoid colon | No | Surgery | NED at 5 mo |
| Gross et al. [7] | 2010 | Male | 5.5 | Abdominal pain and fever | 5 cm | Ascending colon | No | Surgery | NED at 2 yr |
| Freeman et al. [8] | 2010 | Female | 17 | Rectal bleeding | 4–6 cm | Sigmoid colon | No | Surgery | NED at 15 yr |
| Park et al. [9] | 2010 | Male | 7 | Abdominal pain | 3.9 cm | Ascending colon | No | Surgery | NED at 26 mo |
| Shi et al. [27] | 2010 | Female | 38 | - | 6.0 cm | Ascending colon | No | Surgery | NED at 8 mo |
| Shi et al. [27] | 2010 | Male | 42 | - | 4.5 cm | Sigmoid colon | No | Surgery | NED at 15 mo |
| Shi et al. [27] | 2010 | Male | 36 | - | 4.8 cm | Descending colon | No | Surgery | NED at 32 mo |
| Shi et al. [27] | 2010 | Female | 45 | - | 3.5 cm | Ascending colon | No | Surgery | NED at 36 mo |
| Maran-Gonzalez et al. [6] | 2011 | Female | 11 | Prolapsed mass | 2 cm | Rectum | No | Endoscopic resection; | NA |
| Lee et al. [28] | 2012 | Female | 62 | Bleeding | 5 cm | Sigmoid | No | Surgery | NED at 16 mo |
| Lee et al. [28] | 2012 | Female | 62 | Abdominal pain and melena | 5.5 × 4.2 × 2.5 cm | Sigmoid colon | No | Surgery | NED at 4 mo |
| Scheppach et al. [29] | 2013 | Male | 23 | Abdominal pain and melaena | 5.5 cm | Cecum | Lymph node and liver metastasis | Surgery and adjuvant chemotherapy | DOD |
| Im et al. [5] | 2013 | Male | 17 | Hematochezia | 3 cm | Rectum | No | Endoscopic resection | NED at 10 mo |
| Korytnaya et al. [30] | 2014 | Female | 47 | Amenorrhea, galactorrhea, abdominal pain | 17 × 13 × 8 cm | Distal stomach, proximal duodenum, and right colon | No | Surgery | NED |
| Kanazawa et al. [31] | 2014 | Female | 55 | None (physical examination) | 3 cm | Rectum | No | Endoscopic resection | NED at 15 mo |
| Balta et al. [32] | 2015 | Male | 49 | Rectum bleeding | - | - | - | - | - |
| Iwamoto et al. [33] | 2016 | Female | 42 | None ( annual medical checkup) | 5 cm | Descending colon | No | Surgery | NED at 5 mo. |
| Cheng et al. [34] | 2016 | Male | 40 | Dyschezia | 6 × 7 cm | Sigmoid colon | No | Surgery | Recurrence and pancreatic metastasis at 27 mo |
| Batereau et al. [35] | 2016 | Female | 26 | Localized abdominal pain | - | Transverse colon | No | Surgery | AWD, multiple relapses |
| Kolin et al. [36] | 2017 | Female | 18 | Rectal bleeding | 1.8 cm | Rectum | - | Surgery | NA |
| Neuhaus et al. [37] | 2018 | Female | 36 | Abdominal and suprapubic pain | 10 cm | Transverse colon | No | Surgery | NA |
| Lin et al. [38] | 2018 | Male | 28 | Abdominal pain and intermittent melena | 8.9 × 7.2 cm | Cecum | No | Surgery | Liver metastasis at 49 mo NED at 28 mo. after the second operation |
| Iwa et al. [39] | 2019 | Male | 69 | None ( annual medical checkup) | 4.1 × 4.0 × 3.2 cm | Cecum | No | Surgery | NA |
| Uhlenhopp et al. [16] | 2020 | Male | 64 | Left lower quadrant pain and nausea with vomiting | 12.6 ×10 × 9 cm | Sigmoid colon | Liver metastasis | Surgery | DOD |
| Bennett et al. [40] | 2020 | Female | 67 | No gastrointestinal symptoms | 0.8 cm | Ascending colon | No | Endoscopic dissection | NED |
| Fuse et al. [41] | 2021 | Female | 53 | - | - | Colon | - | Surgery | Lung metastasis at 8 yr |
| Cheng et al. [4] | 2021 | Female | 17 | Painless hematochezia | 3.5 × 3.1 × 2.8 cm | Rectosigmoid Colon | Lymph node metastasis | Surgery and adjuvant chemotherapy | NED at 24 mo |
| Razak et al. [42] | 2022 | Female | 69 | None (annual medical checkup) | - | Cecum | No | Surgery | NED at 48 mo |
| Kou et al. [3] | 2022 | Female | 12 | Abdominal pain | 5.0 × 4.5 × 3.0 cm | Transverse colon | No | Surgery | NED at 4 mo |
| Chen et al. [43] | 2023 | Female | 55 | Abdominal pain, weight loss | 12.0 × 6.7 cm | Ascending Colon | No | Surgery | NA |
| Yan et al. [12] | 2023 | Female | 47 | - | 1.5 × 2.0 cm | Sigmoid colon | No | Endoscopic resection | NED at 3 mo |
| Sugimura et al. [44] | 2024 | Male | 64 | None (follow-up colonoscopy) | 0.4 cm | Transverse colon | No | Polypectomy | NED |
| Current report | 2024 | Female | 61 | Defecation with blood clots | 3 × 3 × 3 cm | Sigmoid colon | No | Surgery | NED at 9 mo |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slice, G.; Stulpinas, R.; Poskus, T.; Kryzauskas, M. Perivascular Epithelioid Cell Tumor (PEComa) of the Sigmoid Colon: Case Report and Literature Review. Curr. Oncol. 2025, 32, 330. https://doi.org/10.3390/curroncol32060330
Slice G, Stulpinas R, Poskus T, Kryzauskas M. Perivascular Epithelioid Cell Tumor (PEComa) of the Sigmoid Colon: Case Report and Literature Review. Current Oncology. 2025; 32(6):330. https://doi.org/10.3390/curroncol32060330
Chicago/Turabian StyleSlice, Gintare, Rokas Stulpinas, Tomas Poskus, and Marius Kryzauskas. 2025. "Perivascular Epithelioid Cell Tumor (PEComa) of the Sigmoid Colon: Case Report and Literature Review" Current Oncology 32, no. 6: 330. https://doi.org/10.3390/curroncol32060330
APA StyleSlice, G., Stulpinas, R., Poskus, T., & Kryzauskas, M. (2025). Perivascular Epithelioid Cell Tumor (PEComa) of the Sigmoid Colon: Case Report and Literature Review. Current Oncology, 32(6), 330. https://doi.org/10.3390/curroncol32060330





