Abstract
Introduction: Prostate cancer with spinal metastases (PCSM) is associated with high morbidity and mortality. The impact of biomarkers on the prognosis of spinal metastases, however, remains unclear. Objective: This study explored associations between potential biomarkers, treatment modalities, survival, and neurological outcomes in PCSM patients. Methods: We conducted a retrospective analysis of 68 patients as part of a neurosurgical cohort with PCSM at a comprehensive cancer center from 2013 to 2023, examining the influence of potential biomarkers, treatment modalities, and demographics on prognosis. The primary outcomes were the identification of biomarkers, overall survival (OS) in years, survival after spinal metastasis in years, spinal metastasis recurrence, and postoperative neurological outcomes via Frankel scores. Results: All the patients (n = 68) had adenocarcinoma, and the median age was 69 years. The mortality rate was 66% with a median OS of 6 years. Seventy-two biomarkers were identified. An accelerated failure time model (AFT) showed that radiotherapy to the prostate increased the OS (TR = 1.805, p = 0.001), while smoking status (TR = 0.625, p < 0.001) and PTEN gene mutations (TR = 0.504, p = 0.006) were associated with decreased OS. Kaplan–Meier analysis associated PTEN mutations with reduced median OS using the Gehan–Breslow–Wilcoxon test (3.50 vs. 9.49 years; p = 0.001). PTEN mutations were trending towards but were not significant for decreased survival following spinal metastases (2.04 vs. 3.15 years; p = 0.08). Both PTEN (p = 0.02) and Tumor Protein 53 (TP53, p = 0.01) mutations were associated with increased spinal metastasis recurrence when analyzed using Fisher’s exact test. No differences were observed in the median OS or survival after spinal metastases among patients with or without androgen receptor splice variant-7 (AR-V7), prostate-specific membrane antigen (PSMA), TP53, or other analyzed biomarkers. Similarly, neither age, receipt of chemotherapy, nor radiotherapy to the spine correlated with OS. Only chemotherapy was associated with a decreased postoperative Frankel Score (p = 0.002). Conclusions: PTEN mutations and smoking status were associated with decreased OS in patients with PCSM. Both PTEN and TP53 mutations were associated with increased spinal metastasis recurrence. Receipt of radiotherapy to the prostate was correlated with prolonged survival, whereas receipt of radiotherapy to the spine was not. Chemotherapy was associated with decreased postoperative neurological outcomes.
Keywords:
genetic biomarkers; prostate cancer; spine; malignancy; metastasis; mortality; motor; sensory 1. Introduction
Prostate cancer remains the most frequently diagnosed cancer in men worldwide, with an annual incidence of over 1.4 million cases [1]. Early-stage prostate cancer typically has a favorable prognosis due to its slow progression. Yet, survival rates decline significantly once spinal metastasis occurs, rendering treatment options largely palliative [2].
Spinal metastases often lead to morbidity by inducing pathologic fractures, spinal cord compression, and high levels of pain [3]. Associated neurological degeneration and loss of ambulation typically result in an inability to complete activities of daily living [4]. The risk of neurological deterioration underscores the need to identify novel biomarkers that could more accurately predict morbidity and mortality once spinal metastases occur [5].
Recent work has identified genetic mutations that can serve as biomarkers for diagnosing and prognosticating prostate cancer, such as the fusion of Transmembrane Serine Protease 2 (TMPRSSS2) and E26 Transformation-Specific (ETS) family genes, MYC amplification, and alterations in Phosphatase and Tensin Homolog (PTEN) and Tumor Protein 53 (TP53) [6]. These biomarkers, including mutations in androgen receptor (AR)-regulated genes and the identification of specific markers like Kinesin family member 11 (KIF11), have paved the way for targeted therapies and personalized treatment strategies, particularly for bone metastases [6,7,8]. However, the mutational profiles of prostate cancer with spinal metastasis (PCSM) have not been extensively studied in a neurosurgical context, where the clinical management and treatment strategies are unique.
In this study, we fill this gap by examining the mutational profiles of prostate cancer with spinal metastasis (PCSM), specifically in a neurosurgical cohort, providing novel insights into how these mutations correlate with treatment strategies and clinical outcomes. This work is among the first to explore these associations within this specific cohort, potentially leading to more tailored and effective treatments for patients with PCSM.
2. Methods
2.1. Study Design and Ethical Considerations
This study has a retrospective design. After obtaining institutional review board (IRB) approval (IRB00378753), we reviewed the medical records of adult patients as part of a neurosurgical cohort with PCSM at a single comprehensive cancer center from 2013 to 2022. The cohort consisted of all patients with spinal metastasis, both oligometastatic and polymetastatic.
2.2. Data Extraction and Outcomes of Interest
Data collected from the medical records by the authors included patient demographics, functional neurological outcomes, treatment modalities, mutations from genetic sequencing reports, and protein expression from immunohistochemistry data contained in pathology reports. Biomarker data consisted of spine metastasis resection pathology analysis. Each patient was retrospectively analyzed to assess whether next-generation sequencing (NGS) information, performed by a third party at the time of primary cancer diagnosis, was available. If so, sequencing data was obtained in the form of sequencing reports, which contained information on biomarkers of clinical interest and biomarkers with unknown significance. The same methods were used to assess immunohistochemistry data from pathology reports. All the biomarkers included in the reports were listed, though only biomarkers with at least five occurrences were incorporated into our statistical analysis.
The demographic variables included smoking status and age at time of surgical intervention, or if no surgical intervention was performed, at diagnosis. Surgical data included spinal procedure(s) performed and associated complications. Functional neurological outcomes included postoperative changes in Frankel scores (Grade A–E) and ambulatory status, as compared to the associated preoperative status [9]. Treatment modalities consisted of chemotherapy, radiotherapy (RT), immunotherapy, and targeted therapy received at any point during the disease course.
Outcomes of interest included the identification of potential biomarkers, overall survival (OS) in years, survival time after first spinal metastases in years, spinal metastasis recurrence, and postoperative neurological outcomes as assessed by Frankel scores. The OS was defined as the time from the initial diagnosis of prostate cancer until death. Survival time after the first spinal metastasis was defined as the period from the initial occurrence of prostate cancer metastasis to the spine until death. Spinal metastasis recurrence was defined as radiographic evidence of spinal metastasis recurrence after initial surgical intervention.
2.3. Statistical Analysis
The data were collected using Excel (Microsoft Corp., Redmond, WA, USA) and analyzed using Prism 10 (GraphPad, Boston, MA, USA) and R Studio 4.3.0 (RStudio Inc., Boston, MA, USA). The continuous variables are reported as the median and interquartile range (IQR), while the categorical data are presented as counts and percentages. Kaplan–Meier curves were generated to assess the OS and survival time after spinal metastases. Comparisons were made using the Wilcoxon–Breslow–Gehan test, with a significance level set at 0.05. Patients with biomarkers or who received certain treatments were compared to their counterparts without the same biomarkers or treatment status. An accelerated failure time (AFT) model was developed using stepwise backward selection to explore the associations between potential biomarkers with at least five observations, treatment modalities, and demographics with OS. The AFT model included variables such as age, smoking status, treatment modality, and biomarkers with at least five observations that met a p-value of <0.2 in univariate Kaplan–Meier analysis. Time ratios (TR), standard errors (SE), and p-values were reported. Time ratios (TR) greater than 1 indicate that the event of interest took longer to occur compared to the specified reference group, and vice versa. Additionally, an ordinal logistic regression model was employed to identify significant predictors of the postoperative Frankel score. The covariates included in the analysis were biomarkers with at least five observations, treatment modalities, smoking status, and preoperative Frankel score. The model generated odds ratios, 95% confidence intervals (CI), and p-values, with a significance level set at 0.05. Spinal metastasis recurrence was analyzed for all potential biomarkers with at least five observations using Fisher’s exact test with a significance level set at 0.05. A multiple comparison adjustment was not performed given the size of the dataset and the exploratory nature of the analysis.
3. Results
3.1. Baseline Characteristics
We identified sixty-eight eligible patients with prostatic adenocarcinoma metastasis to the spine in our dataset, all of whom were included in our study (Table 1). The median age was 69 years, with an IQR of 63–75 years. The follow-up period for each patient extended from the time of initial prostate cancer diagnosis until death or the last recorded encounter.

Table 1.
Demographic and clinical characteristics of study population (n = 68). * Represents other metastatic lesions discovered at the time of spinal metastases. ** Represents top 10 most frequently prescribed treatments.
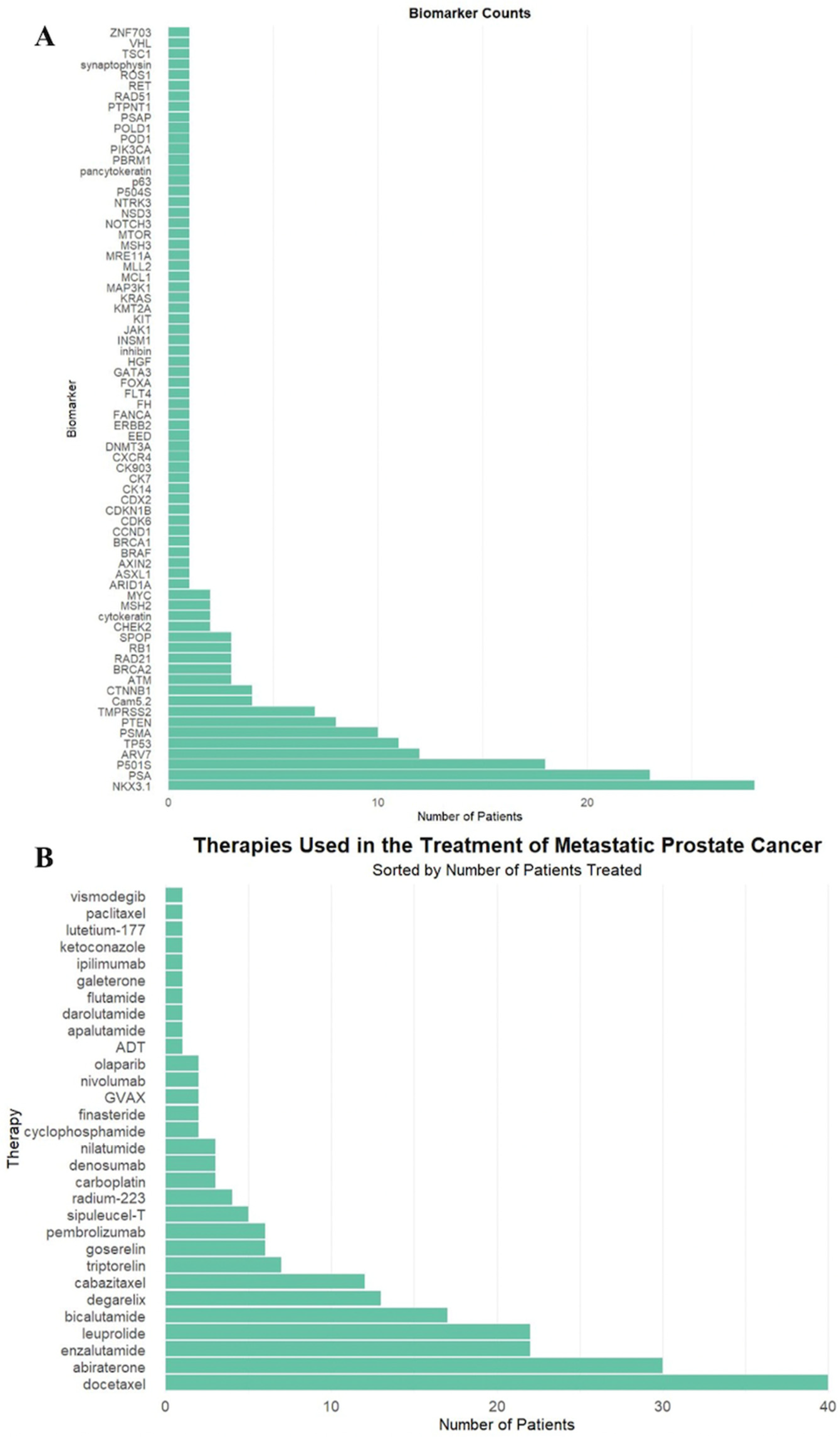
In total, 72 unique biomarkers were identified (Figure 1A). These biomarkers were deemed clinically significant due to their inclusion in the pathology and genetic sequencing reports, retrospectively reviewed by authors in patient electronic medical records, as biomarkers of interest.
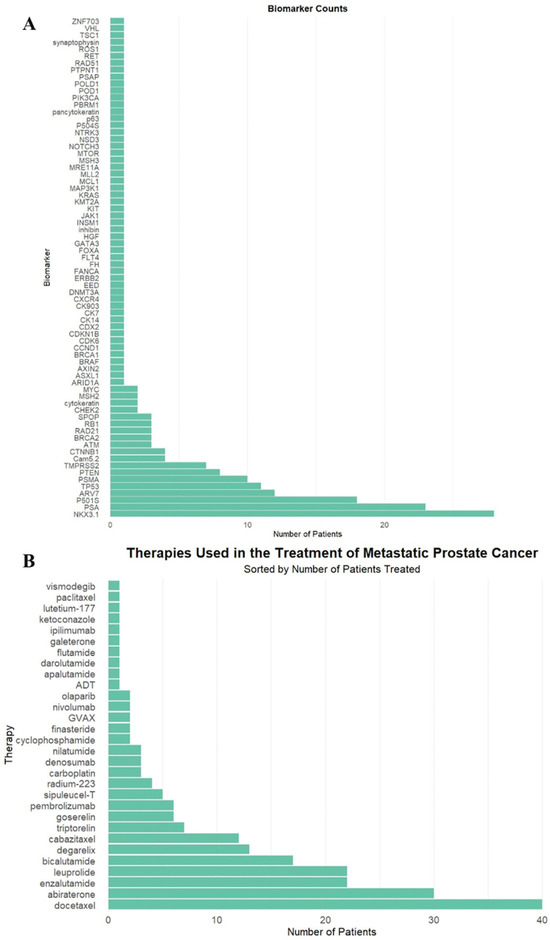
Figure 1.
(A) Biomarker frequency among all patients in the study population. (B) Frequency of therapeutic agents administered to patients in the study population.
The most commonly used therapeutics were docetaxel (40, 59%), abiraterone (30, 44%), and enzalutamide (22, 32%), as demonstrated in Figure 1B. Twenty-three patients (34%) received RT targeted at the primary tumor site, and 59 patients (87%) received RT to the spine.
Sixty-two patients received surgical intervention (91%). Of these patients, the most common surgical interventions were laminectomy (52, 84%), fusion (39, 63%), and corpectomy (10, 16%). The vast majority of patients had no surgical complications (59, 95%). Table 1 lists the complications for the remaining patients.
Metastasis included sites other than the osseous spine, listed in Table 1. The predominant levels of spine metastasis were thoracic (29, 68%), lumbar (10, 24%), cervical (3, 6%), and sacral (1, 3%). The most common (mode) preoperative and postoperative Frankel score was D. The differences in the preoperative and postoperative ambulatory status were not significant (p = 0.81) (Table 1).
3.2. Survival Analysis
The median OS was 6.44 years, with an IQR of 3.32 to 10.82 years. The median time to spinal metastasis was 4.83 years, with an IQR of 2.33 to 9.37 years. Following the development of spinal metastases, the median OS was 2.28 years, with an IQR of 1.04 to 4.53 years.
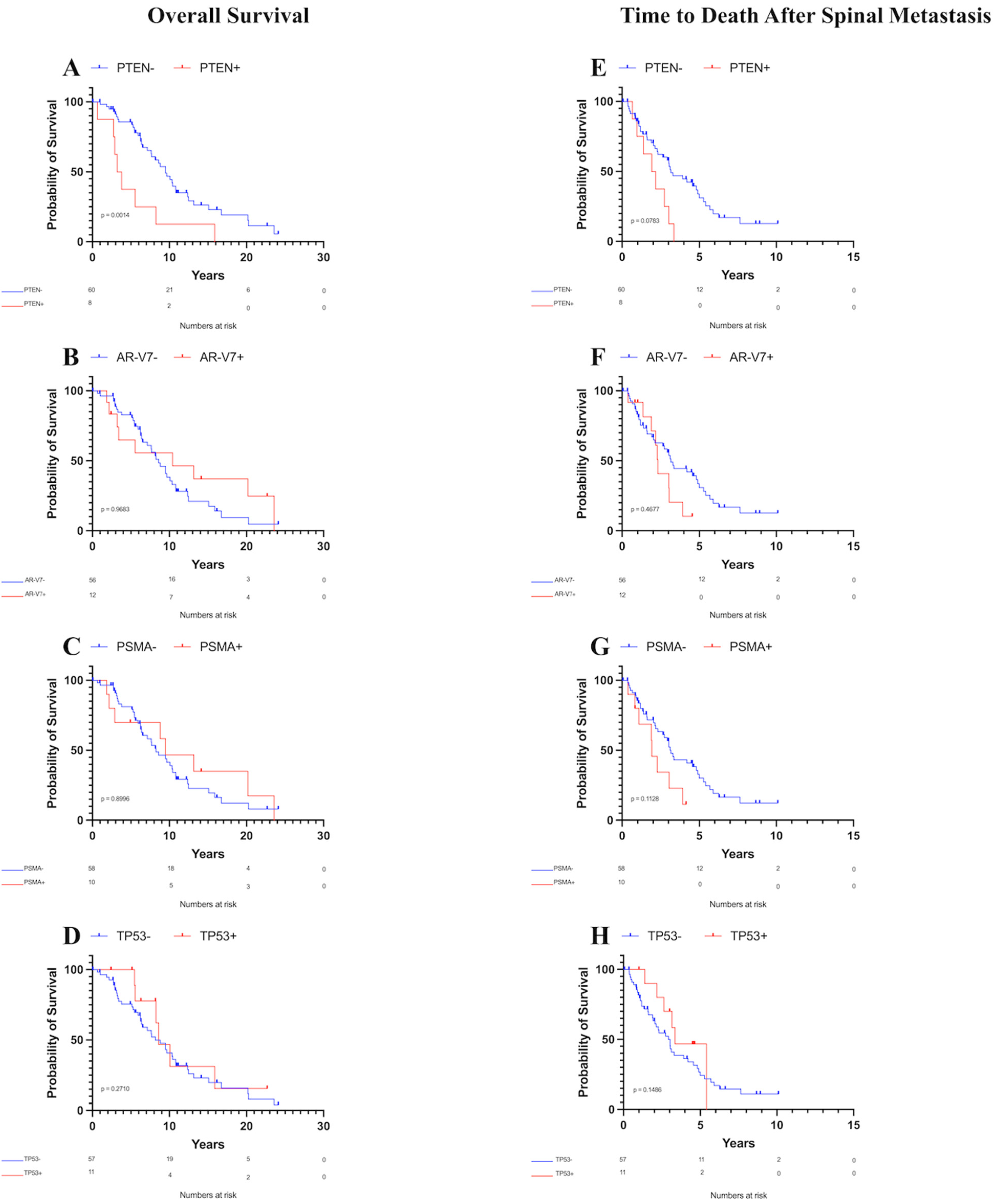
Patients with PTEN mutations exhibited a decreased median OS of 3.50 years compared to 9.49 years for those without PTEN mutations (Figure 2A, p = 0.001). Conversely, no significant differences in the median OS were observed between patients with or without androgen receptor splice variant-7 (AR-V7, Figure 2B, p = 0.97), prostate-specific membrane antigen (PSMA, Figure 2C, p = 0.90), TP53 mutations (Figure 2D, p = 0.27), or any other analyzed biomarker with at least five observations. Furthermore, age at time of surgical intervention (or at diagnosis for patients who did not receive surgical intervention) did not significantly affect the OS according to Kaplan–Meier survival analysis (p = 0.08).
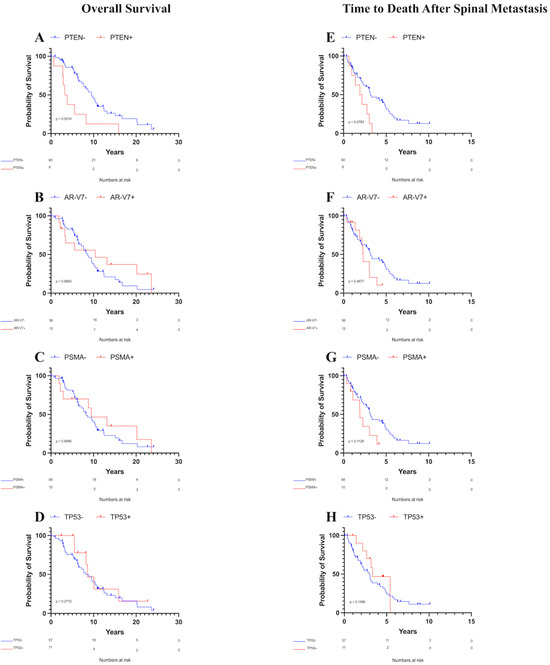
Figure 2.
Kaplan–Meier overall survival and survival after spinal metastasis curves for patients with metastatic prostate cancer to the spine. Only PTEN was associated with decreased OS. (A) OS by PTEN status, (B) OS by AR-V7 status, (C) OS by PSMA status, (D) OS by TP53 status, (E) Survival time after spinal metastasis by PTEN status, (F) Survival time after spinal metastasis by AR-V7 status, (G) Survival time after spinal metastasis by PSMA status, (H) Survival time after spinal metastasis by TP53 status.
Analysis of survival time after spinal metastasis revealed that patients with PTEN mutations had a median OS of 2.04 years compared to 3.15 years for those without PTEN mutations. This difference was not significant using the Gehan–Breslow–Wilcoxon test, although it was trending towards significance (Figure 2E, p = 0.08). No significant differences in the median OS after spinal metastasis were found between patients with or without AR-V7 (Figure 2F, p = 0.47), PSMA (Figure 2G, p = 0.11), TP53 mutations (Figure 2H, p = 0.15), or any other analyzed biomarker with at least five observations. Additionally, age showed no association with OS after spinal metastasis according to Kaplan–Meier survival analysis (p = 0.59).
Finally, spinal metastasis recurrence was analyzed using Fisher’s exact test. In total, 11 (18%) patients experienced spinal metastasis recurrence after surgical intervention, of which 5 (45%) occurred at the same spinal level, 4 (36%) occurred at an adjacent level, and 8 (73%) occurred at a distant level. Both PTEN (p = 0.02) and TP53 mutations (p = 0.01) were associated with increased spinal metastasis recurrence (Table 2).

Table 2.
Fisher’s exact test was conducted to correlate spinal metastasis recurrence with biomarker status. Both PTEN and TP53 were associated with recurrence at a significance level of 0.05.
3.3. Predictive Models
An ordinal logistic regression model was utilized to assess the impact of various factors on the postoperative Frankel score, considering the preoperative Frankel score as a covariate (Table 3). The factors included age, smoking status, treatment modality, and biomarkers with at least five observations. Notably, treatment with chemotherapy was associated with a decreased postoperative Frankel score (p = 0.002). Additionally, an accelerated failure time (AFT) model employing stepwise backward selection was applied to explore the influence of factors such as age, smoking status, treatment modality, and biomarkers with at least five observations and p < 0.2 from the Wilcoxon–Breslow–Gehan test during Kaplan–Meier analysis on the OS. RT to the prostate was associated with increased OS (Table 4, TR = 1.805, p = 0.001). Conversely, smoking status (TR = 0.625, p < 0.001) and PTEN gene mutations (TR = 0.504, p = 0.006) were negatively associated with OS. Table 5 summarizes the major findings.

Table 3.
An ordinal logistic regression model (backwards selection) was performed to predict the postoperative Frankel Score using biomarkers (n ≥ 5) and various factors, with the preoperative Frankel Score acting as a covariate. Age, radiotherapy to spine and radiotherapy to prostate, immunotherapy, and targeted therapy were included in the original model and eliminated through backwards selection. The preoperative Frankel score and chemotherapy use were associated with predicting the postoperative Frankel score at a 0.05 significance level.

Table 4.
An accelerated failure time model (backwards selection) was used to assess the influence of biomarkers (n ≥ 5) and various covariates on the overall survival. Age, chemotherapy, and radiotherapy to spine were included in the initial model but were found to be insignificant and removed through backward selection. Smoking status, radiotherapy to prostate, and PTEN were associated with overall survival at a 0.05 significance level.

Table 5.
Summary of major findings.
4. Discussion
Metastatic prostate cancer (mPC) is common, with almost one-fifth of patients presenting with metastasis at diagnosis [10]. Prostate cancer has a predilection for osseous metastases, of which the spine is the most common site [2]. Biomarkers offer a novel avenue for the prognostication of morbidity and mortality in patients with PCSM.
4.1. Biomarkers
To the best of our knowledge, our results are the first to demonstrate that mutations in PTEN, a tumor-suppressor gene, are associated with decreased OS or increased spinal metastasis recurrence in a surgical cohort consisting entirely of prostate cancer patients who developed spinal metastases. Prior research has identified PTEN as a gene inactivated by mutations or deletions in patients with prostate cancer [6]. Yoshimoto et al. demonstrated PTEN to be associated with a higher Gleason Score and tumor stage, indicating a higher-grade tumor and more aggressive disease course [11]. Ferraldeschi et al. demonstrated that PTEN inactivation is associated with worse OS in metastatic castration-resistant prostate cancer (mCRPC) [12]. Likewise, Zhang et al. found PTEN mutations to be associated with decreased progression-free survival (PFS) and OS in a group of 205 patients with metastatic castration-naïve prostate cancer (mCNPC) [13]. Further research should explore the potential prognostic value of identifying PTEN mutations in patients with PCSM and any associations between PTEN mutations and an increased risk of metastasis to other locations.
Our results also demonstrated no difference in OS or survival after spinal metastases in patients with mutations in AR-V7, PSMA, or TP53. AR-V7 is an androgen receptor splice variant that is hypothesized to be a potential molecular contributor to prostate cancer progression, given its role in conferring resistance to enzalutamide and abiraterone in preclinical studies [14]. Antonarakis et al. illustrated that patients with the AR-V7 mCRPC variant had shorter PFS and no appreciable clinical benefit with the administration of abiraterone/enzalutamide compared to their counterparts without AR-V7 [15].
The expression of prostate-specific membrane antigen (PSMA), which is upregulated in prostate cancer, has been studied as a prognostic factor. Sweat et al. analyzed 232 patients with node-positive prostatic adenocarcinoma and noted that PSMA expression is significantly greater in prostate adenocarcinoma and lymph node metastases compared to benign prostatic tissue [16]. Likewise, Perner et al. demonstrated that high PSMA levels are associated with increased rates of prostate cancer recurrence [17]. In contrast, Pomykala et al. suggest that PSMA identification does not significantly influence mPC management [18].
Finally, mutations in TP53, a tumor-suppressor gene, have been postulated to be associated with decreased survival in patients with prostate cancer. Zhang et al. demonstrated that TP53 mutations in circulating tumor DNA of Chinese patients with prostate cancer were associated with a higher rate of metastases and castration resistance, in addition to decreased PFS [13]. Zhou et al. identified TP53 mutations as a negative independent predictor in mPC, especially when co-occurring with other genetic alterations, such as AR-amplification and PTEN deletion [19]. Our data suggests that TP53 mutations may be associated with increased risk of spinal metastasis recurrence.
Therefore, since our results demonstrate no correlation between survival and AR-V7, PSMA, or TP53 mutation status, this likely reflects the limited statistical power of our study based on our sample size. This is especially true regarding AR-V7 and TP53, whose statuses as negative prognostic factors are better established in the literature.
4.2. Treatments
Our analysis revealed that prostate-directed radiation therapy (RT) was associated with improved OS. Mohiuddin et al. concluded that external beam radiation therapy (EBRT) provided a survival benefit when combined with androgen deprivation therapy (ADT) in high-risk prostate cancer patients compared to conservative management [20]. Zelefsky et al. demonstrated in a cohort of 2047 patients with clinically localized prostate cancer that high-dose intensity modulated radiation therapy (IMRT) improved biochemical control and decreased the risk of distant metastases [21].
In contrast, we found that radiation therapy of the spine was not associated with improved OS. While this may represent a true lack of association, it may also represent the limited power of the study based on the sample size. Fischer-Valuck et al. noted improved OS in patients with prostate cancer and bone (but not exclusively spine) metastasis receiving long-course radiation therapy (LC-RT) but not short-course radiation therapy (SC-RT) [22]. Abugharib et al. found spine-directed stereotactic body radiation therapy (SBRT) to be associated with improved 1- and 2-year OS rates in a hormone-sensitive prostate cancer cohort (98% and 95%) compared to a castration-resistant prostate cancer cohort (79% and 65%) [23]. Still, the literature suggests that the timing and staging of prostate cancer are critical in determining the survival benefits of RT, as patients with a higher disease volume burden typically fare worse following RT administration [24].
In comparison to RT, chemotherapy was not associated with OS but was correlated with worse postoperative neurological outcomes. Originally used as a palliative measure, chemotherapy in prostate cancer has evolved to offer survival advantages, largely due to the implementation of docetaxel [25]. Clinical trials suggest that docetaxel is mainly beneficial in lowering prostate-specific antigen (PSA) values but may offer increased survival in patients with prostate cancer early in their disease course [25]. One clinical trial found no significant survival benefit in mCRPC patients, whereas others reported an increase in OS in mCNPC [26,27,28]. Despite these benefits, chemotherapeutic agents are associated with gastrointestinal and cardiovascular toxicities, thromboembolic events, infections, and neuropathy in patients with prostate cancer [25]. Consequently, their side effect profiles may contribute to worse postoperative neurological outcomes with prolonged use. Chemotherapy is also not a first-line treatment for prostate cancer but is, instead, reserved for advanced cases. The observed decline in neurological outcomes among patients with PCSM receiving chemotherapy may, therefore, be more directly attributable to the extent of metastasis rather than the impact of chemotherapy itself.
Finally, our study did not find a survival benefit associated with the use of immunotherapy for patients with prostate cancer and spinal metastases. Again, this may represent a lack of true association or the limited power of the study to detect a true correlation given the sample size. Immunotherapy, which has been found to improve OS in patients with prostate cancer, is an ideal treatment modality as prostate cancer cells express unique proteins, such as PSA and PSMA, that can act as therapeutic targets for the immune system [29]. However, due to the heterogeneity found within the tumor microenvironment in prostate cancer, establishing a standard immunotherapy treatment has been difficult [29]. In mCRPC patients, Sipuleucel-T, an immunotherapy vaccine for prostate cancer, was found to improve OS, though not the PFS or PSA value [30]. Consequently, National Comprehensive Cancer Center Network (NCCN) guidelines recommend using Sipuleucel-T early in the disease course when the immune system retains higher functionality. This is in contrast to the lower immune system functionality observed in mPC patients, as may have been the case in our patient population [31].
4.3. Demographic Factors
Our results demonstrated no survival difference based on the age of PCSM patients at the time of surgical intervention or diagnosis. Hamsta et al. reported that younger individuals (less than age 70) are diagnosed with metastatic lesions in prostate cancer at a higher rate when compared to geriatric patients [32]. Although these tumors may present more aggressively in younger patients, this population may be offered more aggressive treatment options [32]. Studies have also linked older age to biases in oncological treatments, which have, at times, resulted in under-treatment [33]. As a result, elderly patients may not be offered more aggressive therapeutic regimens and, subsequently, fare worse. In addition, older age is linked to a higher comorbidity burden, which is often associated with worse outcomes.
Finally, our data indicate that smoking history is associated with decreased OS. This is in line with Foerster et al., who found smoking status to be associated with a higher risk of recurrence, metastasis, and reduced survival in patients with prostate cancer [34]. Kenfield et al. also recognized that smoking status at the time of prostate cancer diagnosis is associated with increased overall mortality, cardiovascular-disease-specific mortality, and prostate-cancer-specific mortality [35]. Smokers are reported to undergo routine PSA testing less frequently and may, as a result, be more likely to be diagnosed at a later stage, increasing their risk of mortality [36]. In addition, Plaskon et al. observed a dose–response relationship between pack-years and prostate cancer risk in middle-aged men, suggesting that smoking may directly influence tumor growth [37].
4.4. Limitations
While our study outlines several factors associated with survival and postoperative neurological outcomes in PCSM, it is not without limitations. The scope of our analysis was constrained by a relatively small patient cohort sourced from a single institution, thereby limiting our statistical power. We also combined our analysis to include both protein expression from immunohistochemical data and genetic mutations from sequencing data in an exploratory fashion, which limited our ability to comprehensively analyze each individually. In addition, the retrospective nature of our study allowed us to demonstrate correlation but not causation. Moreover, the small sample size prevented the analysis of specific gene mutations and differences in the chemotherapy and RT received. Therefore, larger multi-institutional studies are needed to better understand how genetic mutations and other potential biomarkers, treatment modalities, and demographic factors affect survival and postoperative neurological outcomes in PCSM patients. Larger studies would also aid in investigating the relationship between primary tumor biomarkers and biomarkers from the resected spinal cord metastatic tumor tissue, and understanding the relationships and interactions between different mutations. Lastly, a homogenous patient population was used, neurosurgical PCSM patients, which may reduce the general validity of our results. Further, large institutional studies not limited to solely neurosurgical PCSM patients are warranted.
5. Conclusions
The use of mutation status may aid in the prognostication of morbidity and mortality in patients with PCSM. This study provides novel insights into how specific genetic mutations, particularly PTEN and TP53 mutations, influence clinical outcomes in this unique patient population. In this study, we demonstrated that PTEN mutations and smoking status were associated with decreased overall survival (OS) in PCSM patients. These findings suggest that PTEN mutations, along with smoking history, could be critical factors in early risk stratification. Both PTEN and TP53 mutations were associated with increased spinal metastasis recurrence, highlighting the potential of these mutations as biomarkers for recurrence risk in PCSM patients. Further analyses with larger sample sizes for sufficient power should be conducted to identify specific PTEN mutations associated with poor survival outcomes.
Additionally, our study presents new evidence that radiation therapy (RT) to the prostate was correlated with enhanced survival, whereas RT to the spine did not show the same benefit, suggesting the need for more targeted radiation strategies in the management of spinal metastases. Only chemotherapy was associated with decreased postoperative neurological outcomes, underscoring the complex interplay between systemic treatments and neurological health in this cohort.
By elucidating these associations, our work offers novel insights that could guide clinicians in developing more personalized treatment plans for patients with PCSM, ultimately improving clinical decision-making and patient outcomes. These findings provide a foundation for future studies exploring targeted therapies tailored to the mutational profiles of PCSM patients.
Author Contributions
Conceptualization, A.A. and Y.X.; Data curation, A.A., M.A.H., and S.S.; Formal analysis, A.A.; Investigation, Y.X.; Methodology, A.A., P.P., S.A.S., and C.W.-L.; Project administration, S.L., K.J.R., A.B., T.F.W., N.T., and D.L.; Resources, A.-H.A.-M.; Supervision, S.L., K.J.R., A.B., T.F.W., N.T., and D.L.; Writing—original draft, A.A., P.P., M.A.H., S.S., S.A.S., S.N., and B.Z.M.; Writing—review and editing, A.A., Y.X., P.P., M.A.H., S.A.S., C.W.-L., S.N., B.Z.M., S.L., K.J.R., A.B., T.F.W., N.T., and D.L. All the authors will be updated at each stage of manuscript processing, including submission, revision, and revision reminder, via emails from our system or the assigned Assistant Editor. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All procedures involving human participants in this study were reviewed and approved by the Institutional Review Board (IRB00378753, approved date: 8 March 2023). This study followed the 1975 Helsinki Declarations, revised in 2008, and their later amendments or comparable ethical standards.
Informed Consent Statement
Informed consent was waived due to the study’s retrospective design.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| PCSM | Prostate cancer with spinal metastasis |
| IRB | Institutional review board |
| RT | Radiotherapy |
| OS | Overall survival |
| IQR | Interquartile range |
| AFT | Accelerated failure time |
| TR | Time ratio |
| SE | Standard error |
| CI | Confidence interval |
| AR-V7 | Androgen receptor splice variant-7 |
| PSMA | Prostate-specific membrane antigen |
| mPC | Metastatic prostate cancer |
| mCRPC | Metastatic castration-resistant prostate cancer |
| PFS | Progression-free survival |
| mCNPC | Metastatic castration-naïve prostate cancer |
| EBRT | External beam radiation therapy |
| ADT | Androgen deprivation therapy |
| IMRT | Intensity modulated radiation therapy |
| LC-RT | Long-course radiation therapy |
| SC-RT | Short-course radiation therapy |
| SBRT | Stereotactic body radiation therapy |
| PSA | Prostate-specific antigen |
| NCCN | National Comprehensive Cancer Center Network |
References
- Leslie, S.W.; Soon-Sutton, T.L.; Sajjad, H.; Skelton, W.P. Prostate Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024; Available online: http://www.ncbi.nlm.nih.gov/books/NBK470550/ (accessed on 4 March 2024).
- Kadeerhan, G.; Xue, B.; Wu, X.-L.; Chen, W.-N.; Wang, D.-W. Incidence trends and survival of metastatic prostate cancer with bone and visceral involvement: 2010-2019 surveillance, epidemiology, and end results. Front. Oncol. 2023, 13, 1201753. [Google Scholar] [CrossRef]
- Tarawneh, A.M.; Pasku, D.; Quraishi, N.A. Surgical complications and re-operation rates in spinal metastases surgery: A systematic review. Eur. Spine J. 2020, 30, 2791–2799. [Google Scholar] [CrossRef]
- Zhang, D.; Yin, H.; Wu, Z.; Yang, X.; Liu, T.; Xiao, J. Surgery and survival outcomes of 22 patients with epidural spinal cord compression caused by thyroid tumor spinal metastases. Eur. Spine J. 2012, 22, 569–576. [Google Scholar] [CrossRef]
- Alvarez-Cubero, M.J.; Arance, E.; de Santiago, E.; Sanchez, P.; Sepúlveda, M.R.; Marrero, R.; Lorente, J.A.; Gonzalez-Cabezuelo, J.M.; Cuenca-Lopez, S.; Cozar, J.M.; et al. Follow-Up Biomarkers in the Evolution of Prostate Cancer, Levels of S100A4 as a Detector in Plasma. Int. J. Mol. Sci. 2022, 24, 547. [Google Scholar] [CrossRef]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate cancer. Nat. Rev. Dis. Prim. 2021, 7, 9. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Liu, B.; Wei, S.; Wang, T.; Li, T.; Lin, J.; Ni, X. KIF11: A potential prognostic biomarker for predicting bone metastasis-free survival of prostate cancer. Oncol. Lett. 2022, 24, 312. [Google Scholar] [CrossRef]
- Ylitalo, E.B.; Thysell, E.; Jernberg, E.; Lundholm, M.; Crnalic, S.; Egevad, L.; Stattin, P.; Widmark, A.; Bergh, A.; Wikström, P. Subgroups of Castration-resistant Prostate Cancer Bone Metastases Defined Through an Inverse Relationship Between Androgen Receptor Activity and Immune Response. Eur. Urol. 2017, 71, 776–787. [Google Scholar] [CrossRef]
- Frankel, H.L.; OHancock, D.; Hyslop, G.; Melzak, J.; Michaelis, L.S.; Ungar, G.H.; Vernon, J.D.S.; Walsh, J.J. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Spinal Cord. 1969, 7, 179–192. [Google Scholar] [CrossRef]
- La Manna, F.; Karkampouna, S.; Zoni, E.; De Menna, M.; Hensel, J.; Thalmann, G.N.; Julio, M.K.-D. Metastases in Prostate Cancer. Cold Spring Harb. Perspect. Med. 2018, 9, a033688. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Cunha, I.W.; ACoudry, R.; Fonseca, F.P.; Torres, C.H.; ASoares, F.; ASquire, J. FISH analysis of 107 prostate cancers shows that PTEN genomic deletion is associated with poor clinical outcome. Br. J. Cancer 2007, 97, 678–685. [Google Scholar] [CrossRef]
- Ferraldeschi, R.; Rodrigues, D.N.; Riisnaes, R.; Miranda, S.; Figueiredo, I.; Rescigno, P.; Ravi, P.; Pezaro, C.; Omlin, A.; Lorente, D.; et al. PTEN Protein Loss and Clinical Outcome from Castration-resistant Prostate Cancer Treated with Abiraterone Acetate. Eur. Urol. 2015, 67, 795–802. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Kong, Y.-Y.; Wang, Q.-F.; Yang, Y.-J.; Liu, Z.; Lin, N.; Ye, D.-W.; Dai, B. Prognostic value of PTEN in de novo diagnosed metastatic prostate cancer. Asian J. Androl. 2022, 24, 50–55. [Google Scholar] [CrossRef]
- Nakazawa, M.; Antonarakis, E.S.; Luo, J. Androgen Receptor Splice Variants in the Era of Enzalutamide and Abiraterone. Discov. Oncol. 2014, 5, 265–273. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and Resistance to Enzalutamide and Abiraterone in Prostate Cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef]
- Sweat, S.D.; Pacelli, A.; Murphy, G.P.; Bostwick, D.G. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology 1998, 52, 637–640. [Google Scholar] [CrossRef]
- Perner, S.; Hofer, M.D.; Kim, R.; Shah, R.B.; Li, H.; Möller, P.; Hautmann, R.E.; Gschwend, J.E.; Kuefer, R.; Rubin, M.A. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum. Pathol. 2007, 38, 696–701. [Google Scholar] [CrossRef]
- Pomykala, K.L.; Czernin, J.; Grogan, T.R.; Armstrong, W.R.; Willliams, J.; Calais, J. Total-Body 68Ga-PSMA-11 PET/CT for Bone Metastasis Detection in Prostate Cancer Patients: Potential Impact on Bone Scan Guidelines. J. Nucl. Med. 2020, 61, 405–411. [Google Scholar] [CrossRef]
- Zhou, J.; Lai, Y.; Peng, S.; Tang, C.; Chen, Y.; Li, L.; Huang, H.; Guo, Z. Comprehensive analysis of TP53 and SPOP mutations and their impact on survival in metastatic prostate cancer. Front. Oncol. 2022, 12, 957404. [Google Scholar] [CrossRef]
- Mohiuddin, J.J.; Baker, B.R.; Chen, R.C. Radiotherapy for high-risk prostate cancer. Nat. Rev. Urol. 2015, 12, 145–154. [Google Scholar] [CrossRef]
- Zelefsky, M.J.; Yamada, Y.; Fuks, Z.; Zhang, Z.; Hunt, M.; Cahlon, O.; Park, J.; Shippy, A. Long-term results of conformal radiotherapy for prostate cancer: Impact of dose escala-tion on biochemical tumor control and distant metastases-free survival outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1028–1033. [Google Scholar] [CrossRef]
- Fischer-Valuck, B.W.; Baumann, B.C.; Apicelli, A.; Rao, Y.J.; Roach, M.; Daly, M.; Dans, M.C.; White, P.; Contreras, J.; Henke, L.; et al. Palliative radiation therapy (RT) for prostate cancer patients with bone metastases at diagnosis: A hospital-based analysis of patterns of care, RT fractionation scheme, and overall survival. Cancer Med. 2018, 7, 4240–4250. [Google Scholar] [CrossRef]
- Abugharib, A.; Zeng, K.L.; Tseng, C.L.; Soliman, H.; Myrehaug, S.; Husain, Z.; Maralani, P.J.; Larouche, J.; Cheung, P.; Emmenegger, U.; et al. Spine Stereotactic Body Radiotherapy for Prostate Cancer Metastases and the Impact of Hormone Sensitivity Status on Local Control. Neurosurgery 2022, 90, 743–749. [Google Scholar] [CrossRef]
- Venugopal, B.; Shahhat, S.; Beck, J.; Hanumanthappa, N.; Ong, A.D.; Dubey, A.; Koul, R.; Bashir, B.; Chowdhury, A.; Sivananthan, G.; et al. Factors Associated with Long-Term Prostate Cancer Survival after Palliative Radiother-apy to a Bone Metastasis and Contemporary Palliative Systemic Therapy: A Retrospective, Population-Based Study. Curr Oncol. 2023, 30, 5560–5573. [Google Scholar] [CrossRef]
- Nader, R.; El Amm, J.; Aragon-Ching, J.B. Role of chemotherapy in prostate cancer. Asian J. Androl. 2018, 20, 221–229. [Google Scholar] [CrossRef]
- Gravis, G.; Boher, J.M.; Joly, F.; Soulié, M.; Albiges, L.; Priou, F.; Latorzeff, I.; Delva, R.; Krakowski, I.; Laguerre, B.; et al. Androgen Deprivation Therapy (ADT) Plus Docetaxel Versus ADT Alone in Metastatic Non castrate Prostate Cancer: Impact of Metastatic Burden and Long-term Survival Analysis of the Randomized Phase 3 GETUG-AFU15 Trial. Eur. Urol. 2016, 70, 256–262. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.-H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.S.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef]
- Mitsogiannis, I.; Tzelves, L.; Dellis, A.; Issa, H.; Papatsoris, A.; Moussa, M. Prostate cancer immunotherapy. Expert. Opin. Biol. Ther. 2022, 22, 577–590. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Prostate Cancer. Version 2. **Important Practical Guidelines for PCa Treatment from the Oncologist Perspective. In NCCN Clinical Practive Guidelines in Oncology (NCCN Guidelines); NCCN: Plymouth Meeting, PA, USA, 2021.
- Hamstra, D.A.; Bae, K.; Pilepich, M.V.; Hanks, G.E.; Grignon, D.J.; McGowan, D.G.; Roach, M.; Lawton, C.; Lee, R.J.; Sandler, H. Older age predicts decreased metastasis and prostate cancer-specific death for men treated with radiation therapy: Meta-analysis of radiation therapy oncology group trials. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1293–1301. [Google Scholar] [CrossRef]
- Yancik, R.; Yates, J.W.; Cumberlin, R. Research recommendations for radiation therapy in older cancer patients. Report from the National Institute on Aging, National Cancer Institute, and American College of Radiology Workshop: Radiation therapy and cancer in older persons. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Foerster, B.; Pozo, C.; Abufaraj, M.; Mari, A.; Kimura, S.; D’Andrea, D.; John, H.; Shariat, S.F. Association of Smoking Status With Recurrence, Metastasis, and Mortality Among Pa-tients With Localized Prostate Cancer Undergoing Prostatectomy or Radiotherapy: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Kenfield, S.A.; Stampfer, M.J.; Chan, J.M.; Giovannucci, E. Smoking and prostate cancer survival and recurrence. JAMA 2011, 305, 2548–2555. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.M.; Davila, E.P.; Zhao, W.; Parker, D.; Hooper, M.W.; Caban-Martinez, A.; Dietz, N.; Huang, Y.; Messiah, A.; Lee, D.J. Cancer screening behaviors among smokers and non-smokers. Cancer Epidemiol. 2010, 34, 611–617. [Google Scholar] [CrossRef]
- Plaskon, L.A.; Penson, D.F.; Vaughan, T.L.; Stanford, J.L. Cigarette smoking and risk of prostate cancer in middle-aged men. Cancer Epidemiol. Biomarkers Prev. 2003, 12, 604–609. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).