18F-FDG PET/CT Semiquantitative and Radiomic Features for Assessing Pathologic Axillary Lymph Node Status in Clinical Stage I–III Breast Cancer Patients: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
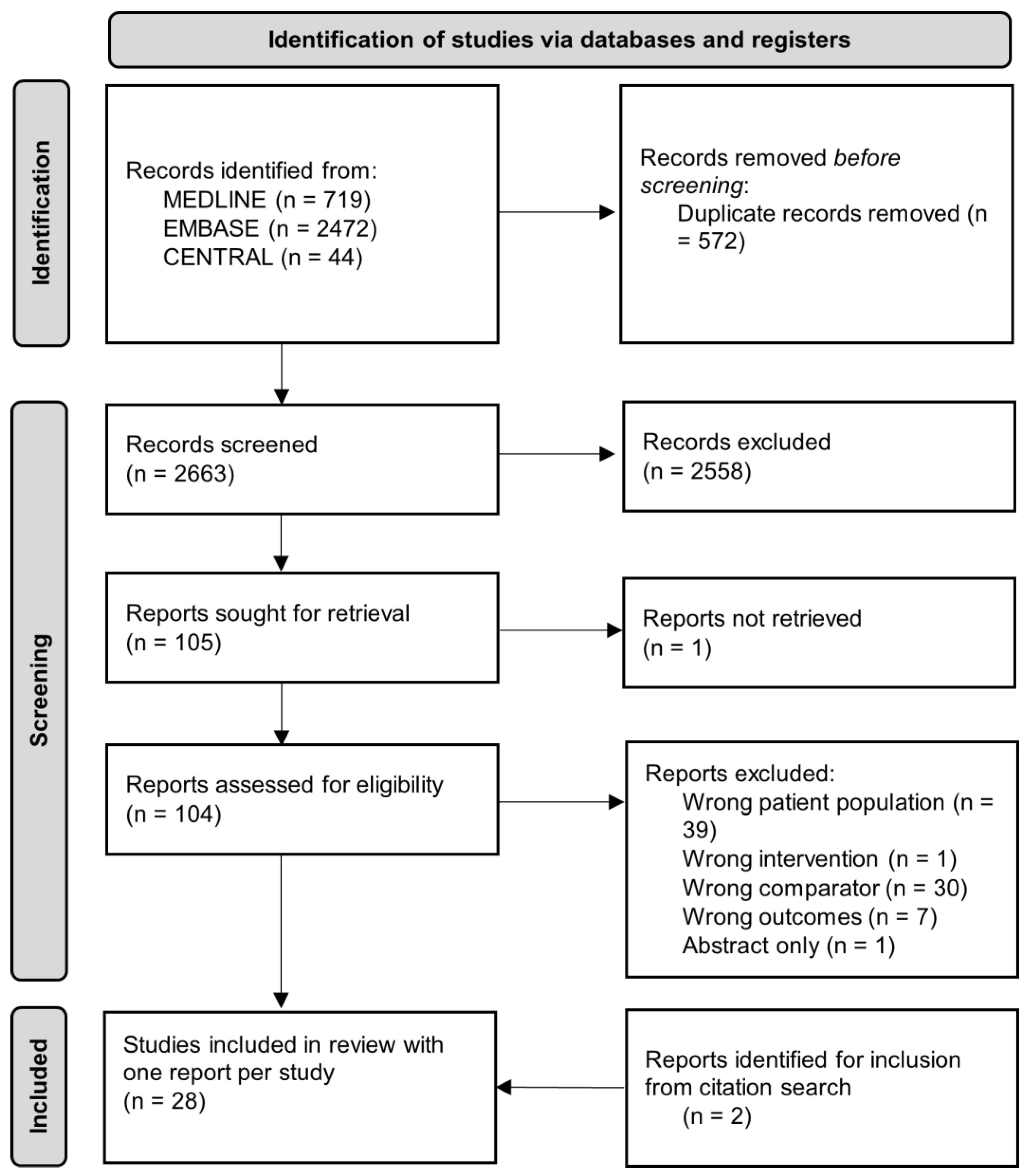
3.1. Search and Eligible Studies
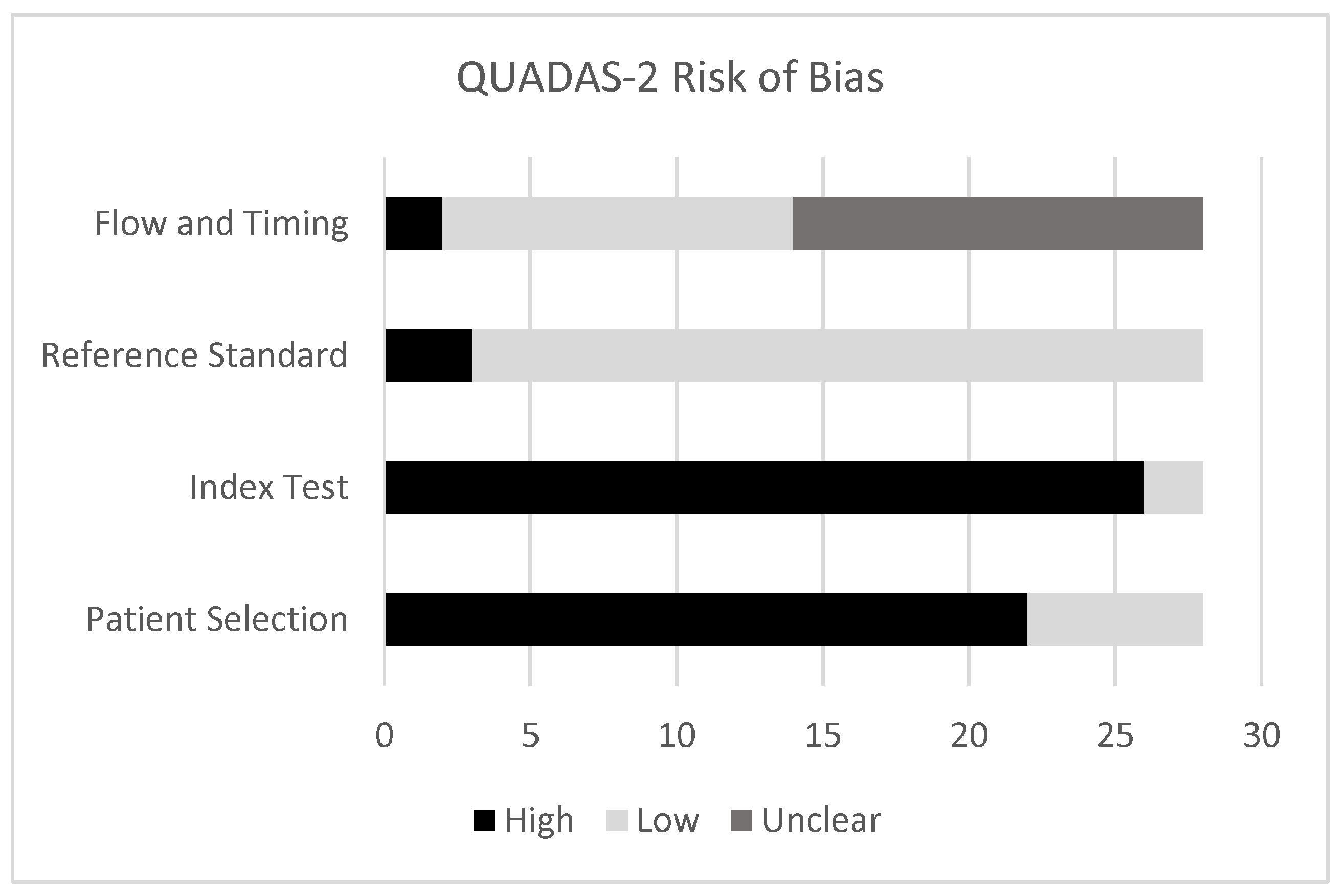
3.2. Quality Assessment
3.3. SUVmax of the Primary Tumour
3.4. MTV and TLG of the Primary Tumour
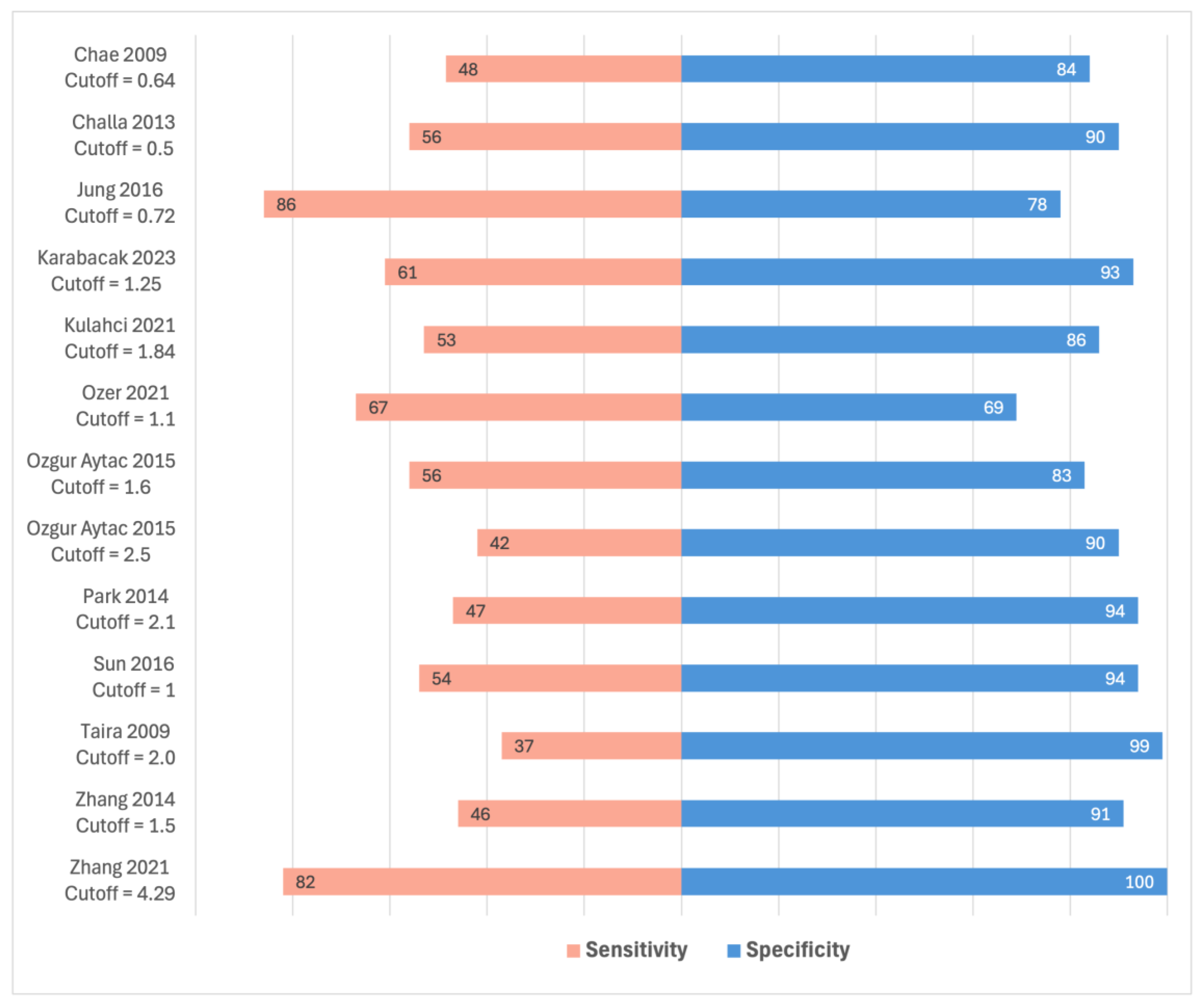
3.5. SUVmax of ALNs
3.6. Semiquantitative Features from Other ROIs
3.7. Radiomic Features
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALN | Axillary lymph node |
| SUV | Standardized uptake value |
| MTV | Metabolic tumour volume |
| TLG | Total lesion glycolysis |
| ROI | Region of interest |
| ROC | Receiver-operating characteristics |
Appendix A
| Study Type | Country | Size (Patients) | Age (Mean or Median) | Clinical Stage | Axillary Nodal Status | Details of PET/CT Acquisition | Region of Interest (Method of Delineation) | Features | |
|---|---|---|---|---|---|---|---|---|---|
| An 2017 [21] | Retrospective cohort | South Korea | 173 | 49 | Not specified | 128 ALN−, 45 ALN+ | Dosage: 5 MBq/kg Blood glucose: <150 mg/dL Acquisition parameters: 60 min uptake, 3 min/frame, 7–8 frames, 600 mm FOV Reconstruction parameters: OSEM algorithm, 2 iterations, 20 subsets, slice thickness 3.27 mm | Breast tumour (semi-automatic) | Semiquantitative (SUVmax, SUVmean, MTV, TLG) |
| Can 2019 [22] | Retrospective cohort | Turkey | 129 | 49 | Not specified | 61 ALN−, 68 ALN+ | Dosage: 5.5 MBq/kg Blood glucose: <=140 mg/dL Acquisition parameters: 60 min uptake, 3 min/bed position, 15.5 cm FOV Reconstruction parameters: OSEM algorithm, 2 iterations, 8 subsets, FWHM 5 mm | Breast tumour (manual) | Semiquantitative (SUVmax, MTV, TLG) |
| Cetindag 2023 [23] | Retrospective cohort | Turkey | 67 | 56 | Not specified | 33 ALN−, 34 ALN+ | Dosage: Not specified Blood glucose: <150 mg/dL Acquisition parameters: Not specified Reconstruction parameters: Not specified | Most prominent lymph node (manual) | Semiquantitative (SUVmax) |
| Chae 2009 [24] | Retrospective cohort | South Korea | 108 | 49 | 77 T1, 29 T2, 2 T3 18 N1, 10 N2, 5 N3 | 75 ALN−, 33 ALN+ | Dosage: 5 mCi Blood glucose: <120 mg/dL Acquisition parameters: 60 min uptake Reconstruction parameters: Not specified | Axilla (not specified) | Semiquantitative (SUVmax) |
| Challa 2013 [25] | Prospective cohort | India | 37 | 51 | T1 or T2 with N0 | 21 ALN−, 16 ALN+ | Dosage: 370 Mbq Blood glucose: <140 mg/dl Acquisition parameters: 45–60 min uptake, 2–3 min/bed position Reconstruction parameters: OSEM algorithm, 2 iterations, 8 subsets, slice thickness 1.5 mm, matrix of 128 × 128 pixels. | Breast tumour and axilla (manual) | Semiquantitative (SUVmax) |
| Chen 2022 [26] | Retrospective cohort | China | 180 | 55 | cN0 | 28 ALN−, 152 ALN+ | Dosage: 0.10–0.15 mCi/kg Blood glucose: <11.1 mmol/L Acquisition parameters: 60 min uptake, 2 min/bed position, 8 bed positions Reconstruction parameters: OSEM algorithm, 6 mm FWHM, voxel size 5.3 × 5.3 × 2.5 mm | Breast tumour (manual) | Radiomics Features of first order, shape, and texture (1562 PET features and 1562 CT features, 3124 PET/CT features total) |
| Erol 2021 [27] | Retrospective cohort | Turkey | 143 | 52 | Not specified | 65 ALN−, 78 ALN+ | Dosage: Not specified Blood glucose: <150 mg/dL Acquisition parameters: 60 min uptake Reconstruction parameters: Not specified | Breast tumour (manual) | Semiquantitative (SUVmax, SUVmean, MTV, TLG) |
| Jung 2016 [28] | Retrospective cohort | South Korea | 428 | 50 | Not specified | 261 ALN−, 167 ALN+ | Dosage: 370–550 MBq Blood glucose: <130 mg/dL Acquisition parameters: 60 min uptake, 2 min/bed position, 7–8 bed positions Reconstruction parameters: Not specified | Breast tumour and axilla (manual) | Semiquantitative (SUVmax) |
| Karabacak 2023 [29] | Retrospective cohort | Turkey | 104 | 57 | Not specified (b) | 42 ALN−, 62 ALN+ | Dosage: 0.14 mCi/kg Blood glucose: <180 mg/dL Acquisition parameters: 60 min uptake, 3 min/bed position Reconstruction parameters: Not specified | Breast tumour and axilla (semi-automatic) | Semiquantitative (SUVmax) |
| Karan 2016 [30] | Retrospective cohort | Turkey | 70 | 47 | Not specified | 19 ALN−, 51 ALN+ | Dosage: 297–370 MBq Blood glucose: <150 mg/dL Acquisition parameters: 60 min uptake, 2.5 min/bed position, 6–7 bed positions Reconstruction parameters: Not specified | Breast tumour (automatic) | Semiquantitative (SUVmax) |
| Kim 2015 [31] | Retrospective cohort | South Korea | 671 | 52 | Not specified | 392 ALN−, 279 ALN+ | Dosage: 5.2 MBq/kg Blood glucose <120 mg/dL Acquisition parameters: 60 min uptake Reconstruction parameters: Iterative algorithms 2 iterations, 21 subsets, 168 × 168 image matrices | Breast tumour (manual) | Semiquantitative (SUVmax) |
| Kong 2021 [32] | Retrospective cohort | South Korea | 221 | 49 | T1 174, T2 50 | 161 ALN−, 63 ALN+ | Dosage: 4.44 MBq/kg Blood glucose: <160 mg/dL Acquisition parameters: 60 min, 3–4 min/frame, 7–8 frames Reconstruction parameters: Iterative algorithms 2 iterations, 8 subsets. FWHM 5.0 mm | Breast tumour (not specified) | Semiquantitative (SUVmax) |
| Kulahci 2021 [33] | Retrospective cohort | Turkey | 113 | 52 | Not specified | 64 ALN−, 49 ALN+ | Dosage: 5 MBq/kg Blood glucose: <200 mg/dL Acquisition parameters: 60 min uptake, 2–4 min/bed position Reconstruction parameters: Not specified | Axilla (manual) | Semiquantitative (SUVmax) |
| Kutluturk 2019 [34] | Retrospective cohort | Turkey | 232 | 51 | Mean clinical tumour size: 2.4 cm | 68 ALN−, 164 ALN+ | Dosage: 0.1 mg/kg Blood glucose: Not specified Acquisition parameters: 60 min uptake Reconstruction parameters: OSEM algorithm | Breast tumour and axilla (not specified) | Semiquantitative (SUVmax) |
| Monzawa 2009 [35] | Retrospective cohort | Japan | 50 | 59 | Stage I in 34 patients, II in 15 patients, and III in 1 patient | 35 ALN−, 15 ALN+ | Dosage: 185 mBq Blood glucose: Not specified Acquisition parameters: 50–60 min uptake, 2 min/bed position Reconstruction parameters: 128 × 128 matrix, section thickness of 3.27 mm | Breast tumour (manual) | Semiquantitative (SUVmax) |
| Ozen 2024 [36] | Retrospective cohort | Turkey | 40 | 52 | Stage 1A: 12, 1B 1, 2A 10, 2B 8, 3A 6, 3C 3 | 18 ALN−, 22 ALN+ | Dosage: 2.5 MBq/kg Blood glucose: Not specified Acquisition parameters: 60 min uptake, 3 min/bed position Reconstruction parameters: Iterative algorithm | Breast tumour, axilla, normal parenchyma, liver (manual) | Semiquantitative (SUVmax, tumour to parenchyma SUVmax ratio, tumour to liver SUVmax ratio, axilla to parenchyma SUVmax ratio, axilla to liver SUVmax ratio) |
| Ozer 2021 [37] | Retrospective cohort | Turkey | 90 | 55 | Early breast cancer | 36 ALN−, 54 ALN+ | Dosage: 369 MBq Blood glucose: <200 mg/dL Acquisition parameters: 60 min uptake Reconstruction parameters: Thinned slices up to 2 mm, using xSHARP technology and Richardson-Lucy maximum likelihood algorithm | Breast tumour and axilla (Manual) | Semiquantitative (SUVmax) |
| Ozgur Aytac 2015 [38] | Retrospective cohort | Turkey | 273 | 50 | 131 T1, 142 T2 | 105 ALN−, 168 ALN+ | No details specified | Axilla (not specified) | Semiquantitative (SUVmax) |
| Ozkan 2019 [39] | Retrospective cohort | Turkey | 192 | 51 | Stage IB to IIIA | 80 ALN−, 112 ALN+ | Dosage: 3.7–5.5 MBq/kg Blood glucose: <150 g/mL Acquisition parameters: 60 min uptake Reconstruction parameters: Not specified | Breast tumour and axilla (manual) | Semiquantitative (SUVmax) |
| Pahk 2020 [40] | Retrospective cohort | South Korea | 173 | 62 | Not specified | 108 ALN−, 65 ALN+ | Dosage: 5.29 MBq/kg Blood glucose: Not specified Acquistion parameters: 60 min uptake, 1 min/bed position, 9 bed positions, 18 cm axial FOV, 4.4 mm spatial resolution, Reconstruction parameters: 3D row-action maximum likelihood algorithm | Breast tumour, visceral (V) and subcutaneous (S) adipose tissue (manual) | Semiquantitative (SUVmax, V/S SUVmax ratio) |
| Park 2014 [41] | Retrospective cohort | South Korea | 136 | 50 | Not specified | 66 ALN−, 70 ALN+ | Dosage: 7.4 MBq/kg Blood glucose: <7.2 mmol/L Acquisition parameters: 60 min uptake, 3.5 min/bed position, 5–6 bed positions, 16.2 cm axial FOV Reconstruction parameters: OSEM algorithm, 2 iterations, 8 subsets | Breast tumour and axilla (manual) | Semiquantitative (SUVmax, lymph node to tumour SUVmax ratio) |
| Song 2021 [42] | Retrospective cohort | South Korea | 100 | Not specified | Not specified (b) | 57 ALN−, 43 ALN+ | Dosage: 5.5 MBq/kg Blood glucose: <8.3 mmol/L Acquisition parameters: 60 min uptake, 3 min/bed position Reconstruction parameters: OSEM algorithm | Breast tumour (automatic) | Radiomics (792 radiomics features) |
| Song 2017 [43] | Retrospective cohort | South Korea | 128 | 53 | Not specified (b) | 76 ALN−, 52 ALN+ | Dosage: 5.5 MBq/kg Blood glucose: <8.3 mmol/L Acquisition parameters: 60 min uptake, 3 min/bed position Reconstruction parameters: OSEM algorithm | Breast tumour (manual) | Semiquantitative (SUVmax) |
| Sun 2016 [44] | Retrospective cohort | South Korea | 196 | 54 | cT1-3 N0-3 TNM stage I: 89, II: 88, III: 19 | 131 ALN−, 65 ALN+ | Dosage: 555 MBq/kg Blood glucose: <200 mg/dL Acquisition parameters: 50 min uptake, 2.5 min/bed position, 6–8 bed positions Reconstruction parameters: Not specified | Breast tumour and axilla (manual) | Semiquantitative (SUVmax, ALN SUVmax ratio) |
| Taira 2009 [45] | Retrospective cohort | Japan | 90 | 55 | cN0 | 65 ALN−, 27 ALN+ | Dosage: 3 MBq/kg Blood glucose: Not specified Acquisition parameters: 60 min uptake Reconstruction parameters: Not specified | Breast tumour and axilla (manual) | Semiquantitative (SUVmax) |
| Yoo 2018 [46] | Retrospective cohort | South Korea | 135 | 54 | cN0 | 104 ALN−, 31 ALN+ | Dosage: 3.7 MBq/kg Blood glucose: <140 mg/dL Acquisition parameters: 60 min uptake Reconstruction parameters: OSEM algorithm, 2 iterations, 21 subsets, 200 × 200 matrices, 3.4 × 3.4 mm pixel size, slice thickness 3.0 mm | Breast tumour (semi-automatic) | Semiquantitative (SUVmax, MTV, TLG) |
| Zhang 2021 [47] | Retrospective cohort | China | 40 patients, 209 lymph nodes | 56 | All stage III (N2) disease (pathologic) | 112 ALN−, 97 ALN+ (Individual lymph nodes) | Dosage: 4.81 MBq/kg Blood glucose: <8.2 mmol/L Acquisition parameters: 60–70 min uptake, 2 min/body position, 6–7 positions Reconstruction parameters: 5 mm slice thickness | ALNs (manual) | Semiquantitative (SUVmax, early phase of dual-point PET-CT) |
| Zhang 2014 [48] | Retrospective cohort | China | 164 | 45 | cN0 | 123 ALN−, 41 ALN+ | Dosage: 5.3 MBq/kg ± 10% Blood glucose: <11.0 mmol/L Acquisition parameters: 60 min uptake, 5–7 bed positions Reconstruction parameters: OSEM algorithm, 2 iterations, 28 subsets. FWHM 8 mm | Axilla (manual) | Semiquantitative (SUVmax) |
| Mean or Median ALN SUVmax in ALN+ vs. ALN− Cases | Multivariable Analysis of ALN SUVmax [Other Variables Included in the Model] | ROC Analysis on ALN SUVmax | |
|---|---|---|---|
| Cetindag 2023 [23] | 6.3 vs. 1.6 p = 0.001 | - | - |
| Chae 2009 [24] | - | - | Cutoff 0.64 Accuracy 73.2%, sensitivity 48.5%, specificity 84% |
| Challa 2013 [25] | - | - | Cutoff 0.5 AUC 75.4%, sensitivity 56%, specificity 90% |
| Jung 2016 [28] | - | Cutoff 0.72, OR 5.37, p < 0.005 [age, tumour SUVmax, size, lymphatic invasion, nuclear grade, histologic grade, HER2] | Cutoff 0.72 AUC 78.3%, accuracy 65%, sensitivity 86%, specificity 78% |
| Karabacak 2023 [29] | 2.2 vs. 1.0 p < 0.001 | - | Cutoff 1.25 AUC 77.6%, accuracy 74%, sensitivity 61%, specificity 93% |
| Kulahci 2021 [33] | - | - | Cutoff: 1.84 AUC 75.6%, sensitivity 53%, specificity: 86% |
| Kutluturk 2019 [34] | - | Cutoff 3.2, OR 15.7, p < 0.001 [largest tumour size] | - |
| Ozer 2021 [37] | ALN+ nodal SUVmax was 1.939 times higher than ALN− nodal SUVmax (p = 0.001) | - | Cutoff 1.1 Sensitivity 67%, specificity 69% |
| Ozgur Aytac 2015 [38] | Cutoff 2.5, OR 14, p = 0.005 [tumour stage, hormone receptor status, histology type, age, SUVmax cutoff of 1] | Cutoff 1.6 Sensitivity: 56%, specificity: 83% Cutoff 2.5 AUC 71.5%, sensitivity 42%, specificity 90% | |
| Park 2014 [41] | - | - | Cutoff 2.1 AUC 70.5%, accuracy 70%, sensitivity 47%, specificity 94% |
| Sun 2016 [44] | - | Cutoff of 1 was significant for ALN metastases with p < 0.001 (details not specified). [primary tumour SUVmax, SUVmax ratio] | Cutoff 1 AUC 74.6%, sensitivity 54%, specificity 94% |
| Taira 2009 [45] | - | - | Cutoff 2.0 Sensitivity 37%, specificity 99% |
| Zhang 2021 [47] | SUVmax of matched lymph nodes: 5.45 vs. 2.79 p < 0.001 | - | Cutoff 4.29 AUC 96.1%, sensitivity 82%, specificity 100% |
| Zhang 2014 [48] | - | - | Cutoff 1.5 Accuracy 80%, sensitivity 46%, specificity 91% |
References
- Brackstone, M.; Baldassarre, F.G.; Perera, F.E.; Cil, T.; Chavez Mac Gregor, M.; Dayes, I.S.; Engel, J.; Horton, J.K.; King, T.A.; Kornecki, A.; et al. Management of the Axilla in Early-Stage Breast Cancer: Ontario Health (Cancer Care Ontario) and ASCO Guideline. J. Clin. Oncol. 2021, 39, 3056–3082. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.; Anorbe, E.; Alcorta, P.; Lopez, F.; Alonso, I.; Cortes, J. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: A systematic review. AJR Am. J. Roentgenol. 2006, 186, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Riedel, F.; Schaefgen, B.; Sinn, H.P.; Feisst, M.; Hennigs, A.; Hug, S.; Binnig, A.; Gomez, C.; Harcos, A.; Stieber, A.; et al. Diagnostic accuracy of axillary staging by ultrasound in early breast cancer patients. Eur. J. Radiol. 2021, 135, 109468. [Google Scholar] [CrossRef]
- Wilke, L.G.; McCall, L.M.; Posther, K.E.; Whitworth, P.W.; Reintgen, D.S.; Leitch, A.M.; Gabram, S.G.; Lucci, A.; Cox, C.E.; Hunt, K.K.; et al. Surgical complications associated with sentinel lymph node biopsy: Results from a prospective international cooperative group trial. Ann. Surg. Oncol. 2006, 13, 491–500. [Google Scholar] [CrossRef]
- Dayes, I.S.; Metser, U.; Hodgson, N.; Parpia, S.; Eisen, A.F.; George, R.; Blanchette, P.; Cil, T.D.; Arnaout, A.; Chan, A.; et al. Impact of (18)F-Labeled Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography Versus Conventional Staging in Patients With Locally Advanced Breast Cancer. J. Clin. Oncol. 2023, 41, 3909–3916. [Google Scholar] [CrossRef]
- Groheux, D.; Hindie, E. Breast cancer: Initial workup and staging with FDG PET/CT. Clin. Transl. Imaging 2021, 9, 221–231. [Google Scholar] [CrossRef]
- Kasem, J.; Wazir, U.; Mokbel, K. Sensitivity, Specificity and the Diagnostic Accuracy of PET/CT for Axillary Staging in Patients With Stage I–III Cancer: A Systematic Review of The Literature. Vivo 2021, 35, 23–30. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Luo, H.; Zhang, J. PET/CT and MRI for Identifying Axillary Lymph Node Metastases in Breast Cancer Patients: Systematic Review and Meta-Analysis. J. Magn. Reson. Imaging 2020, 52, 1840–1851. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, K.I.; Julian, J.A.; Holloway, C.M.; McCready, D.; Gulenchyn, K.Y.; George, R.; Hodgson, N.; Lovrics, P.; Perera, F.; Elavathil, L.; et al. Prospective study of 2-[(1)(8)F]fluorodeoxyglucose positron emission tomography in the assessment of regional nodal spread of disease in patients with breast cancer: An Ontario clinical oncology group study. J. Clin. Oncol. 2012, 30, 1274–1279. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Dong, D.; Wei, J.; Fang, C.; Zhou, X.; Sun, K.; Li, L.; Li, B.; Wang, M.; et al. The Applications of Radiomics in Precision Diagnosis and Treatment of Oncology: Opportunities and Challenges. Theranostics 2019, 9, 1303–1322. [Google Scholar] [CrossRef]
- Bera, K.; Braman, N.; Gupta, A.; Velcheti, V.; Madabhushi, A. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat. Rev. Clin. Oncol. 2022, 19, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.-J.; Su, G.-H.; You, C.; Zhang, X.; Xiao, Y.; Jiang, Y.-Z.; Shao, Z.-M. Radiomics in breast cancer: Current advances and future directions. Cell Rep. Med. 2024, 5, 101719. [Google Scholar] [CrossRef] [PubMed]
- Urso, L.; Evangelista, L.; Alongi, P.; Quartuccio, N.; Cittanti, C.; Rambaldi, I.; Ortolan, N.; Borgia, F.; Nieri, A.; Uccelli, L.; et al. The Value of Semiquantitative Parameters Derived from 18F-FDG PET/CT for Predicting Response to Neoadjuvant Chemotherapy in a Cohort of Patients with Different Molecular Subtypes of Breast Cancer. Cancers 2022, 14, 5869. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Sollini, M.; Cozzi, L.; Ninatti, G.; Antunovic, L.; Cavinato, L.; Chiti, A.; Kirienko, M. PET/CT radiomics in breast cancer: Mind the step. Methods 2021, 188, 122–132. [Google Scholar] [CrossRef]
- Urso, L.; Manco, L.; Castello, A.; Evangelista, L.; Guidi, G.; Castellani, M.; Florimonte, L.; Cittanti, C.; Turra, A.; Panareo, S. PET-Derived Radiomics and Artificial Intelligence in Breast Cancer: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 13409. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Pollock, A.; Berge, E. How to do a systematic review. Int. J. Stroke 2017, 13, 138–156. [Google Scholar] [CrossRef]
- Veritas Health Innovation. Covidence Systematic Review Software; Veritas Health Innovation: Melbourne, Australia; Available online: www.covidence.org.
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- An, Y.S.; Kang, D.K.; Jung, Y.; Kim, T.H. Volume-based metabolic parameter of breast cancer on preoperative 18F-FDG PET/CT could predict axillary lymph node metastasis. Medicine 2017, 96, e8557. [Google Scholar] [CrossRef]
- Can, C.; Komek, H. Metabolic and volume-based parameters of (18F)FDG PET/CT for primary mass and axillary lymph node metastasis in patients with invasive ductal carcinoma: A retrospective analysis in relation to molecular subtype, axillary lymph node metastasis and immunohistochemistry and inflammatory markers. Nucl. Med. Commun. 2019, 40, 1051–1059. [Google Scholar] [PubMed]
- Cetindag, O.; Avsar, G.; Hakseven, M.; Deryol, R.; Ertekin, S.C.; Karasoy, D.; Eroglu, A.; Bayar, S. The predictive value, sensitivity, specificity, and accuracy of PET CT in the evaluation of axillary metastases in breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 10008–10015. [Google Scholar] [PubMed]
- Chae, B.J.; Bae, J.S.; Kang, B.J.; Kim, S.H.; Jung, S.S.; Song, B.J. Positron emission tomography-computed tomography in the detection of axillary lymph node metastasis in patients with early stage breast cancer. Jpn. J. Clin. Oncol. 2009, 39, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Challa, V.R.; Srivastava, A.; Dhar, A.; Parshad, R.; Bal, C.; Mohan Reddy Gona, R.; Kumar, R.; Datta Gupta, S.; Sharma, P. Role of fluorine-18-labeled 2-fluoro-2-deoxy-D-glucose positron emission tomography-computed tomography in the evaluation of axillary lymph node involvement in operable breast cancer in comparison with sentinel lymph node biopsy. Indian J. Nucl. Med. 2013, 28, 138–143. [Google Scholar]
- Chen, K.; Yin, G.; Xu, W. Predictive Value of (18)F-FDG PET/CT-Based Radiomics Model for Occult Axillary Lymph Node Metastasis in Clinically Node-Negative Breast Cancer. Diagnostics 2022, 12, 997. [Google Scholar] [CrossRef]
- Erol, M.; Onner, H.; Eren Karanis, M.I. Evaluation of the Histopathological Features of Early-stage Invasive Ductal Breast Carcinoma by (18)Fluoride-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography. Mol. Imaging Radionucl. Ther. 2021, 30, 129–136. [Google Scholar] [CrossRef]
- Jung, N.Y.; Kim, S.H.; Kang, B.J.; Park, S.Y.; Chung, M.H. The value of primary tumor (18)F-FDG uptake on preoperative PET/CT for predicting intratumoral lymphatic invasion and axillary nodal metastasis. Breast Cancer 2016, 23, 712–717. [Google Scholar] [CrossRef]
- Karabacak, U.; Turkan, H.; Coskun, G.; Mollaoglu, M.C.; Hasbek, Z.; Karadayi, K. The Role of Axillary SUV(max) min (18)F-FDG PET/CT in Predicting the Number of Axillary Metastases of Breast Cancer. J. Coll. Physicians Surg. Pak. 2023, 33, 374–379. [Google Scholar]
- Karan, B.; Pourbagher, A.; Torun, N. Diffusion-weighted imaging and (18) F-fluorodeoxyglucose positron emission tomography/computed tomography in breast cancer: Correlation of the apparent diffusion coefficient and maximum standardized uptake values with prognostic factors. J. Magn. Reson. Imaging 2016, 43, 1434–1444. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, S.H.; Kim, S.; Kang, T.; Bae, Y.T. Tumour 18 F-FDG Uptake on preoperative PET/CT may predict axillary lymph node metastasis in ER-positive/HER2-negative and HER2-positive breast cancer subtypes. Eur. Radiol. 2015, 25, 1172–1181. [Google Scholar] [CrossRef]
- Kong, E.; Choi, J. The new perspective of PET/CT for axillary nodal staging in early breast cancer patients according to ACOSOG Z0011 trial PET/CT axillary staging according to Z0011. Nucl. Med. Commun. 2021, 42, 1369–1374. [Google Scholar] [CrossRef]
- Kulahci, O.; Irkorucu, O.; Deveci, E.K.; Tas, Z.A. Relationship between lymph node metastasis and a low 18F-FDG PET/CT axillary suvmax value in breast cancer. J. Coll. Physicians Surg. Pak. 2021, 30, 511–516. [Google Scholar]
- Kutluturk, K.; Simsek, A.; Comak, A.; Gonultas, F.; Unal, B.; Kekilli, E. Factors affecting the accuracy of (18)F-FDG PET/CT in evaluating axillary metastases in invasive breast cancer. Niger. J. Clin. Pract. 2019, 22, 63–68. [Google Scholar] [CrossRef]
- Monzawa, S.; Adachi, S.; Suzuki, K.; Hirokaga, K.; Takao, S.; Sakuma, T.; Hanioka, K. Diagnostic performance of fluorodeoxyglucose-positron emission tomography/computed tomography of breast cancer in detecting axillary lymph node metastasis: Comparison with ultrasonography and contrast-enhanced CT. Ann. Nucl. Med. 2009, 23, 855–861. [Google Scholar] [CrossRef]
- Ozen, A.; Sayin, T.; Kandemir, O.; Ekmekcioglu, O.; Altınay, S.; Bastug, E.; Muhammedoglu, A.; Celik, A.; Albayrak, R. Comparison between 18F-FDG PET/CT and diffusion-weighted imaging in detection of invasive ductal breast carcinoma. Asia Ocean. J. Nucl. Med. Biol. 2024, 12, 11. [Google Scholar]
- Ozer, N.; Sahin, A. Correlation of Breast Cancer Subgroups and Axillary Metastases with 18F-FDG PET/CT and the Contribution of 18F-FDG PET/CT in the Management of the Axilla. J. Coll. Physicians Surg. Pak. 2021, 31, 150–155. [Google Scholar]
- Ozgur Aytac, H.; Colacoglu, T.; Nihal Nursal, G.; Zafer Nursal, T.; Aka Bolat, F.; Yabanoglu, H.; Yildirim, S.; Moray, G. Predictors determining the status of axilla in breast cancer: Where is PET/CT on that? J. BUON 2015, 20, 1295–1303. [Google Scholar]
- Ozkan, E.E.; Sengul, S.S.; Erdogan, M.; Gurdal, O.; Eroglu, H.E. 18F-fluorodeoxyglucose PET/computed tomography in locoregional staging and assessment of biological and clinical aggressiveness of breast cancer subtypes. Nucl. Med. Commun. 2019, 40, 1043–1050. [Google Scholar] [CrossRef]
- Pahk, K.; Joung, C.; Kim, S. Visceral fat metabolic activity evaluated by preoperative (18)F-FDG PET/CT significantly affects axillary lymph node metastasis in postmenopausal luminal breast cancer. Sci. Rep. 2020, 10, 1348. [Google Scholar] [CrossRef]
- Park, J.; Byun, B.H.; Noh, W.C.; Lee, S.S.; Kim, H.A.; Kim, E.K.; Choi, C.W.; Lim, S.M. Lymph node to primary tumor SUV ratio by 18F-FDG PET/CT and the prediction of axillary lymph node metastases in breast cancer. Clin. Nucl. Med. 2014, 39, e249–e253. [Google Scholar] [CrossRef]
- Song, B.I. A machine learning-based radiomics model for the prediction of axillary lymph-node metastasis in breast cancer. Breast Cancer 2021, 28, 664–671. [Google Scholar] [CrossRef]
- Song, B.I.; Kim, H.W.; Won, K.S. Predictive Value of (18)F-FDG PET/CT for Axillary Lymph Node Metastasis in Invasive Ductal Breast Cancer. Ann. Surg. Oncol. 2017, 24, 2174–2181. [Google Scholar] [CrossRef]
- Sun, W.Y.; Choi, Y.J.; Song, Y.-J. Prediction of Axillary Nodal Status according to the Axillary Lymph Node to Primary Breast Tumor Maximum Standardized Uptake Value Ratio on 18 F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography. J. Breast Dis. 2016, 4, 92–99. [Google Scholar] [CrossRef]
- Taira, N.; Ohsumi, S.; Takabatake, D.; Hara, F.; Takashima, S.; Aogi, K.; Takashima, S.; Inoue, T.; Sugata, S.; Nishimura, R. Determination of indication for sentinel lymph node biopsy in clinical node-negative breast cancer using preoperative 18F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging. Jpn. J. Clin. Oncol. 2009, 39, 16–21. [Google Scholar] [CrossRef]
- Yoo, J.; Kim, B.S.; Yoon, H.J. Predictive value of primary tumor parameters using (18)F-FDG PET/CT for occult lymph node metastasis in breast cancer with clinically negative axillary lymph node. Ann. Nucl. Med. 2018, 32, 642–648. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, X.; Xiao, Y.; Ma, C.; Cao, G.; Liu, Y.; Li, Y. Early SUV(max) is the best predictor of axillary lymph node metastasis in stage III breast cancers. Quant. Imaging Med. Surg. 2021, 11, 1680–1691. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, F.; Han, P. The role of (18)F-FDG PET/CT in the diagnosis of breast cancer and lymph nodes metastases and micrometastases may be limited. Hell. J. Nucl. Med. 2014, 17, 177–183. [Google Scholar]
- Spak, D.A.; Plaxco, J.S.; Santiago, L.; Dryden, M.J.; Dogan, B.E. BI-RADS® fifth edition: A summary of changes. Diagn. Interv. Imaging 2017, 98, 179–190. [Google Scholar] [CrossRef]
- Zarcaro, C.; Santonocito, A.; Zeitouni, L.; Ferrara, F.; Kapetas, P.; Milos, R.-I.; Helbich, T.; Baltzer, P.; Clauser, P. Inter-reader agreement of the BI-RADS CEM lexicon. Eur. Radiol. 2024, 35, 2378–2386. [Google Scholar] [CrossRef]
- Christensen, J.; Prosper, A.E.; Wu, C.C.; Chung, J.; Lee, E.; Elicker, B.; Hunsaker, A.R.; Petranovic, M.; Sandler, K.L.; Stiles, B.; et al. ACR Lung-RADS v2022: Assessment Categories and Management Recommendations. Chest 2024, 165, 738–753. [Google Scholar] [CrossRef]
- Zhan, Y.; Song, F.; Zhang, W.; Gong, T.; Zhao, S.; Lv, F. Prediction of benign and malignant pulmonary nodules using preoperative CT features: Using PNI-GARS as a predictor. Front. Immunol. 2024, 15, 2024. [Google Scholar] [CrossRef] [PubMed]
- Spadarella, G.; Stanzione, A.; Akinci D’Antonoli, T.; Andreychenko, A.; Fanni, S.C.; Ugga, L.; Kotter, E.; Cuocolo, R. Systematic review of the radiomics quality score applications: An EuSoMII Radiomics Auditing Group Initiative. Eur. Radiol. 2023, 33, 1884–1894. [Google Scholar] [CrossRef]
- Talari, K.; Goyal, M. Retrospective Studies—Utility and Caveats. J. R. Coll. Physicians Edinb. 2020, 50, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Tofthagen, C. Threats to validity in retrospective studies. J. Adv. Pract. Oncol. 2012, 3, 181–183. [Google Scholar] [PubMed]
- Adams, M.C.; Turkington, T.G.; Wilson, J.M.; Wong, T.Z. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am. J. Roentgenol. 2010, 195, 310–320. [Google Scholar] [CrossRef]
- Reiazi, R.; Abbas, E.; Famiyeh, P.; Rezaie, A.; Kwan, J.Y.Y.; Patel, T.; Bratman, S.V.; Tadic, T.; Liu, F.F.; Haibe-Kains, B. The impact of the variation of imaging parameters on the robustness of Computed Tomography radiomic features: A review. Comput. Biol. Med. 2021, 133, 104400. [Google Scholar] [CrossRef]
- Whybra, P.; Zwanenburg, A.; Andrearczyk, V.; Schaer, R.; Apte, A.P.; Ayotte, A.; Baheti, B.; Bakas, S.; Bettinelli, A.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Convolutional Filters for Reproducible Radiomics and Enhanced Clinical Insights. Radiology 2024, 310, e231319. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
- Berenguer, R.; Pastor-Juan, M.d.R.; Canales-Vázquez, J.; Castro-García, M.; Villas, M.V.; Mansilla Legorburo, F.; Sabater, S. Radiomics of CT Features May Be Nonreproducible and Redundant: Influence of CT Acquisition Parameters. Radiology 2018, 288, 407–415. [Google Scholar] [CrossRef]
- Kocak, B.; Akinci D’Antonoli, T.; Mercaldo, N.; Alberich-Bayarri, A.; Baessler, B.; Ambrosini, I.; Andreychenko, A.E.; Bakas, S.; Beets-Tan, R.G.H.; Bressem, K.; et al. METhodological RadiomICs Score (METRICS): A quality scoring tool for radiomics research endorsed by EuSoMII. Insights Imaging 2024, 15, 8. [Google Scholar] [CrossRef]
- Caulley, L.; Catalá-López, F.; Whelan, J.; Khoury, M.; Ferraro, J.; Cheng, W.; Husereau, D.; Altman, D.G.; Moher, D. Reporting guidelines of health research studies are frequently used inappropriately. J. Clin. Epidemiol. 2020, 122, 87–94. [Google Scholar] [CrossRef]
- Logullo, P.A.-O.; MacCarthy, A.; Kirtley, S.; Collins, G.S. Reporting guideline checklists are not quality evaluation forms: They are guidance for writing. Health Sci. Rep. 2020, 3, e165. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, D.; Kim, H.S.; Park, S.Y.; Kim, J.Y.; Cho, S.J.; Shin, J.H.; Kim, J.H. Quality of science and reporting of radiomics in oncologic studies: Room for improvement according to radiomics quality score and TRIPOD statement. Eur. Radiol. 2020, 30, 523–536. [Google Scholar] [CrossRef]
- Davey, M.G.; Davey, M.S.; Boland, M.R.; Ryan, É.J.; Lowery, A.J.; Kerin, M.J. Radiomic differentiation of breast cancer molecular subtypes using pre-operative breast imaging—A systematic review and meta-analysis. Eur. J. Radiol. 2021, 144, 109996. [Google Scholar] [CrossRef]
- Gong, X.; Guo, Y.; Zhu, T.; Peng, X.; Xing, D.; Zhang, M. Diagnostic performance of radiomics in predicting axillary lymph node metastasis in breast cancer: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 2022. [Google Scholar] [CrossRef]
- Leung, J.-H.; Karmakar, R.; Mukundan, A.; Thongsit, P.; Chen, M.-M.; Chang, W.-Y.; Wang, H.-C. Systematic Meta-Analysis of Computer-Aided Detection of Breast Cancer Using Hyperspectral Imaging. Bioengineering 2024, 11, 1060. [Google Scholar] [CrossRef]
- Bouron, C.; Mathie, C.; Seegers, V.; Morel, O.; Jezequel, P.; Lasla, H.; Guillerminet, C.; Girault, S.; Lacombe, M.; Sher, A.; et al. Prognostic Value of Metabolic, Volumetric and Textural Parameters of Baseline [(18)F]FDG PET/CT in Early Triple-Negative Breast Cancer. Cancers 2022, 14, 637. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, C.J.; Hsu, W.L.; Chang, S.M.; Huang, Y.F.; Tyan, Y.C. Prognostic Significance of Metabolic Parameters and Textural Features on (18)F-FDG PET/CT in Invasive Ductal Carcinoma of Breast. Sci. Rep. 2019, 9, 10946. [Google Scholar]
- Satoh, Y.; Hirata, K.; Tamada, D.; Funayama, S.; Onishi, H. Texture Analysis in the Diagnosis of Primary Breast Cancer: Comparison of High-Resolution Dedicated Breast Positron Emission Tomography (dbPET) and Whole-Body PET/CT. Front. Med. 2020, 7, 603303. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, D.; Shen, C.; Duan, X. Construction of a comprehensive predictive model for axillary lymph node metastasis in breast cancer: A retrospective study. BMC Cancer 2023, 23, 1028. [Google Scholar] [CrossRef]

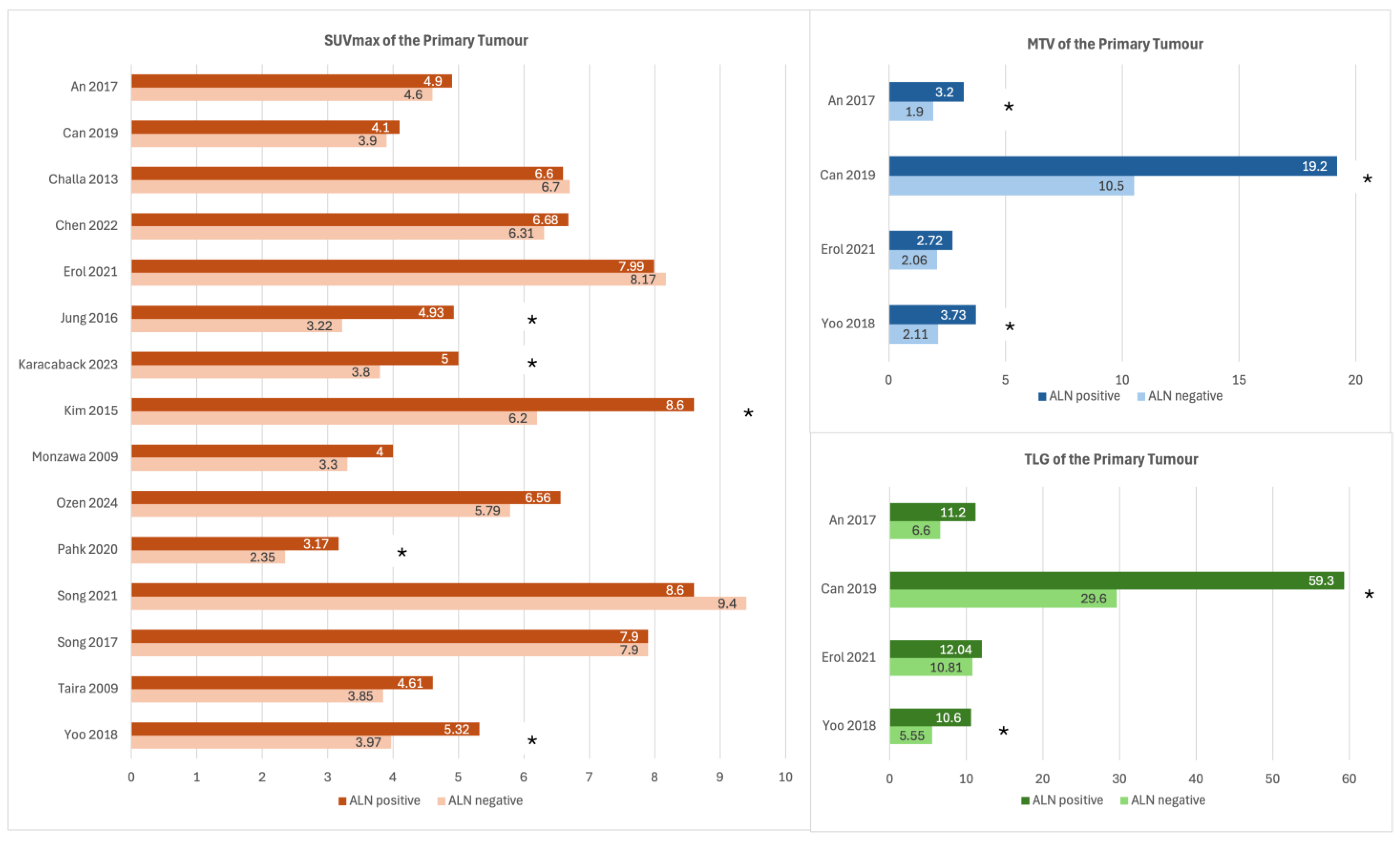
| Mean or Median Tumour SUVmax in ALN+ vs. ALN− Cases | Multivariable Analysis of Tumour SUVmax [Other Variables Included in the Model] | ROC Analysis on Tumour SUVmax | |
|---|---|---|---|
| An 2017 [21] | 4.9 vs. 4.6 Not significant | - | - |
| Can 2019 [22] | 4.1 vs. 3.9 Not significant | - | - |
| Challa 2013 [25] | 6.6 vs. 6.7 p not reported | - | - |
| Chen 2022 [26] | 6.68 vs. 6.31 Not significant | - | - |
| Erol 2021 [27] | 7.99 vs. 8.17 Not significant | - | - |
| Jung 2016 [28] | 4.93 vs. 3.22 p < 0.0001 | With Cutoff 2.8, OR: 4 (p = 0.04) [age, SUVmax of ALN (cutoff: 0.72), Size, LVI, Nuclear grade, Histologic grade, HER2] | Cutoff: 2.8, AUC: 67.7%, accuracy 67.7%, sensitivity 63.2%, specificity 65% |
| Karabacak 2023 [29] | 5 vs. 3.8 p = 0.042 | - | Cutoff 4.05, AUC: 61.8%, accuracy 57%, sensitivity 58%, specificity 55% |
| Karan 2016 [30] | N0 patients: 4.30 N1 patients: 6.18 N2 patients: 10.80 N3 patients: 10.53 p = 0.015 | - | - |
| Kim 2015 [31] | 8.6 vs. 6.2 p < 0.001 | Cutoff: 4.25, OR: 3.497 (2.245–5.446), p < 0.001. [Age, Tumour size > 2 cm, Histological grade (grade 3 vs. 2), ER Status, PR status, HER2 status, P53 status, Ki67 status, LVI, Histology (ductal versus other)] | - |
| Kong 2021 [32] | N0: 3.44 Low burden: 6.04 High burden: 5.82 Not significant | - | - |
| Monzawa 2009 [35] | 4.0 vs. 3.3 Not significant | - | - |
| Ozen 2024 [36] | 6.56 vs. 5.79 Not significant | - | - |
| Ozkan 2019 [39] | - | - | Cutoff 1.79, AUC 84.7%, accuracy 84%, sensitivity 79%, specificity 93% |
| Pahk 2020 [40] | 3.17 vs. 2.35 p = 0.012 | Cutoff: determined based on molecular subtype. Not significant. [BMI, T stage, LVI, visceral/subcutaneous fat ratio] | - |
| Song 2021 [42] | 8.6 vs. 9.4 Not significant | - | - |
| Song 2017 [43] | 7.9 vs. 7.9 Not significant | No significant results with a cutoff of 3.9. If cutoff determined by molecular subtype, OR 4.87, p = 0.0037 [LVI, tumour size, SUVmax cutoff 3.9, nodal uptake] | Cutoff 3.9, AUC 59.7% |
| Taira 2009 [45] | 4.61 vs. 3.85 Not significant | - | - |
| Yoo 2018 [46] | 5.32 vs. 3.97 p = 0.022 | No significant results [tumour size, TLG] | Cutoff 3.11, AUC 63.6% |
| Sensitivity | Specificity | PPV | NPV | Accuracy | AUC | |
|---|---|---|---|---|---|---|
| Chen 2022 [26] RF Model | - | - | - | - | 81.2% (65.3–93.9%) | 81.7% (66.1–92.9%) |
| Chen 2022 [26] SGD Model | - | - | - | - | 74.5% (50–87.5%) | 77.5% (50.6–89.2%) |
| Chen 2022 [26] KNN Model | - | - | - | - | 78.5% (64.3–89.3%) | 79.5% (64.5–88.5%) |
| Chen 2022 [26] SVM Model | - | - | - | - | 75.6% (60.7–89.3%) | 78.3% (66.0–87.7%) |
| Song 2021 [42] | 90.9% | 71.4% | 71.4% | 90.9% | 80% | 89.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, A.; Rashid, S.; Shi, S.; Blew, C.; Levine, M.; Saha, A. 18F-FDG PET/CT Semiquantitative and Radiomic Features for Assessing Pathologic Axillary Lymph Node Status in Clinical Stage I–III Breast Cancer Patients: A Systematic Review. Curr. Oncol. 2025, 32, 300. https://doi.org/10.3390/curroncol32060300
Hwang A, Rashid S, Shi S, Blew C, Levine M, Saha A. 18F-FDG PET/CT Semiquantitative and Radiomic Features for Assessing Pathologic Axillary Lymph Node Status in Clinical Stage I–III Breast Cancer Patients: A Systematic Review. Current Oncology. 2025; 32(6):300. https://doi.org/10.3390/curroncol32060300
Chicago/Turabian StyleHwang, Anna, Sana Rashid, Selina Shi, Ciara Blew, Mark Levine, and Ashirbani Saha. 2025. "18F-FDG PET/CT Semiquantitative and Radiomic Features for Assessing Pathologic Axillary Lymph Node Status in Clinical Stage I–III Breast Cancer Patients: A Systematic Review" Current Oncology 32, no. 6: 300. https://doi.org/10.3390/curroncol32060300
APA StyleHwang, A., Rashid, S., Shi, S., Blew, C., Levine, M., & Saha, A. (2025). 18F-FDG PET/CT Semiquantitative and Radiomic Features for Assessing Pathologic Axillary Lymph Node Status in Clinical Stage I–III Breast Cancer Patients: A Systematic Review. Current Oncology, 32(6), 300. https://doi.org/10.3390/curroncol32060300






