Step-by-Step Description of Standardized Technique for Robotic Pancreatoduodenectomy
Abstract
1. Introduction
2. Methods
| Demolitive Phase |
|---|
|
| Reconstructive phase |
|
| Robotic Xi Platform | |
| Maryland Bipolar Forceps | Precise dissection and coagulation |
| Fenestrated Bipolar Forceps | Grasping and coagulation |
| Tip-up Fenestrated Grasper | Gentle tissue grasping |
| Monopolar Curved Scissors | Cutting and dissecting tissue |
| Needle Driver | Suturing during anastomoses |
| Vessel Sealer | Sealing and dividing blood vessels |
| Laparoscopic and Robotic Clip Applier | Applying clips to blood vessels or ducts (e.g., Hem-o-lok clips) |
| Stapling Devices | Linear staplers for transecting the stomach, jejunum, or other structures |
| Laparoscopic Graspers | Retracting, manipulating, and holding tissues |
| Laparoscopic Suction–Irrigation Device | Clearing the surgical field of blood and debris |
| Specimen Retrieval Bag | Removing the resected specimen from the abdominal cavity |
| Drain |
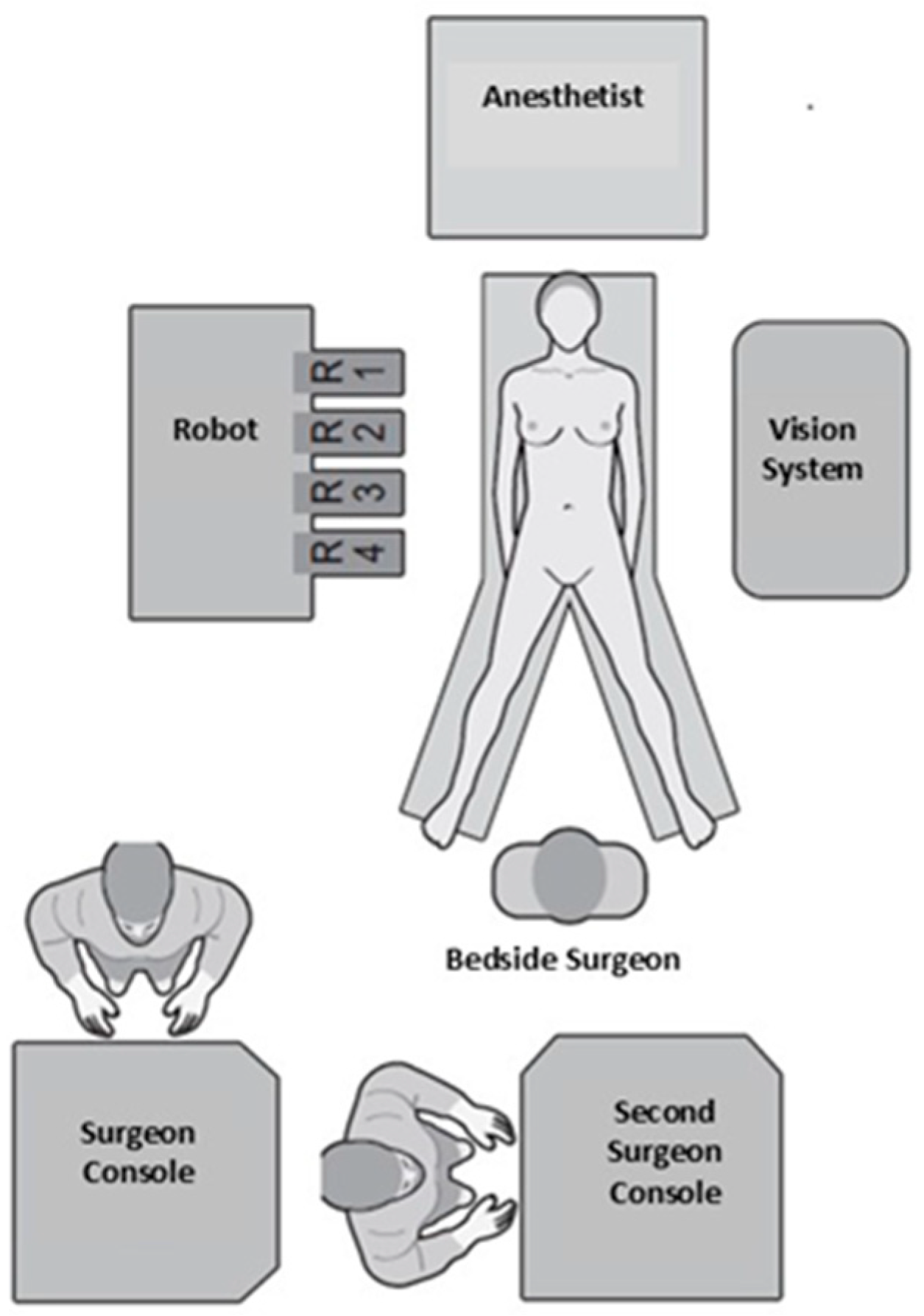
2.1. Operating Room Layout
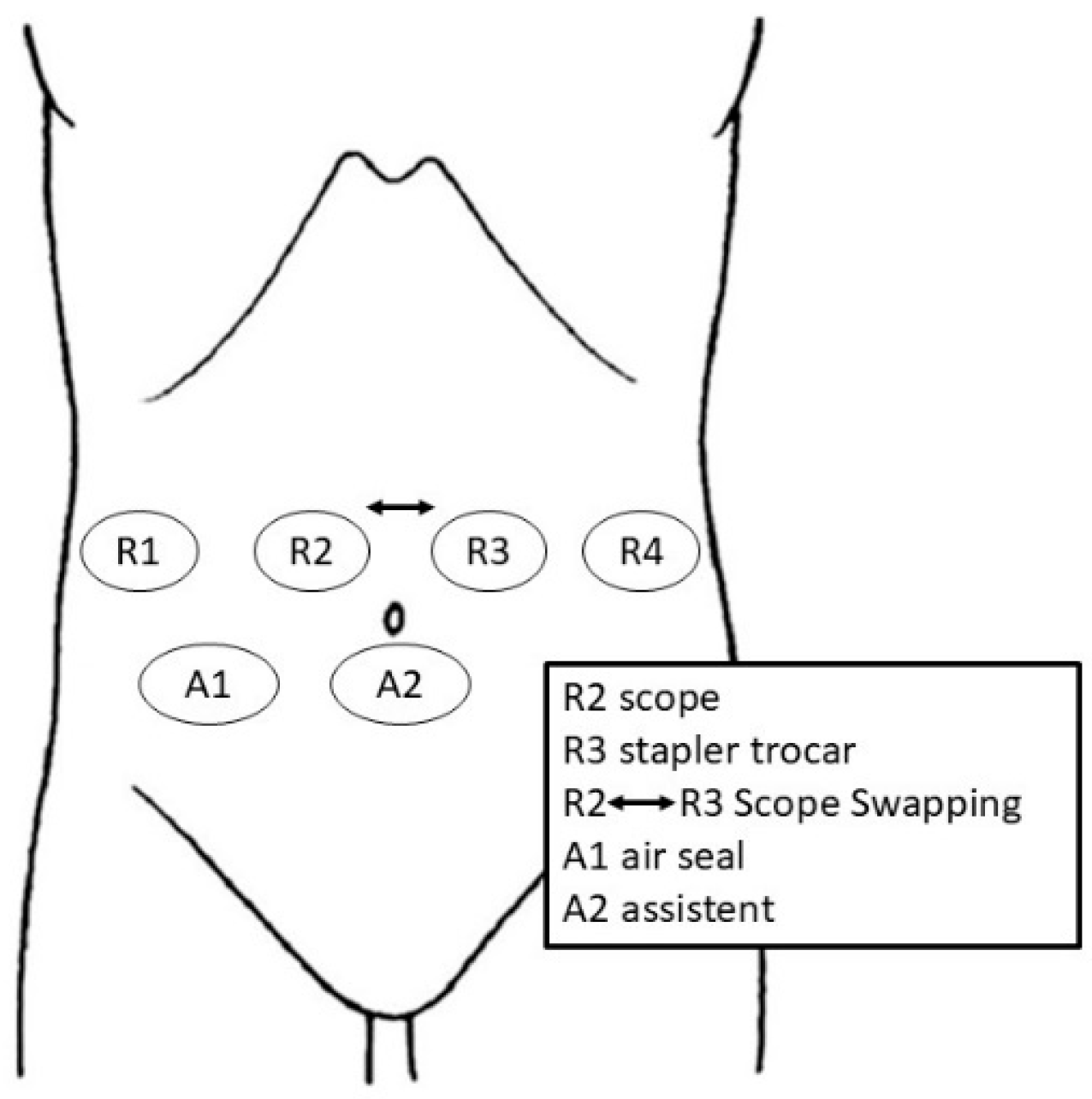
2.2. Trocar Placement and Patient Positioning
3. Operative Steps
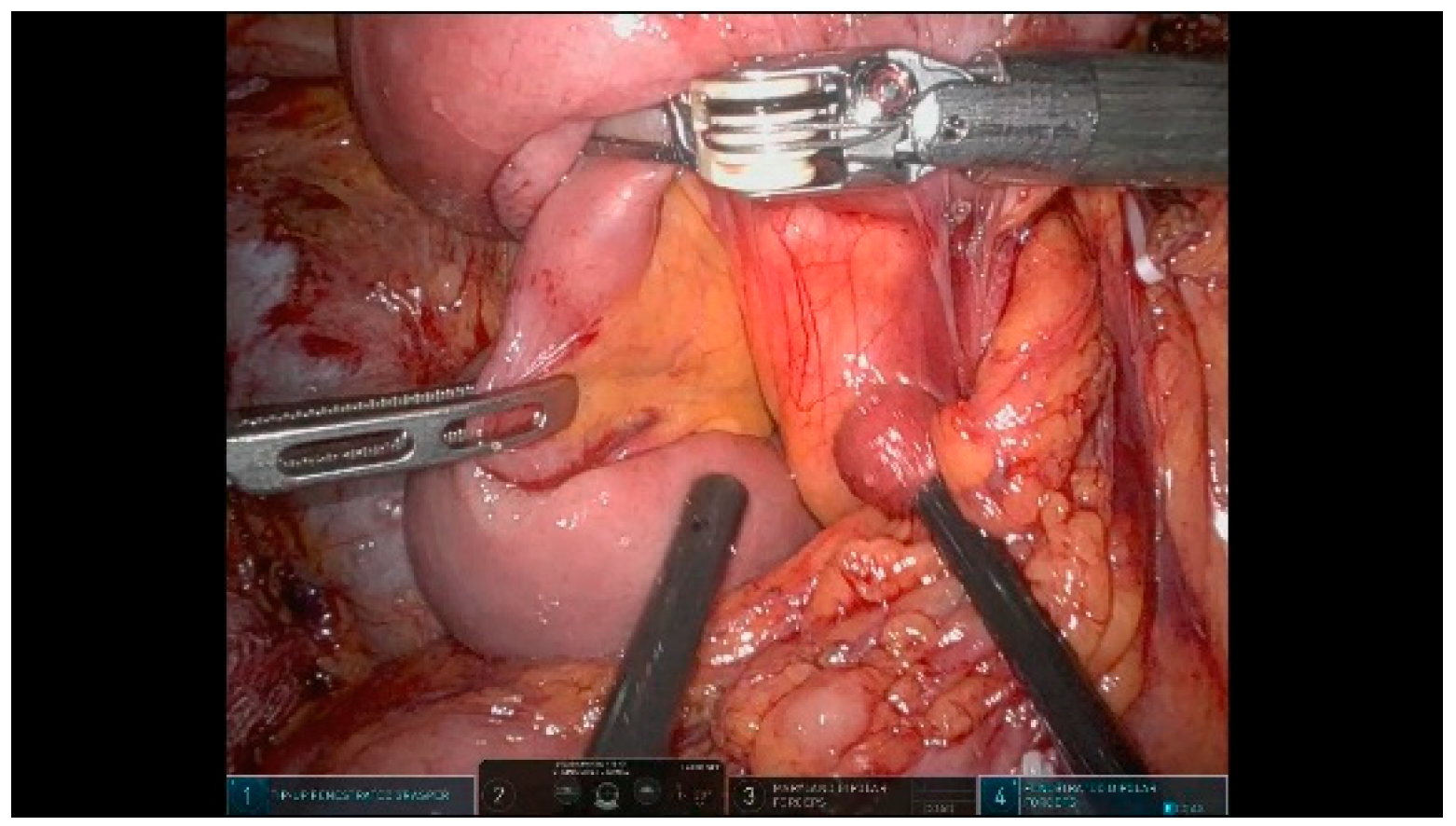
3.1. Dissection Phase
3.1.1. Step 1: Exposure
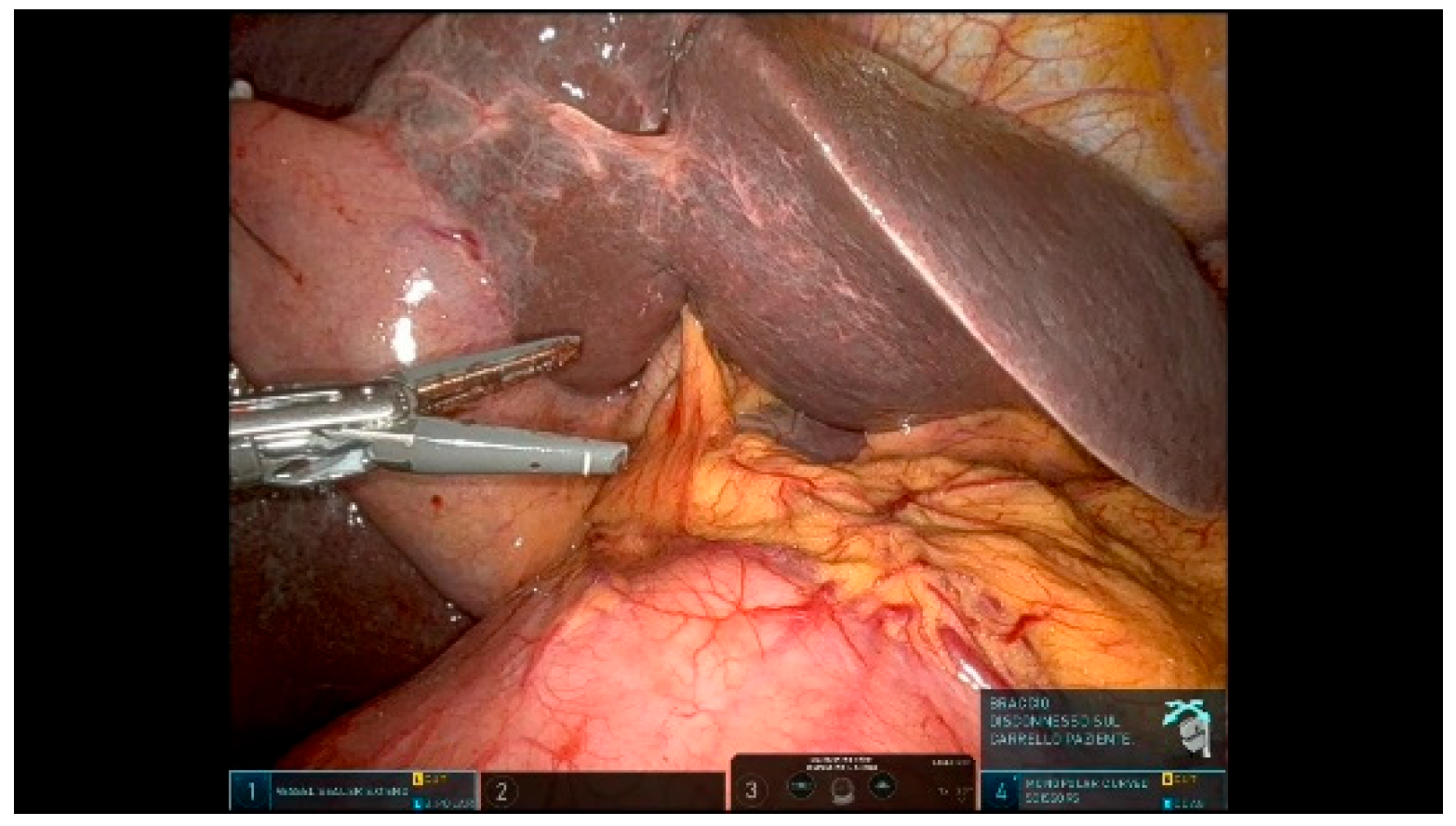
3.1.2. Step 2: Taking Down Hepatic Flexure
3.1.3. Step 3: Extensive Kocher Maneuver
3.1.4. Step 4: First Jejunal Loop Transection
3.1.5. Step 5: Gastrocolic Ligament Division
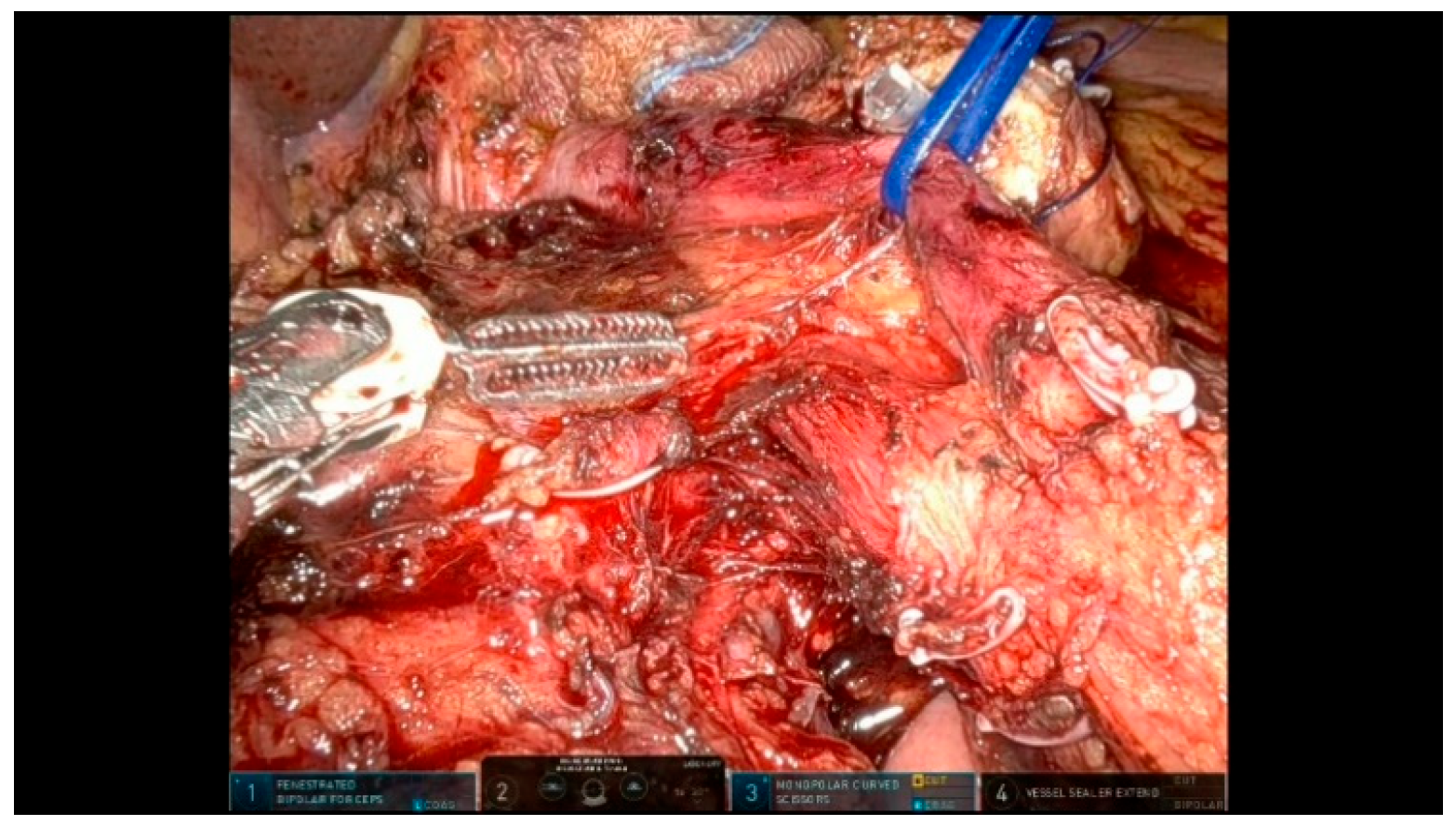
3.1.6. Step 6: Hepatic Hilum Dissection
- Right side: 12b and 12p
- Left side: 12a, 8a, and 8p
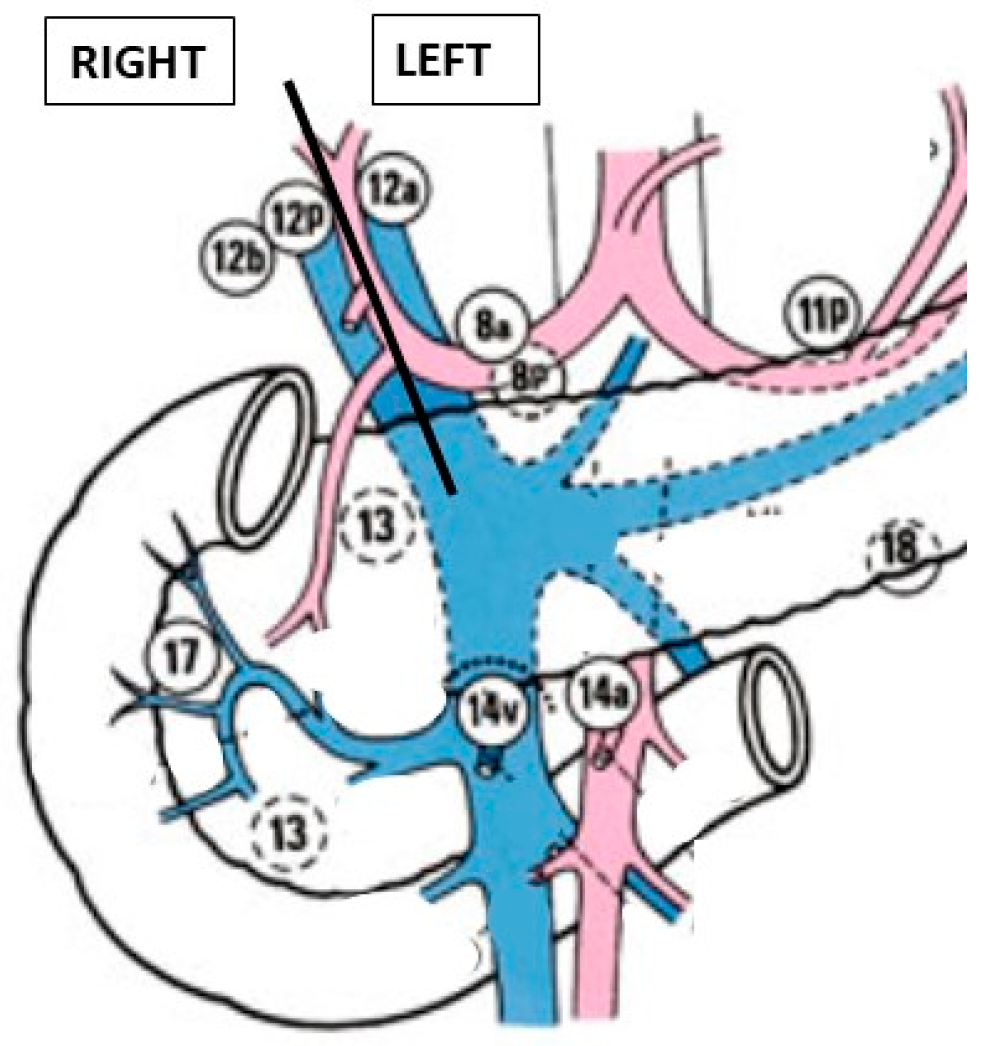
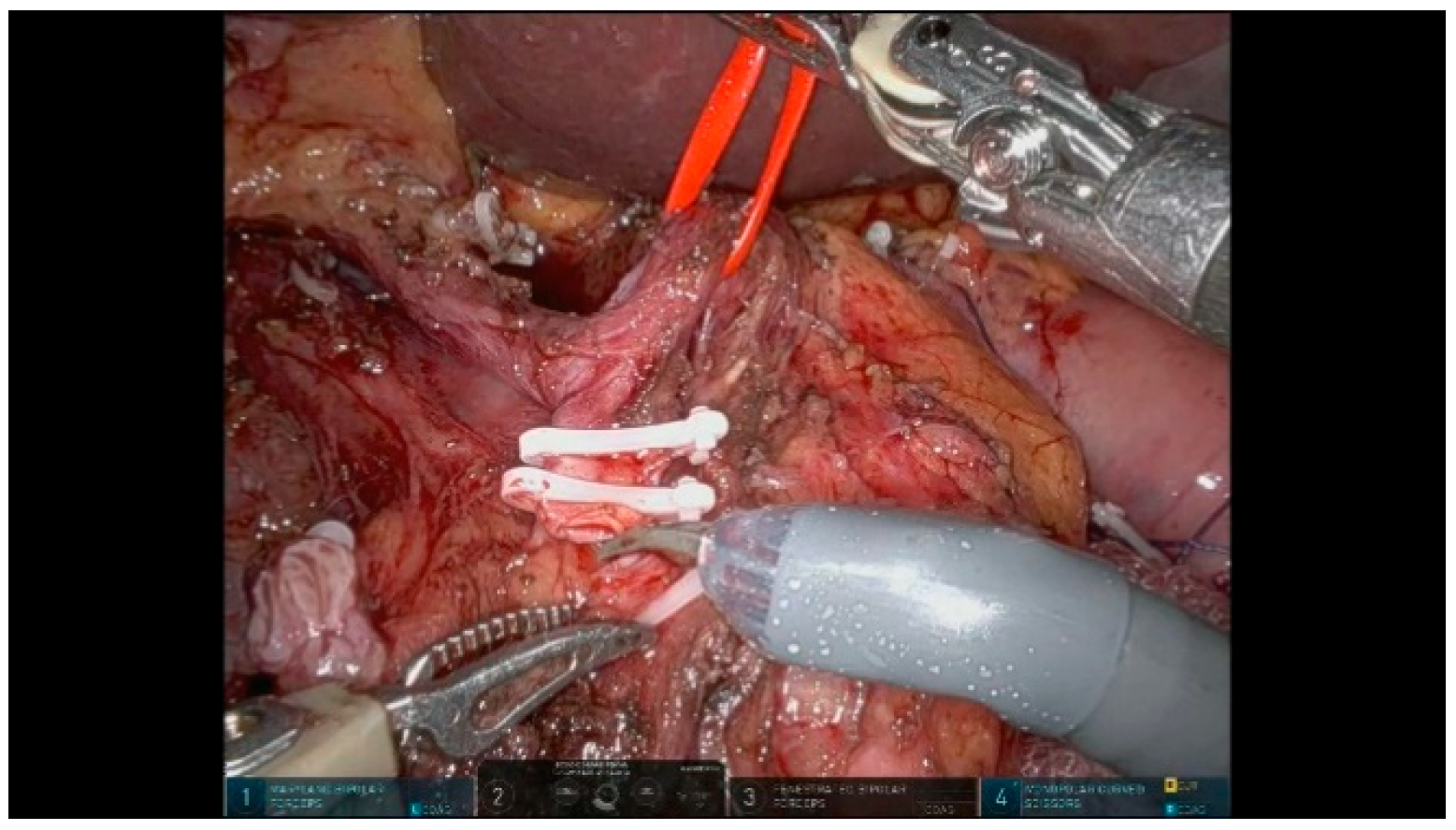
3.1.7. Step 7: Cholecystectomy and Bile Duct Transection
3.1.8. Step 8: Stomach Transection
3.1.9. Step 9: Pancreas Transection
3.1.10. Step 10: Specimen Mobilization
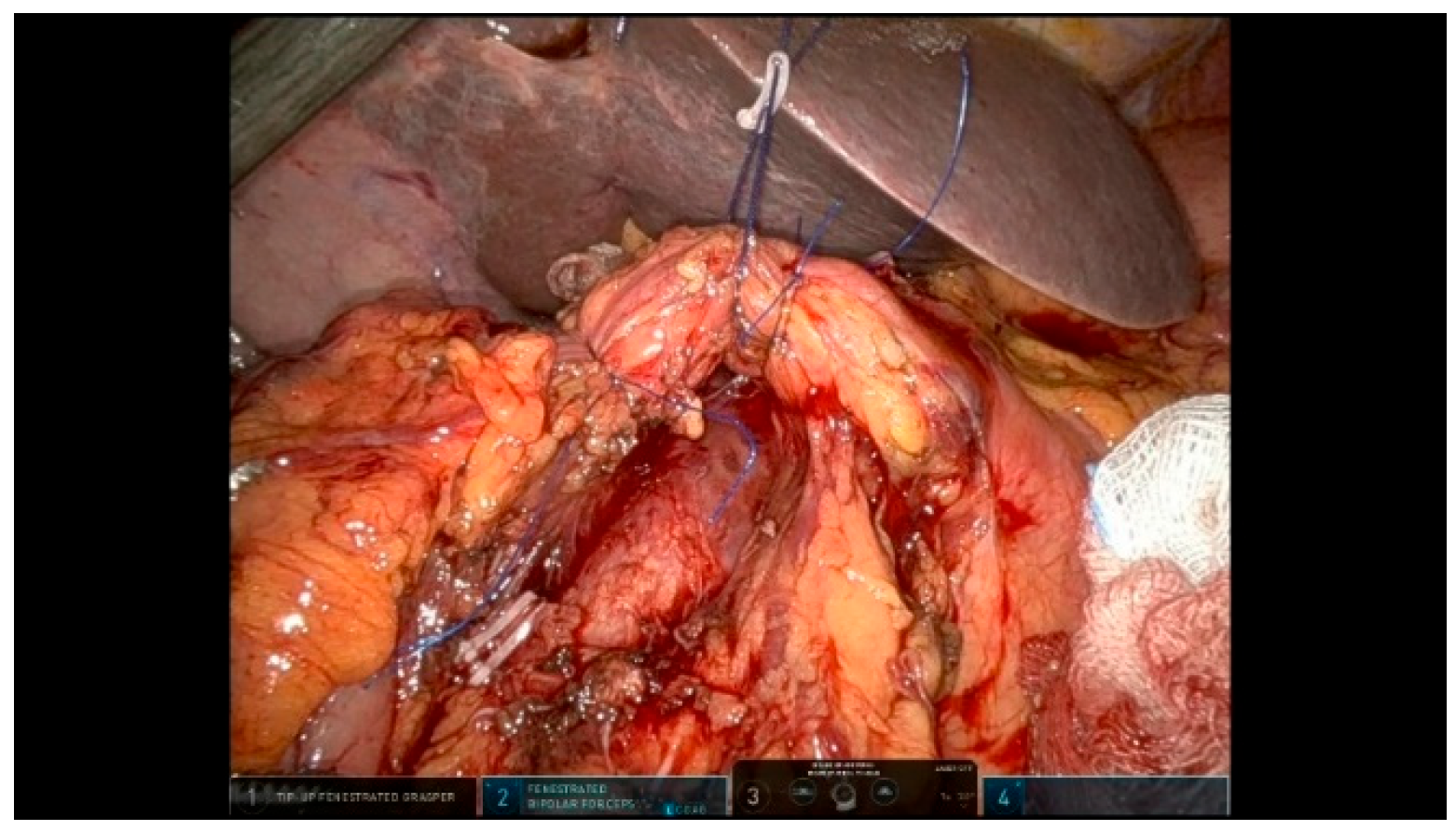
3.2. Reconstructive Phase
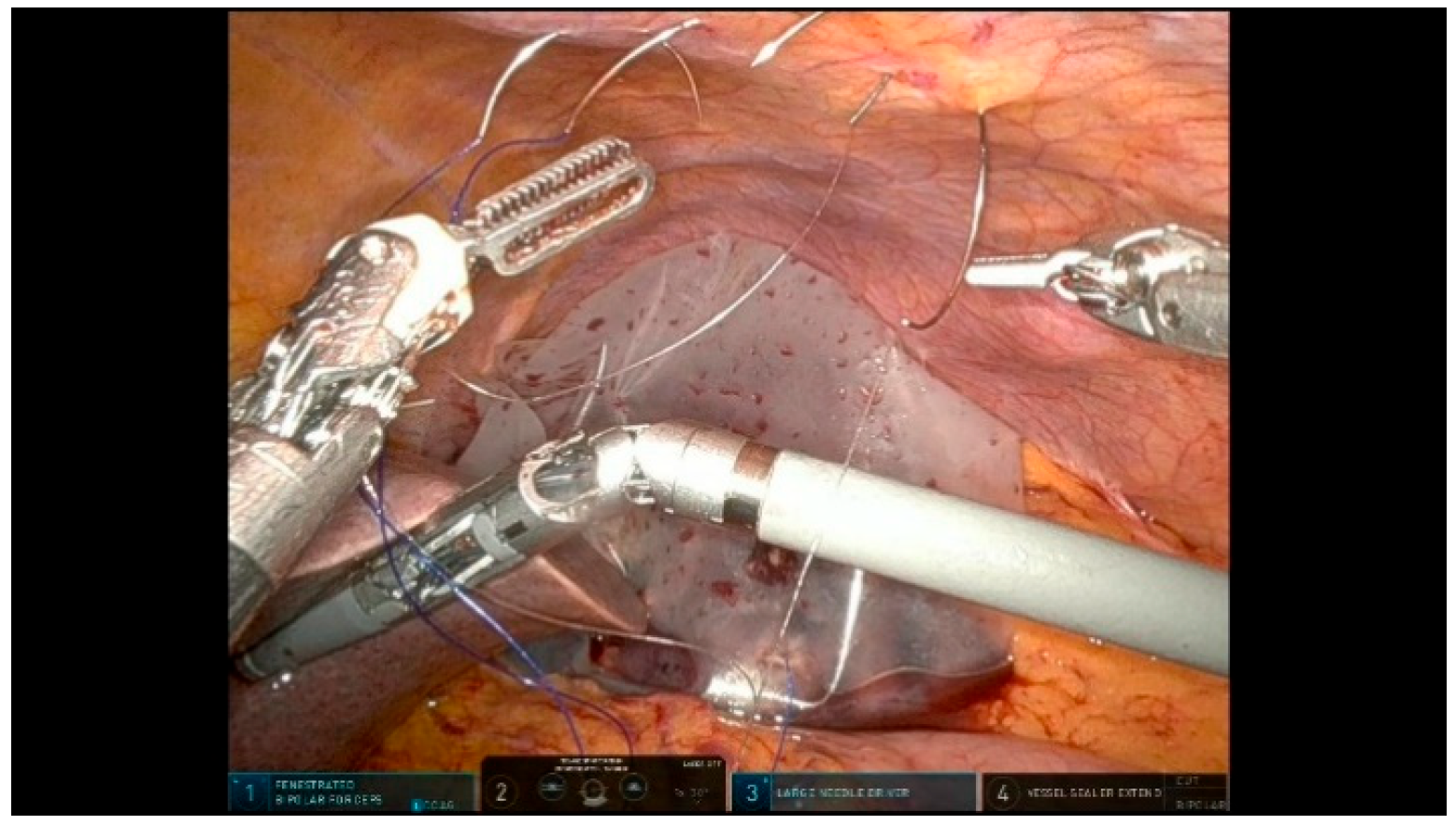
3.2.1. Step 11: Pancreaticojejunostomy
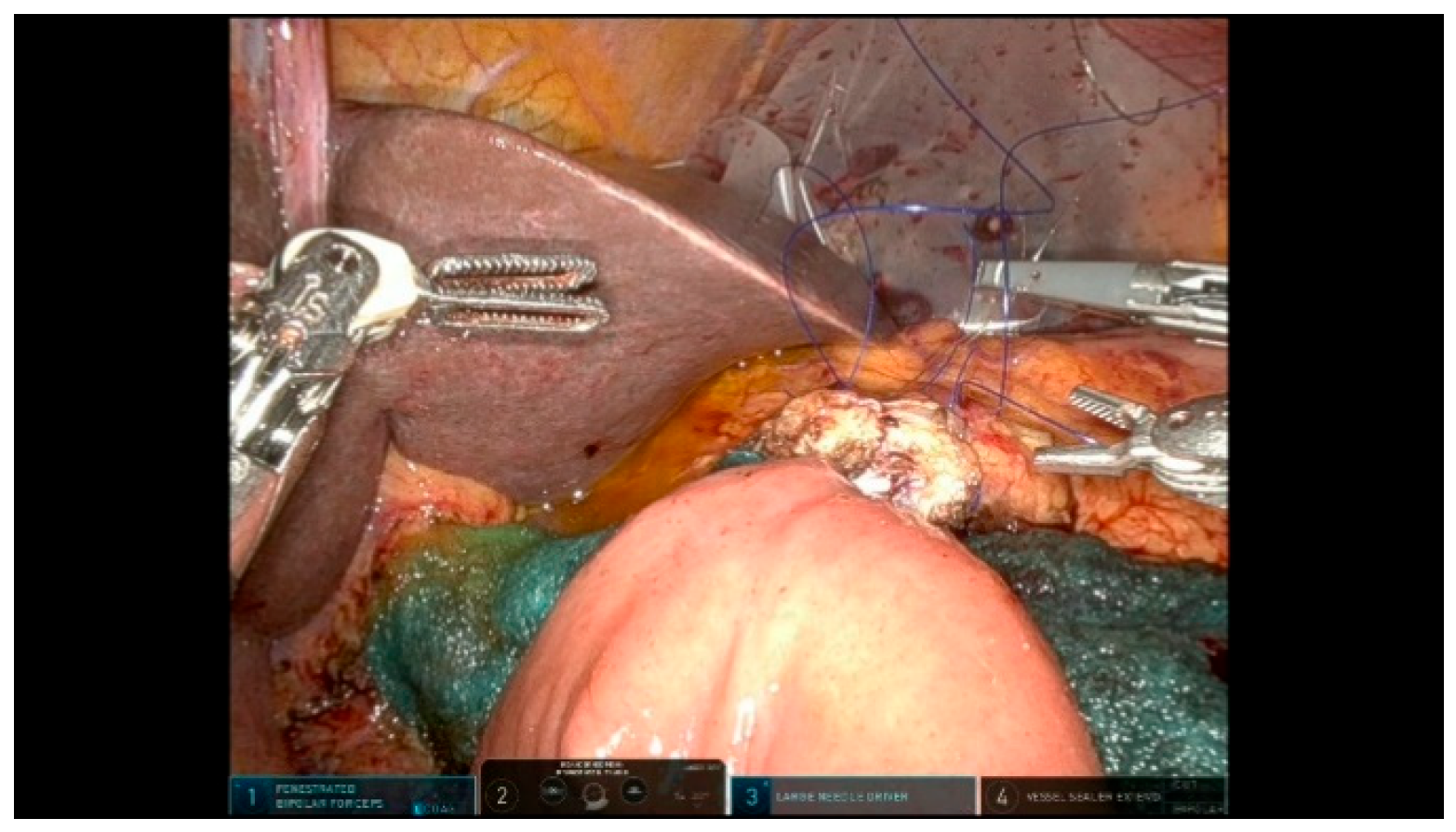
3.2.2. Step 12: Hepaticojejunostomy
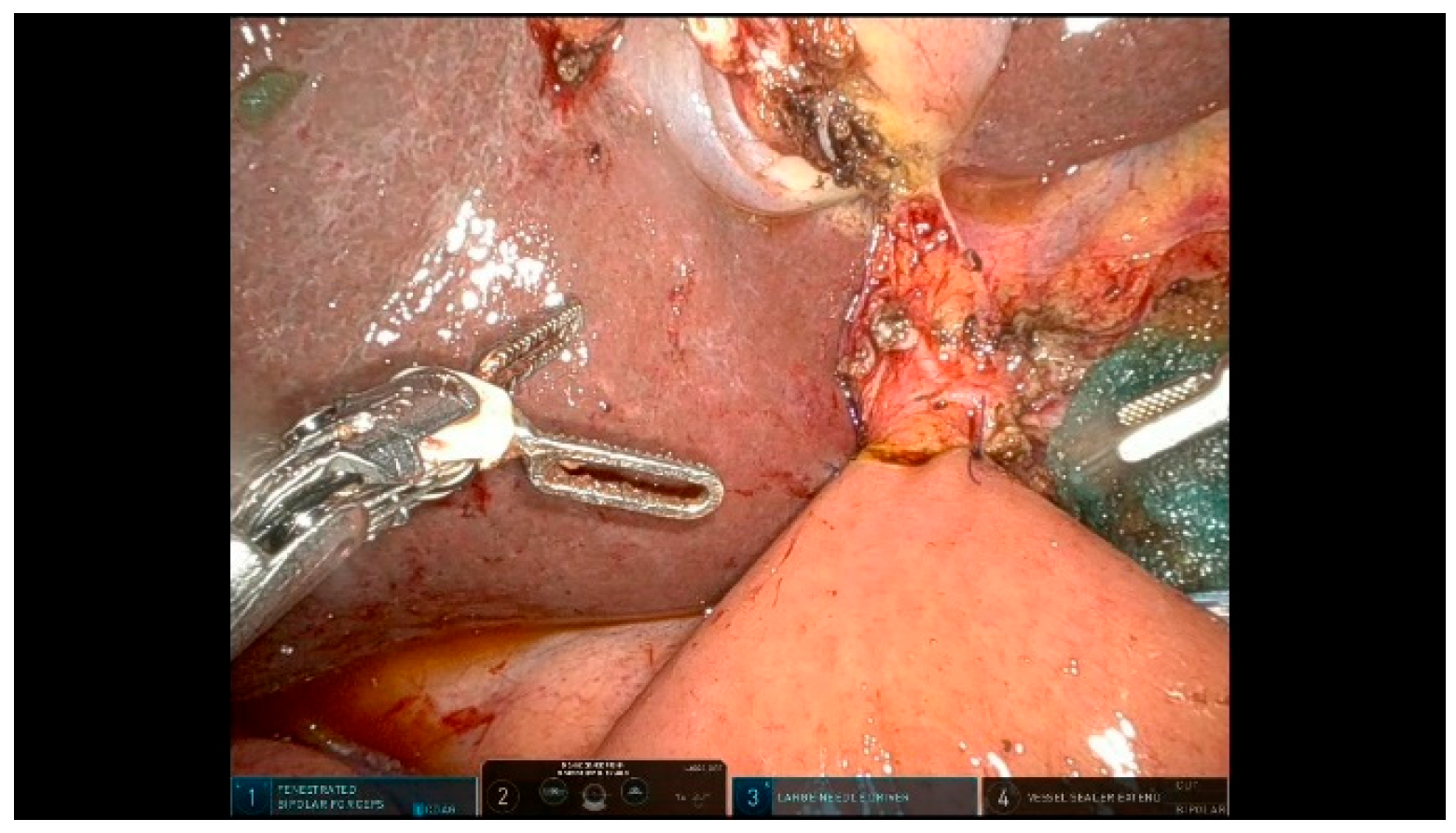
3.2.3. Step 13: Gastrojejunostomy
3.2.4. Step 14: Drain Placement
3.2.5. Step 15: Specimen Removal
4. Discussion
- Trocar Placement: ensure optimal trocar positioning based on the patient size and anatomy to facilitate smooth instrument movement and minimize external collisions between robotic arms.
- Pneumoperitoneum Management: use an AirSeal system to maintain a stable pneumoperitoneum and improve visualization, especially during prolonged dissection phases.
- Double Assistant Trocar: Utilizing two assistant trocars improves the workflow efficiency. One dedicated to suction and irrigation, the other for assisting with exposure, introducing gauze, or passing needles during reconstruction. This setup helps reduce the operative time and enhances surgical precision.
- Liver Retraction: anchor the gallbladder and round ligament to the abdominal wall to free the fourth robotic arm for other tasks.
- Jejunal Resection: perform the jejunal division at the end of an extended Kocher maneuver to facilitate orientation.
- Suspension Techniques: Use resorbable loops or vessel loops to suspend organs and vascular structures, optimizing exposure and safety. These loops help retract and define key landmarks, such as the SMV, without excessive manipulation.
- Pancreatic Neck Transection: ensure the perfect exposure of the inferior and superior pancreatic margins before transection; use vessel loops for better control.
- “Hanging Maneuver”: place a vessel loop around the SMV to facilitate a safe uncinate dissection process.
- Duct-to-Mucosa Anastomosis: use a temporary silicone stent to protect the pancreatic duct and facilitate precise suturing.
- Suture Organization: use different colored sutures to prevent tangling and enhance efficiency during anastomoses.
- Drain Placement: position drains strategically to prevent fluid accumulation and improve postoperative outcomes, particularly near the pancreatojejunostomy and hepaticojejunostomy sites, which can assist in the early identification of potential leaks.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RPD | Robotic pancreaticoduodenectomy |
| LPD | Laparoscopic pancreaticoduodenectomy |
| OPD | Open pancreaticoduodenectomy |
| SMA | Superior mesenteric artery |
| SMV | Superior mesenteric vein |
| PV | Portal vein |
| CHA | Common hepatic artery |
| PHA | Proper hepatic artery |
| CBD | Common bile duct |
| GDA | Gatroduodenal artery |
| MPD | Main pancreatic duct |
| PJ | Pancreaticojejunostomy |
| HJ | Hepaticojejunostomy |
| GJ | Gastrojejunostomy |
References
- Zureikat, A.H.; Beane, J.D.; Zenati, M.S.; Al Abbas, A.I.; Boone, B.A.; Moser, A.J.; Bartlett, D.L.; Hogg, M.E.; Zeh, H.J. 500 Minimally Invasive Robotic Pancreatoduodenectomies: One Decade of Optimizing Performance. Ann. Surg. 2021, 273, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Palanivelu, C.; Senthilnathan, P.; Sabnis, S.C.; Babu, N.S.; Srivatsan Gurumurthy, S.; Anand Vijai, N.; Nalankilli, V.P.; Raj, P.P.; Parthasarathy, R.; Rajapandian, S. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. Br. J. Surg. 2017, 104, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Uijterwijk, B.A.; Wei, K.; Kasai, M.; Ielpo, B.; van Hilst, J.; Chinnusamy, P.; Lemmers, D.H.; Burdio, F.; Senthilnathan, P.; Besselink, M.G.; et al. Minimally invasive versus open pancreatoduodenectomy for pancreatic ductal adenocarcinoma: Individual patient data meta-analysis of randomized trials. Eur. J. Surg. Oncol. 2023, 49, 1351–1361. [Google Scholar] [CrossRef]
- Dokmak, S.; Ftériche, F.S.; Aussilhou, B.; Bensafta, Y.; Lévy, P.; Ruszniewski, P.; Belghiti, J.; Sauvanet, A. Laparoscopic pancreaticoduodenectomy should not be routine for resection of periampullary tumors. J. Am. Coll. Surg. 2015, 220, 831–838. [Google Scholar] [CrossRef]
- Gagner, M.; Pomp, A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg. Endosc. 1994, 8, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Boggi, U.; Amorese, G.; Vistoli, F.; Caniglia, F.; De Lio, N.; Perrone, V.; Barbarello, L.; Belluomini, M.; Signori, S.; Mosca, F. Laparoscopic pancreaticoduodenectomy: A systematic literature review. Surg. Endosc. 2015, 29, 9–23. [Google Scholar] [CrossRef]
- Mabrut, J.Y.; Fernandez-Cruz, L.; Azagra, J.S.; Bassi, C.; Delvaux, G.; Weerts, J.; Fabre, J.; Boulez, J.; Baulieux, J.; Peix, J. Laparoscopic pancreatic resection: Results of a multicenter European study of 127 patients. Surgery 2005, 137, 597–605. [Google Scholar] [CrossRef]
- Napoli, N.; Kauffmann, E.F.; Palmeri, M.; Miccoli, M.; Costa, F.; Vistoli, F.; Amorese, G.; Boggi, U. The Learning Curve in Robotic Pancreaticoduodenectomy. Dig. Surg. 2016, 33, 299–307. [Google Scholar] [CrossRef]
- Giulianotti, P.C. Robotics in General Surgery. Arch. Surg. 2003, 138, 777. [Google Scholar] [CrossRef]
- Kornaropoulos, M.; Moris, D.; Beal, E.W.; Makris, M.C.; Mitrousias, A.; Petrou, A.; Felekouras, E.; Michalinos, A.; Vailas, M.; Schizas, D.; et al. Total robotic pancreaticoduodenectomy: A systematic review of the literature. Surg. Endosc. 2017, 31, 4382–4392. [Google Scholar] [CrossRef]
- Giulianotti, P.C.; Mangano, A.; Bustos, R.E.; Gheza, F.; Fernandes, E.; Masrur, M.A.; Gangemi, A.; Bianco, F.M. Operative technique in robotic pancreaticoduodenectomy (RPD) at University of Illinois at Chicago (UIC): 17 steps standardized technique: Lessons learned since the first worldwide RPD performed in the year 2001. Surg. Endosc. 2018, 32, 4329–4336. [Google Scholar] [CrossRef] [PubMed]
- Giulianotti, P.C.; Mangano, A.; Bustos, R.E.; Fernandes, E.; Masrur, M.A.; Valle, V.; Gangemi, A.; Bianco, F.M. Educational step-by-step surgical video about operative technique in robotic pancreaticoduodenectomy (RPD) at University of Illinois at Chicago (UIC): 17 steps standardized technique—Lessons learned since the first worldwide RPD performed in the year 2001. Surg. Endosc. 2020, 34, 2758–2762. [Google Scholar] [CrossRef]
- van Hilst, J.; Korrel, M.; de Rooij, T.; Lof, S.; Busch, O.R.; Groot Koerkamp, B.; Kooby, D.A.; van Dieren, S.; Abu Hilal, M.; Besselink, M.G. Oncologic outcomes of minimally invasive versus open distal pancreatectomy for pancreatic ductal adenocarcinoma: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2019, 45, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.; Can, M.F.; Bartlett, D.L.; Zeh, H.J.; Zureikat, A.H. The current state of robotic-assisted pancreatic surgery. Nat. Rev. Gastroenterol. Hepatol. 2012, 5, 468–476. [Google Scholar] [CrossRef]
- Asbun, H.J.; Moekotte, A.L.; Vissers, F.L.; Kunzler, F.; Cipriani, F.; Alseidi, A.; D’angelica, M.I.; Balduzzi, A.; Bassi, C.; Björnsson, B.; et al. The Miami International Evidence-based Guidelines on Minimally Invasive Pancreas Resection. Ann. Surg. 2020, 271, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sattari, S.A.; Sattari, A.R.; Makary, M.A.; Hu, C.; He, J. Laparoscopic Versus Open Pancreatoduodenectomy in Patients with Periampullary Tumors: A Systematic Review and Meta-analysis. Ann. Surg. 2023, 277, 742–755. [Google Scholar] [CrossRef]
- Papalampros, A.; Niehaus, K.; Moris, D.; Fard-Aghaie, M.; Stavrou, G.; Margonis, A.G.; Angelou, A.; Oldhafer, K. A safe and feasible “clock-face” duct-to-mucosa pancreaticojejunostomy with a very low incidence of anastomotic failure: A single center experience of 248 patients. J. Visc. Surg. 2016, 153, 425–431. [Google Scholar] [CrossRef]
- Klotz, R.; Mihaljevic, A.L.; Kulu, Y.; Sander, A.; Klose, C.; Behnisch, R.; Joos, M.C.; Kalkum, E.; Nickel, F.; Knebel, P.; et al. Robotic versus open partial pancreatoduodenectomy (EUROPA): A randomised controlled stage 2b trial. Lancet Reg. Health Europe 2024, 39, 100864. [Google Scholar] [CrossRef]
- Boone, B.A.; Zenati, M.; Hogg, M.E.; Steve, J.; Moser, A.J.; Bartlett, D.L.; Zeh, H.J.; Zureikat, A.H. Assessment of quality outcomes for robotic pancreaticoduodenectomy: Identification of the learning curve. JAMA Surg. 2015, 150, 416–422. [Google Scholar] [CrossRef]
- Speicher, P.J.; Nussbaum, D.P.; White, R.R.; Zani, S.; Mosca, P.J.; Blazer, D.G.; Clary, B.M.; Pappas, T.N.; Tyler, D.S.; Perez, A. Defining the Learning Curve for Team-Based Laparoscopic Pancreaticoduodenectomy. Ann. Surg. Oncol. 2014, 21, 4014–4019. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, Q.; Zhao, Z.; Zhang, X.; Gao, Y.; Tan, X.; Liu, R. The standardized technique and surgical video of robotic pancreaticoduodenectomy at the Chinese PLA General Hospital. Updates Surg. 2022, 74, 245–254. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delvecchio, A.; Caringi, S.; De Palma, C.; Brischetto, G.; Filippo, R.; Casella, A.; Ferraro, V.; Stasi, M.; Memeo, R.; Tedeschi, M. Step-by-Step Description of Standardized Technique for Robotic Pancreatoduodenectomy. Curr. Oncol. 2025, 32, 302. https://doi.org/10.3390/curroncol32060302
Delvecchio A, Caringi S, De Palma C, Brischetto G, Filippo R, Casella A, Ferraro V, Stasi M, Memeo R, Tedeschi M. Step-by-Step Description of Standardized Technique for Robotic Pancreatoduodenectomy. Current Oncology. 2025; 32(6):302. https://doi.org/10.3390/curroncol32060302
Chicago/Turabian StyleDelvecchio, Antonella, Silvio Caringi, Cataldo De Palma, Gaetano Brischetto, Rosalinda Filippo, Annachiara Casella, Valentina Ferraro, Matteo Stasi, Riccardo Memeo, and Michele Tedeschi. 2025. "Step-by-Step Description of Standardized Technique for Robotic Pancreatoduodenectomy" Current Oncology 32, no. 6: 302. https://doi.org/10.3390/curroncol32060302
APA StyleDelvecchio, A., Caringi, S., De Palma, C., Brischetto, G., Filippo, R., Casella, A., Ferraro, V., Stasi, M., Memeo, R., & Tedeschi, M. (2025). Step-by-Step Description of Standardized Technique for Robotic Pancreatoduodenectomy. Current Oncology, 32(6), 302. https://doi.org/10.3390/curroncol32060302






