Effects of a Concurrent Mixed-Modality (Telerehabilitation and Face-to-Face) Exercise Rehabilitation Program in a Patient with Multiple Myeloma Prior to Spinal Cord Transplantation: A Case Study
Abstract
1. Introduction
2. Materials and Method
2.1. Study Design
2.2. Patient Information and Clinical Findings
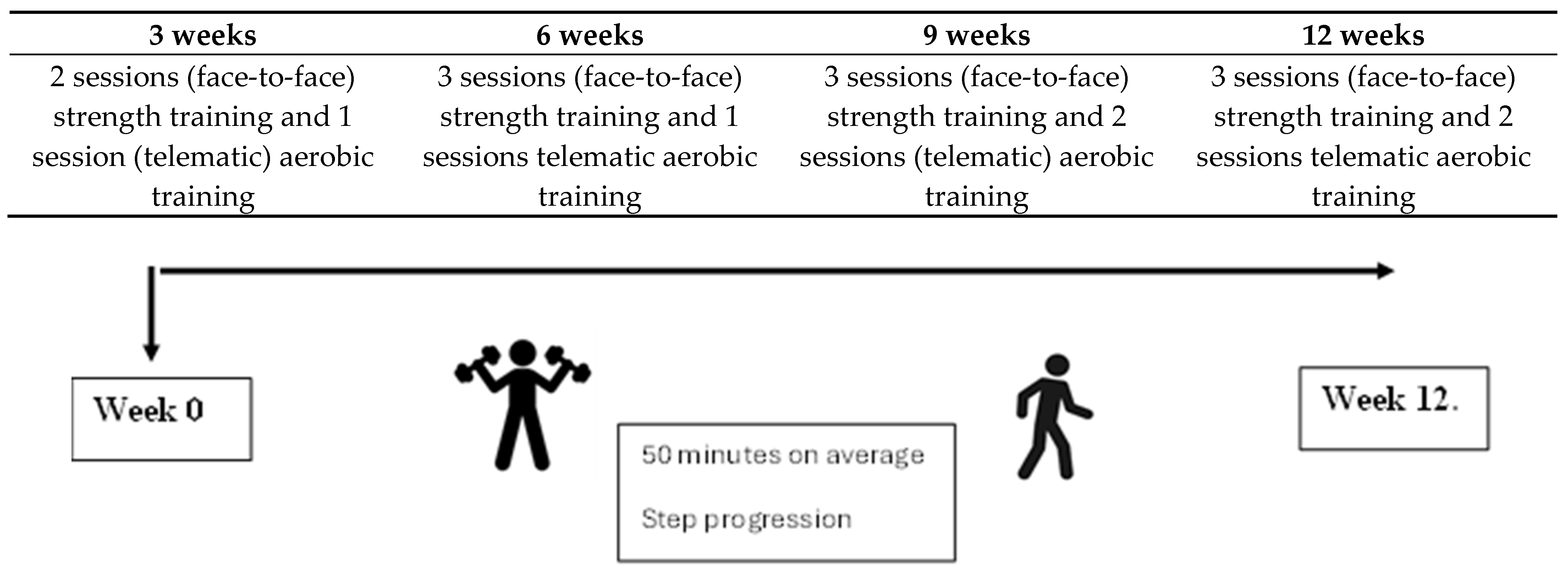
2.3. Study Chronology
2.4. Diagnostic Evaluation
2.5. Therapeutic Intervention
2.5.1. Exercise Protocol
2.5.2. Weeks 1–3
2.5.3. Weeks 4–12
2.6. Evaluation and Outcomes
2.6.1. Dynamometer
2.6.2. Sit-to-Stand Test
2.6.3. Berg Test
2.6.4. 6MWT
2.6.5. SF-36
2.6.6. PIPER
2.6.7. FMS
2.7. Average Effort per Session
3. Results
3.1. Clinical Outcomes
3.1.1. Descriptive Data
3.1.2. Percentage Change Analysis and Bar Charts
3.2. Patient Perspective
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martínez-Guijarro, C.; López-Fernández, M.D.; Lopez-Garzon, M.; Lozano-Lozano, M.; Arroyo-Morales, M.; Galiano-Castillo, N. Feasibility and efficacy of telerehabilitation in the management of patients with head and neck cancer during and after oncological treatment: A systematic review. Eur. J. Oncol. Nurs. 2023, 63, 102279. [Google Scholar] [CrossRef] [PubMed]
- Bladé, J.; Beksac, M.; Caers, J.; Jurczyszyn, A.; von Lilienfeld-Toal, M.; Moreau, P.; Rasche, L.; Rosiñol, L.; Usmani, S.Z.; Zamagni, E.; et al. Extramedullary disease in multiple myeloma: A systematic literature review. Blood Cancer J. 2022, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.; Hayden, P.J.; Will, A.; Wheatley, K.; Coyne, I. Bortezomib for the treatment of multiple myeloma. Cochrane Database Syst. Rev. 2013, CD010816. [Google Scholar]
- Mian, H.; McCurdy, A.; Giri, S.; Grant, S.; Rochwerg, B.; Winks, E.; Rosko, A.E.; Engelhardt, M.; Pawlyn, C.; Cook, G.; et al. The prevalence and outcomes of frail older adults in clinical trials in multiple myeloma: A systematic review. Blood Cancer J. 2023, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Sekine, L.; Ziegelmann, P.K.; Manica, D.; Da Fonte Pithan, C.; Sosnoski, M.; Morais, V.D.; Falcetta, F.S.; Ribeiro, M.R.; Salazar, A.P.; Ribeiro, R.A. Frontline treatment for trans-plant-eligible multiple myeloma: A 6474 patients network meta-analysis. Hematol. Oncol. 2019, 37, 62–74. [Google Scholar] [CrossRef]
- Nørgaard, M.; Skriver, M.V.; Gregersen, H.; Pedersen, G.; Schønheyder, H.C.; Sørensen, H.T. The data quality of haematological malignancy ICD-10 diagnoses in a population-based Hospital Discharge Registry. Eur. J. Cancer Prev. 2005, 14, 201–206. [Google Scholar] [CrossRef]
- Nicol, J.L.; Chong, J.E.; McQuilten, Z.K.; Mollee, P.; Hill, M.M.; Skinner, T.L. Safety, Feasibility, and Efficacy of Exercise In-terventions for People with Multiple Myeloma: A Systematic Review. Clin. Lymphoma Myeloma Leuk. 2023, 23, 86–96. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Dobek, J.; Nail, L.M.; Bennett, J.A.; Leo, M.C.; Torgrimson-Ojerio, B.; Luoh, S.-W.; Schwartz, A. Impact+ resistance training improves bone health and body composition in prematurely menopausal breast cancer survivors: A randomized controlled trial. Osteoporos. Int. 2012, 24, 1637–1646. [Google Scholar] [CrossRef]
- Prado, C.M.; Baracos, V.E.; McCargar, L.J.; Reiman, T.; Mourtzakis, M.; Tonkin, K.; Mackey, J.R.; Koski, S.; Pituskin, E.; Sawyer, M.B. Sarcopenia as a Determinant of Chemotherapy Toxicity and Time to Tumor Progression in Metastatic Breast Cancer Patients Receiving Capecitabine Treatment. Clin. Cancer Res. 2009, 15, 2920–2926. [Google Scholar] [CrossRef]
- van Rooijen, S.J.; Molenaar, C.J.; Fokkenrood, H.J.; Roumen, R.M.; Slooter, G.D. Prehabilitation versus no prehabilitation to improve functional capacity, reduce postoperative complications and improve quality of life in colorectal cancer surgery. Cochrane Database Syst. Rev. 2022, 5, CD013259. [Google Scholar] [CrossRef]
- Michael, C.M.; Lehrer, E.J.; Schmitz, K.H.; Zaorsky, N.G. Prehabilitation exercise therapy for cancer: A systematic review and meta-analysis. Cancer Med. 2021, 10, 4195–4205. [Google Scholar] [CrossRef]
- Carli, F.; Scheede-Bergdahl, C. Prehabilitation to enhance perioperative care. Anesthesiol. Clin. 2015, 33, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Minnella, E.M.; Awasthi, R.; Loiselle, S.E.; Agnihotram, R.V.; Ferri, L.E.; Carli, F. Effect of Exercise and Nutrition Prehabilitation on Functional Capacity in Esophagogastric Cancer Surgery: A Randomized Clinical Trial. JAMA Surg. 2018, 153, 1081. [Google Scholar] [CrossRef] [PubMed]
- Goodhew, R.E.; Edwards, B.A. The effect of exercise interventions on quality of life in patients with multiple myeloma: A systematic review and meta-analysis of randomised controlled trials. Clin. Exp. Med. 2023, 23, 3217–3230. [Google Scholar] [CrossRef]
- Garcia, D.O.; Thomson, C.A. Physical activity and cancer survivorship. Nutr. Clin. Pract. 2014, 29, 768–779. [Google Scholar] [CrossRef]
- Coleman, E.A.; Coon, S.K.; Kennedy, R.L.; Lockhart, K.D.; Stewart, C.B.; Anaissie, E.J.; Barlogie, B. Effects of Exercise in Combination with Epoetin Alfa During High-Dose Chemotherapy and Autologous Peripheral Blood Stem Cell Transplantation for Multiple Myeloma. Oncol. Nurs. Forum 2008, 35, E53–E61. [Google Scholar] [CrossRef]
- Duncan, N. Declaration of Helsinki. World Med. J. 2013, 59, 132. Available online: https://search.ebscohost.com/login.aspx?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=00498122&asa=N&AN=92595558&h=y%2BiBsTY3HDYIIq8fbpL6tuKYCot%2BlQWb2yYwcNYDJaoKWX3%2Fv9lZzfQAlXEx1jCg%2BPpz7tLdE8swVMGotcKWSg%3D%3D&crl=c (accessed on 13 May 2025).
- Kramer, H.S.; Drews, F.A. Checking the lists: A systematic review of electronic checklist use in health care. J. Biomed. Inform. 2017, 71, S6–S12. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-C.; Wu, L.-C.; Chiang, S.-L.; Lu, L.-H.; Chen, C.-Y.; Lin, C.-H.; Ni, C.-H.; Lin, C.-H. Validating the Capability for Measuring Age-Related Changes in Grip-Force Strength Using a Digital Hand-Held Dynamometer in Healthy Young and Elderly Adults. BioMed Res. Int. 2020, 2020, 6936879. [Google Scholar] [CrossRef]
- Lupton-Smith, A.; Fourie, K.; Mazinyo, A.; Mokone, M.; Nxaba, S.; Morrow, B. Measurement of hand grip strength: A cross-sectional study of two dynamometry devices. S. Afr. J. Physiother. 2022, 78, 1768. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Crouch, R. 1-Minute Sit-to-Stand Test: Systematic Review of Procedures, Performance, and Clinimetric Properties. J. Cardiopulm. Rehabil. Prev. 2019, 39, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Montecinos, C.; Torres-Castro, R.; Otto-Yáñez, M.; Barros-Poblete, M.; Valencia, C.; Campos, A.; Jadue, L.; Barros, M.; Solis-Navarro, L.; Resqueti, V. Which Sit-to-Stand Test Best Differentiates Functional Capacity in Older People? Am. J. Phys. Med. Rehabil. 2024, 103, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C.; Trost, S.; Brown, W.; Armit, C. Reliability and validity of physical fitness field tests for adults aged 55 to 70 years. J. Sci. Med. Sport 2005, 8, 61–70. [Google Scholar] [CrossRef]
- Miranda, N.; Tiu, T.K. Berg Balance Testing. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK574518/ (accessed on 13 May 2025).
- Gribble, P.A.; Brigle, J.; Pietrosimone, B.G.; Pfile, K.R.; Webster, K.A. Intrarater Reliability of the Functional Movement Screen. J. Strength Cond. Res. 2013, 27, 978–981. [Google Scholar] [CrossRef]
- Ross, R.M.; Murthy, J.N.; Wollak, I.D.; Jackson, A.S. The six minute walk test accurately estimates mean peak oxygen uptake. BMC Pulm. Med. 2010, 10, 31. [Google Scholar] [CrossRef]
- Felser, S.; Behrens, M.; Liese, J.; Strueder, D.F.; Rhode, K.; Junghanss, C.; Grosse-Thie, C. Feasibility and Effects of a Supervised Exercise Program Suitable for Independent Training at Home on Physical Function and Quality of Life in Head and Neck Cancer Patients: A Pilot Study. Integr. Cancer Ther. 2020, 19, 1534735420918935. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Vogt, L.; Thiel, C.; Jäger, E.; Banzer, W. Validity of the Six-Minute Walk Test in Cancer Patients. Int. J. Sport Med. 2013, 34, 631–636. [Google Scholar] [CrossRef]
- Ware, J.; Kosinski, M.; Gandek, B. SF-36 Health Survey: Manual & Interpretation Guide; Linc RI Qual Inc.: Lincoln, RI, USA, 1993. [Google Scholar]
- Vilagut, G.; Ferrer, M.; Rajmil, L.; Rebollo, P.; Permanyer-Miralda, G.; Quintana, J.M.; Santed, R.; Valderas, J.M.; Ribera, A.; Domingo-Salvany, A.; et al. El Cuestionario de Salud SF-36 español: Una década de experiencia y nuevos desarrollos. Gac. Sanit. 2005, 19, 135–150. [Google Scholar] [CrossRef]
- Ware, J.E., Jr. SF-36 Health Survey Update. Spine 2000, 25, 3130–3139. [Google Scholar] [CrossRef]
- Lins, L.; Carvalho, F.M. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med. 2016, 4, 2050312116671725. [Google Scholar] [CrossRef]
- Brazier, J.E.; Harper, R.; Jones, N.M.; O’Cathain, A.; Thomas, K.J.; Usherwood, T.; Westlake, L. Validating the SF-36 health survey questionnaire: New outcome measure for primary care. BMJ 1992, 305, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Reeve, B.B.; Stover, A.M.; Alfano, C.M.; Smith, A.W.; Ballard-Barbash, R.; Bernstein, L.; McTiernan, A.; Baumgartner, K.B.; Piper, B.F. The Piper Fatigue Scale-12 (PFS-12): Psychometric findings and item reduction in a cohort of breast cancer survivors. Breast Cancer Res. Treat. 2012, 136, 9–20. [Google Scholar] [CrossRef] [PubMed]
- O’ Regan, P.; Hegarty, J. The importance of self-care for fatigue amongst patients undergoing chemotherapy for primary cancer. Eur. J. Oncol. Nurs. 2017, 28, 47–55. [Google Scholar] [CrossRef]
- PhD Dgpgo. Functional Movement Screen (FMS) a la Palestra: ¿Qué nos dice la Ciencia?—Grupo Sobre Entrenamiento. 2024. Available online: https://g-se.com/es/functional-movement-screen-fms-a-la-palestra-que-nos-dice-la-ciencia (accessed on 13 May 2025).
- Ahmadi, M.; Noudehi, M.; Esmaeili, M.; Sadrollahi, A. Comparing the Quality of Life Between Active and Non-Active Elderly Women with an Emphasis on Physical Activity. Salmand 2017, 12, 262–275. [Google Scholar] [CrossRef]
- Chorba, R.S.; Chorba, D.J.; Bouillon, L.E.; Overmyer, C.A.; Landis, J.A. Use of a functional movement screening tool to determine injury risk in female collegiate athletes. N. Am. J. Sports Phys. Ther. NAJSPT 2010, 5, 47–54. [Google Scholar] [PubMed]
- Kiesel, K.; Plisky, P.J.; Voight, M.L. Can Serious Injury in Professional Football be Predicted by a Preseason Functional Move-ment Screen? N. Am. J. Sports Phys. Ther. NAJSPT 2007, 2, 147–158. [Google Scholar]
- Coleman, E.A.; Coon, S.; Hall-Barrow, J.; Richards, K.; Gaylor, D.; Stewart, B. Feasibility of exercise during treatment for multiple myeloma. Cancer Nurs. 2003, 26, 410–419. [Google Scholar] [CrossRef]
- Hillengass, M.; Joseph, J.; McCarthy, J.; Hillengass, J. Physical Activity in Multiple Myeloma: A Review of the Current Literature. J. Adv. Pract. Oncol. 2023, 14, 153–158. [Google Scholar] [CrossRef]

| Season 1 | |||||||
|---|---|---|---|---|---|---|---|
| Exercise | S | Rep | W | I | T | R | |
| Part 1 | Air squats | 3 | 8 | 4/10 | - | 30″ | |
| Band pull apart + Y with theraband | 3 | 8 + 8 | 5/10 | - | 30″ | ||
| Trunk rotation in sitting position | 3 | 10 | 3/10 | - | 30″ | ||
| Part 2 | Balance | 3 | 3× (10 m forward and retun) | 4/10 | - | 30″ | |
| Thoracic extensions | 3 | 8 | 5/10 | - | 30″ | ||
| Front plank | 3 | 20 secs | 7/10 | - | 30″ | ||
| Part 3 | Education | 1 | - | ||||
| Outcome | Initial Value | After 12 Weeks | Difference (%) |
|---|---|---|---|
| Blood Pressure (Systolic/Diastolic) | 132/89 | 120/79 | 9% |
| Weight | 47 kg | 50.6 kg | 7.6% |
| Strength (Right Hand) | 17.9 Kg/cm2 | 19.6 Kg/cm2 | 21.83% |
| Strength (Left Hand) | 22.9 Kg/cm2 | 24 Kg/cm2 | 22.44% |
| STST (Sit to Stand Test) | 7 squats | 17 squats | 58.82% |
| Push-Up Test | 3 repetitions | 8 repetitions | 62.5% |
| Quadriceps (Circumference) | 38 cm | 39 cm | 2.6% |
| Triceps (Circumference) | 19 cm | 19.30 cm | 8.5% |
| Triceps suralis (Circumference) | 30.05 | 31.5 | 2.6% |
| 6MWT (Distance) | 0.3 km | 0.45 km | 50% |
| PIPER | 150 (68.18%) | 80 (36.36%) | 31.82% |
| Berg (Balance Scale) | 40 | 52 | 21% |
| SF-36 (Quality of Life Scale) | 40 | 65 | 25% |
| FMS | 12 (57.14%) | 16 (76.19%) | 19.05% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Sigüenza, J.C.; Blanco-Gimenez, P.; Baraja-Vegas, L.; López-Soler, J.; Falaguera-Vera, F.J.; Jaenada-Carrilero, E.; Vicente-Mampel, J. Effects of a Concurrent Mixed-Modality (Telerehabilitation and Face-to-Face) Exercise Rehabilitation Program in a Patient with Multiple Myeloma Prior to Spinal Cord Transplantation: A Case Study. Curr. Oncol. 2025, 32, 282. https://doi.org/10.3390/curroncol32050282
Hernández-Sigüenza JC, Blanco-Gimenez P, Baraja-Vegas L, López-Soler J, Falaguera-Vera FJ, Jaenada-Carrilero E, Vicente-Mampel J. Effects of a Concurrent Mixed-Modality (Telerehabilitation and Face-to-Face) Exercise Rehabilitation Program in a Patient with Multiple Myeloma Prior to Spinal Cord Transplantation: A Case Study. Current Oncology. 2025; 32(5):282. https://doi.org/10.3390/curroncol32050282
Chicago/Turabian StyleHernández-Sigüenza, Juan Carlos, Paula Blanco-Gimenez, Luis Baraja-Vegas, Josep López-Soler, Francisco Javier Falaguera-Vera, Eloy Jaenada-Carrilero, and Juan Vicente-Mampel. 2025. "Effects of a Concurrent Mixed-Modality (Telerehabilitation and Face-to-Face) Exercise Rehabilitation Program in a Patient with Multiple Myeloma Prior to Spinal Cord Transplantation: A Case Study" Current Oncology 32, no. 5: 282. https://doi.org/10.3390/curroncol32050282
APA StyleHernández-Sigüenza, J. C., Blanco-Gimenez, P., Baraja-Vegas, L., López-Soler, J., Falaguera-Vera, F. J., Jaenada-Carrilero, E., & Vicente-Mampel, J. (2025). Effects of a Concurrent Mixed-Modality (Telerehabilitation and Face-to-Face) Exercise Rehabilitation Program in a Patient with Multiple Myeloma Prior to Spinal Cord Transplantation: A Case Study. Current Oncology, 32(5), 282. https://doi.org/10.3390/curroncol32050282





