ADCs and TCE in SCLC Therapy: The Beginning of a New Era?
Abstract
1. Introduction
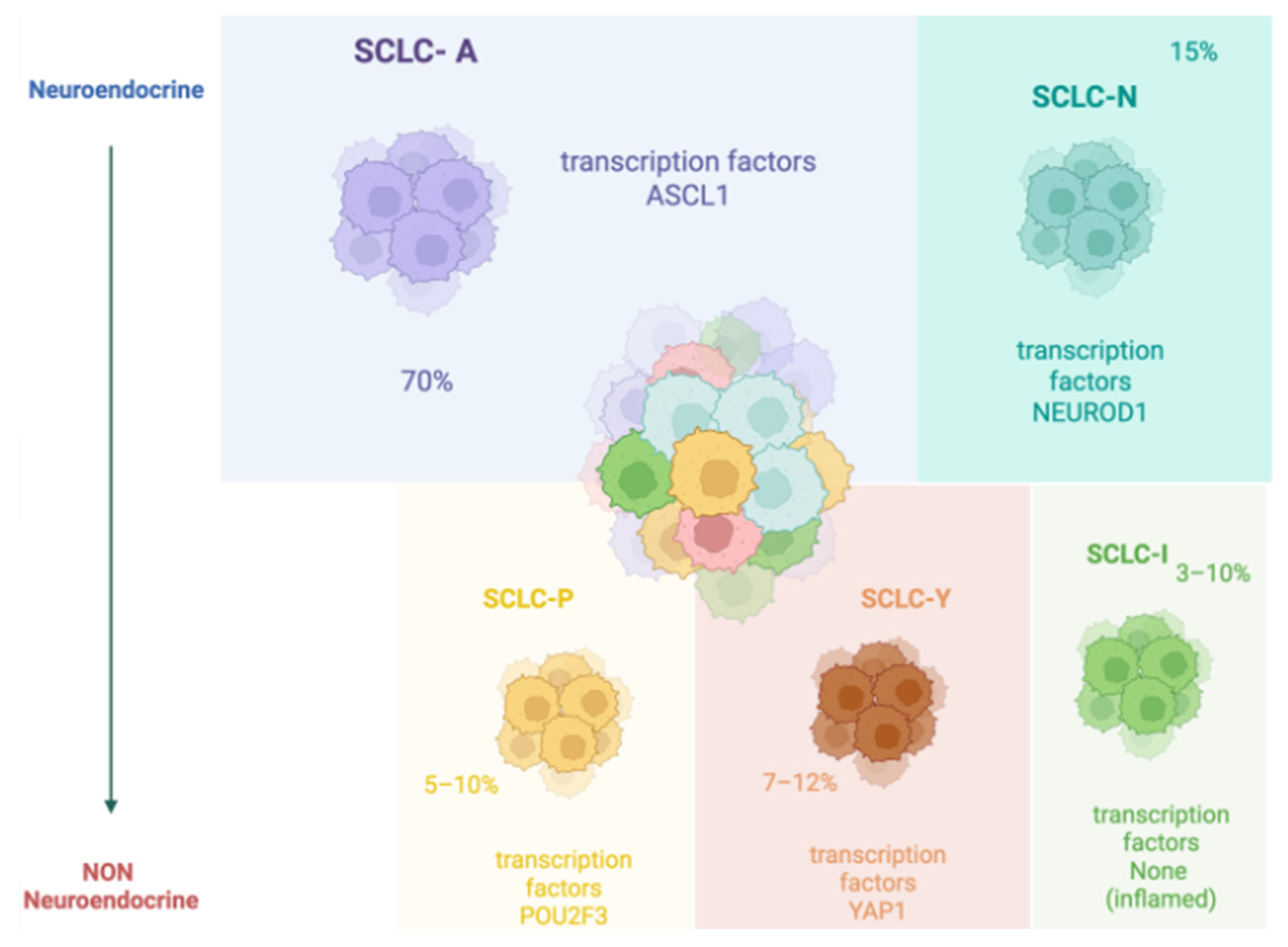
1.1. SCLC Epidemiology and Molecular Classification
1.2. Standard of Care
2. T-Cell Engagers and Antibody-Drug Conjugates: Structure and Function
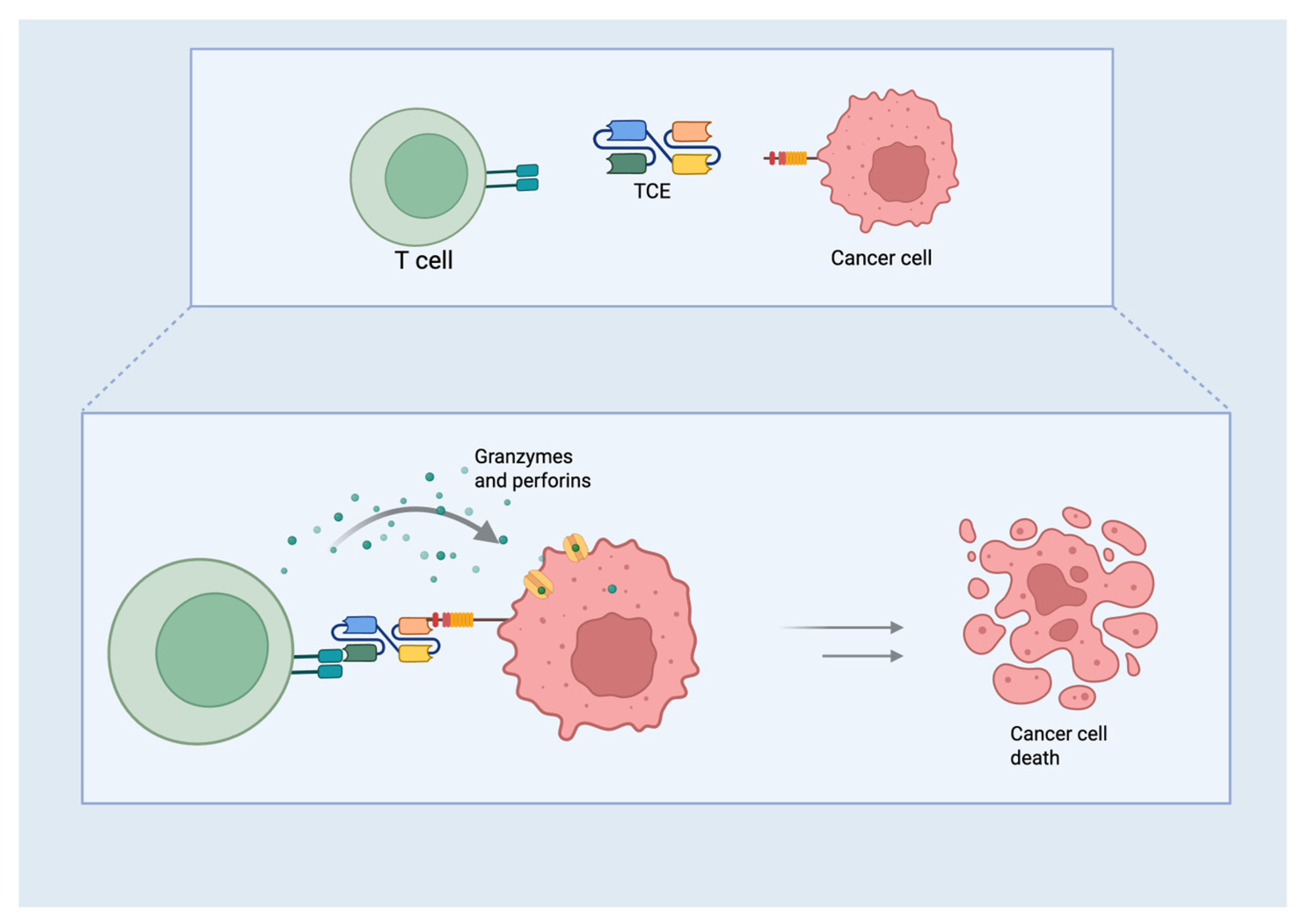
2.1. TCE
2.2. ADCs
2.3. ADCs and TCEs in SCLC
2.3.1. Delta-Like Ligand 3 (DLL3)
DeLLphi-300
Dellphi-301
Other Trials
| Trial Name | Phase Trial | Setting | Drug | n | Primary Endpoint |
|---|---|---|---|---|---|
| DeLLphi-300 | Phase I | Second line—ES | Tb | 269 | Safety [39] |
| DeLLphi-301 | Phase II | Third or next line—ES | Tb | 222 | Safety, ORR [42] |
| DeLLphi-302 | Phase Ib | Second line | Tb + IO | 23 | Safety [46] |
| DeLLphi-303 | Phase Ib | First-line- ES | Tb + ChT + IO or Tb + IO | 269 | Safety [47] |
| DeLLphi-304 | Phase III | Second line—ES | Tb | 509 | OS [43] |
| DeLLphi-305 | Phase III | Maintenance—ES | Tb + IO | OS [48] | |
| DeLLphi-306 | Phase III | Maintenance—LS | Tb | 400 | PFS [49] |
| DeLLphi-307 | Phase IIa | Third or next line—ES (Asiatic) | Tb | 32 | ORR [50] |
| DeLLphi-308 | Phase Ib | ES | SC Tb | 100 | Safety [51] |
| DeLLphi-309 | Phase II | Second line | Tb | 240 | ORR [52] |
2.3.2. Trophoblast Surface Antigen 2 (TROP-2)
TROPiCS-03
Other Trials
2.3.3. B7 Homolog 3 Protein (B7-H3)
IDeate-Lung01, IDeate-Lung02 and IDeate-Lung03
Other Trials
2.3.4. Seizure-Related Homolog Protein 6 (SEZ 6)
Seizure-Related Homolog Protein 6 Is a New Target Expressed in SCLC
3. TCEs vs. ADCs: Therapeutic Scenarios and Challenges
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Saida, Y.; Watanabe, S.; Kikuchi, T. Extensive-Stage Small-Cell Lung Cancer: Current Landscape and Future Prospects. Onco Targets Ther. 2023, 16, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Meijer, J.-J.; Leonetti, A.; Airò, G.; Tiseo, M.; Rolfo, C.; Giovannetti, E.; Vahabi, M. Small Cell Lung Cancer: Novel Treatments beyond Immunotherapy. Semin. Cancer Biol. 2022, 86, 376–385. [Google Scholar] [CrossRef]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-Cell Lung Cancer. Nat. Rev. Dis. Primers 2021, 7, 3. [Google Scholar] [CrossRef]
- Péchoux, C.L.; Sun, A.; Slotman, B.J.; De Ruysscher, D.; Belderbos, J.; Gore, E.M. Prophylactic Cranial Irradiation for Patients with Lung Cancer. Lancet Oncol. 2016, 17, e277–e293. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretić, L.; Kong, G.; Leenders, F.; Lu, X.; Fernández-Cuesta, L.; Bosco, G.; et al. Comprehensive Genomic Profiles of Small Cell Lung Cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef]
- Rudin, C.M.; Poirier, J.T.; Byers, L.A.; Dive, C.; Dowlati, A.; George, J.; Heymach, J.V.; Johnson, J.E.; Lehman, J.M.; MacPherson, D.; et al. Author Correction: Molecular Subtypes of Small Cell Lung Cancer: A Synthesis of Human and Mouse Model Data. Nat. Rev. Cancer 2019, 19, 415. [Google Scholar] [CrossRef]
- Sen, T.; Takahashi, N.; Chakraborty, S.; Takebe, N.; Nassar, A.H.; Karim, N.A.; Puri, S.; Naqash, A.R. Emerging Advances in Defining the Molecular and Therapeutic Landscape of Small-Cell Lung Cancer. Nat. Rev. Clin. Oncol. 2024, 21, 610–627. [Google Scholar] [CrossRef]
- Ganti, A.K.P.; Loo, B.W.; Bassetti, M.; Blakely, C.; Chiang, A.; D’Amico, T.A.; Christopher D’Avella, C.; Dowlati, A.; Downey, R.J.; Hughes, M.; et al. Small Cell Lung Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1441–1464. [Google Scholar] [CrossRef]
- Evans, W.K.; Shepherd, F.A.; Feld, R.; Osoba, D.; Dang, P.; Deboer, G. VP-16 and Cisplatin as First-Line Therapy for Small-Cell Lung Cancer. J. Clin. Oncol. 1985, 3, 1471–1477. [Google Scholar] [CrossRef]
- Schneider, B.J. Management of Recurrent Small Cell Lung Cancer. J. Natl. Compr. Canc Netw. 2008, 6, 323–331. [Google Scholar] [CrossRef]
- Speranza, D.; Santarpia, M.; Luppino, F.; Omero, F.; Maiorana, E.; Cavaleri, M.; Sapuppo, E.; Cianci, V.; Pugliese, A.; Racanelli, V.; et al. Immune Checkpoint Inhibitors and Neurotoxicity: A Focus on Diagnosis and Management for a Multidisciplinary Approach. Expert. Opin. Drug Saf. 2024, 23, 1405–1418. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]
- Reck, M.; Dziadziuszko, R.; Sugawara, S.; Kao, S.; Hochmair, M.; Huemer, F.; de Castro, G.J.; Havel, L.; Bernabé Caro, R.; Losonczy, G.; et al. Five-Year Survival in Patients with Extensive-Stage Small Cell Lung Cancer Treated with Atezolizumab in the Phase III IMpower133 Study and the Phase III IMbrella A Extension Study. Lung Cancer 2024, 196, 107924. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus Platinum-Etoposide versus Platinum-Etoposide in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer (CASPIAN): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; Garassino, M.C.; et al. Durvalumab, with or without Tremelimumab, plus Platinum-Etoposide in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer: 3-Year Overall Survival Update from CASPIAN. ESMO Open 2022, 7, 100408. [Google Scholar] [CrossRef]
- Rolfo, C.; Russo, A. In Search of Lost Biomarker for Immunotherapy in Small Cell Lung Cancer. Clin. Cancer Res. 2024, 30, 652–654. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Garassino, M.C.; Chen, Y.; Reinmuth, N.; Hotta, K.; Poltoratskiy, A.; Trukhin, D.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab ± Tremelimumab + Platinum-Etoposide in Extensive-Stage Small-Cell Lung Cancer (CASPIAN): Outcomes by PD-L1 Expression and Tissue Tumor Mutational Burden. Clin. Cancer Res. 2023, 30, 824–835. [Google Scholar] [CrossRef]
- von Pawel, J.; Schiller, J.H.; Shepherd, F.A.; Fields, S.Z.; Kleisbauer, J.P.; Chrysson, N.G.; Stewart, D.J.; Clark, P.I.; Palmer, M.C.; Depierre, A.; et al. Topotecan versus Cyclophosphamide, Doxorubicin, and Vincristine for the Treatment of Recurrent Small-Cell Lung Cancer. J. Clin. Oncol. 1999, 17, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Trigo, J.; Subbiah, V.; Besse, B.; Moreno, V.; López, R.; Sala, M.A.; Peters, S.; Ponce, S.; Fernández, C.; Alfaro, V.; et al. Lurbinectedin as Second-Line Treatment for Patients with Small-Cell Lung Cancer: A Single-Arm, Open-Label, Phase 2 Basket Trial. Lancet Oncol. 2020, 21, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, B.; Shibamoto, Y.; Nikolic, N.; Milicic, B.; Milisavljevic, S.; Dagovic, A.; Aleksandrovic, J.; Radosavljevic-Asic, G. Role of Radiation Therapy in the Combined-Modality Treatment of Patients with Extensive Disease Small-Cell Lung Cancer: A Randomized Study. J. Clin. Oncol. 1999, 17, 2092–2099. [Google Scholar] [CrossRef] [PubMed]
- Slotman, B.J.; van Tinteren, H.; Praag, J.O.; Knegjens, J.L.; El Sharouni, S.Y.; Hatton, M.; Keijser, A.; Faivre-Finn, C.; Senan, S. Use of Thoracic Radiotherapy for Extensive Stage Small-Cell Lung Cancer: A Phase 3 Randomised Controlled Trial. Lancet 2015, 385, 36–42. [Google Scholar] [CrossRef]
- Longo, V.; Della Corte, C.M.; Russo, A.; Spinnato, F.; Ambrosio, F.; Ronga, R.; Marchese, A.; Del Giudice, T.; Sergi, C.; Casaluce, F.; et al. Consolidative Thoracic Radiation Therapy for Extensive-Stage Small Cell Lung Cancer in the Era of First-Line Chemoimmunotherapy: Preclinical Data and a Retrospective Study in Southern Italy. Front. Immunol. 2023, 14, 1289434. [Google Scholar] [CrossRef] [PubMed]
- Aupérin, A.; Arriagada, R.; Pignon, J.P.; Le Péchoux, C.; Gregor, A.; Stephens, R.J.; Kristjansen, P.E.; Johnson, B.E.; Ueoka, H.; Wagner, H.; et al. Prophylactic Cranial Irradiation for Patients with Small-Cell Lung Cancer in Complete Remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N. Engl. J. Med. 1999, 341, 476–484. [Google Scholar] [CrossRef]
- Wolfson, A.H.; Bae, K.; Komaki, R.; Meyers, C.; Movsas, B.; Le Pechoux, C.; Werner-Wasik, M.; Videtic, G.M.M.; Garces, Y.I.; Choy, H. Primary Analysis of a Phase II Randomized Trial Radiation Therapy Oncology Group (RTOG) 0212: Impact of Different Total Doses and Schedules of Prophylactic Cranial Irradiation on Chronic Neurotoxicity and Quality of Life for Patients with Limited-Disease Small-Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 77–84. [Google Scholar] [CrossRef]
- Ordóñez-Reyes, C.; Garcia-Robledo, J.E.; Chamorro, D.F.; Mosquera, A.; Sussmann, L.; Ruiz-Patiño, A.; Arrieta, O.; Zatarain-Barrón, L.; Rojas, L.; Russo, A.; et al. Bispecific Antibodies in Cancer Immunotherapy: A Novel Response to an Old Question. Pharmaceutics 2022, 14, 1243. [Google Scholar] [CrossRef]
- Huang, Q.; Cai, W.-Q.; Han, Z.-W.; Wang, M.-Y.; Zhou, Y.; Cheng, J.-T.; Zhang, Y.; Wang, Y.-Y.; Xin, Q.; Wang, X.-W.; et al. Bispecific T Cell Engagers and Their Synergistic Tumor Immunotherapy with Oncolytic Viruses. Am. J. Cancer Res. 2021, 11, 2430–2455. [Google Scholar]
- Zhou, S.; Liu, M.; Ren, F.; Meng, X.; Yu, J. The Landscape of Bispecific T Cell Engager in Cancer Treatment. Biomark. Res. 2021, 9, 38. [Google Scholar] [CrossRef]
- Hipp, S.; Voynov, V.; Drobits-Handl, B.; Giragossian, C.; Trapani, F.; Nixon, A.E.; Scheer, J.M.; Adam, P.J. A Bispecific DLL3/CD3 IgG-Like T-Cell Engaging Antibody Induces Antitumor Responses in Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 5258–5268. [Google Scholar] [CrossRef] [PubMed]
- Drago, J.Z.; Modi, S.; Chandarlapaty, S. Unlocking the Potential of Antibody-Drug Conjugates for Cancer Therapy. Nat. Rev. Clin. Oncol. 2021, 18, 327–344. [Google Scholar] [CrossRef]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and Challenges for the next Generation of Antibody-Drug Conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Chari, R.V.J.; Miller, M.L.; Widdison, W.C. Antibody-Drug Conjugates: An Emerging Concept in Cancer Therapy. Angew. Chem. Int. Ed. Engl. 2014, 53, 3796–3827. [Google Scholar] [CrossRef]
- McCombs, J.R.; Owen, S.C. Antibody Drug Conjugates: Design and Selection of Linker, Payload and Conjugation Chemistry. AAPS J. 2015, 17, 339–351. [Google Scholar] [CrossRef]
- Redman, J.M.; Hill, E.M.; AlDeghaither, D.; Weiner, L.M. Mechanisms of Action of Therapeutic Antibodies for Cancer. Mol. Immunol. 2015, 67, 28–45. [Google Scholar] [CrossRef]
- Thomas, A.; Teicher, B.A.; Hassan, R. Antibody-Drug Conjugates for Cancer Therapy. Lancet Oncol. 2016, 17, e254–e262. [Google Scholar] [CrossRef]
- Gay, C.M.; Owonikoko, T.K.; Byers, L.A.; Choudhury, N.J.; Ahmed, S.; Cain, Z.; Qian, X.; Brentnall, M.; Heeke, S.; Poi, M.; et al. Multidimensional Analysis of B7 Homolog 3 (B7-H3) RNA Expression in Small-Cell Lung Cancer (SCLC) Molecular Subtypes. JCO 2024, 42, 8088. [Google Scholar] [CrossRef]
- Alamgir, I.; Alamgir, U.; Alamgir, E.; Qureshi, A.A.; Siddiqui, M.O.; Jaber, M.H.; Motwani, J. Tarlatamab’s FDA Approval: Shaping the Future of Cancer Therapy. Ann. Med. Surg. 2024, 86, 5676–5679. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, J.; Ma, J.; Kang, R.; Liu, Z.; Yu, J. Advances in New Targets for Immunotherapy of Small Cell Lung Cancer. Thorac. Cancer 2024, 15, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Champiat, S.; Lai, W.V.; Izumi, H.; Govindan, R.; Boyer, M.; Hummel, H.-D.; Borghaei, H.; Johnson, M.L.; Steeghs, N.; et al. Tarlatamab, a First-in-Class DLL3-Targeted Bispecific T-Cell Engager, in Recurrent Small-Cell Lung Cancer: An Open-Label, Phase I Study. J. Clin. Oncol. 2023, 41, 2893–2903. [Google Scholar] [CrossRef]
- Dowlati, A.; Hummel, H.-D.; Champiat, S.; Olmedo, M.E.; Boyer, M.; He, K.; Steeghs, N.; Izumi, H.; Johnson, M.L.; Yoshida, T.; et al. Sustained Clinical Benefit and Intracranial Activity of Tarlatamab in Previously Treated Small Cell Lung Cancer: DeLLphi-300 Trial Update. J. Clin. Oncol. 2024, 42, 3392–3399. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.-J.; Cho, B.C.; Felip, E.; Korantzis, I.; Ohashi, K.; Majem, M.; Juan-Vidal, O.; Handzhiev, S.; Izumi, H.; Lee, J.-S.; et al. Tarlatamab for Patients with Previously Treated Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 2063–2075. [Google Scholar] [CrossRef]
- Dingemans, A.-M.C.; Ahn, M.-J.; Blackhall, F.H.; Reck, M.; Hummel, H.-D.; Ramalingam, S.S.; Johnson, M.L.; Akamatsu, H.; Wolf, J.; Sands, J.; et al. DeLLphi-301: Tarlatamab Phase 2 Trial in Small Cell Lung Cancer (SCLC)—Efficacy and Safety Analyzed by Presence of Brain Metastasis. JCO 2024, 42, 8015. [Google Scholar] [CrossRef]
- AMGEN. Available online: https://investors.amgen.com/news-releases/news-release-details/imdelltrar-demonstrated-superior-overall-survival-small-cell/ (accessed on 13 April 2025).
- Available online: https://oncologypro.esmo.org/meeting-resources/esmo-congress-2024/phase-i-trial-of-the-delta-like-ligand-3-dll3-cd3-igg-like-t-cell-engager-bi-764532-in-patients-pts-with-dll3-positive-tumors-updated-data (accessed on 19 April 2025).
- Choudhur, N.J.; Beltran, H.; Johnso, M.L.; Schenk, E.L.; Sanborn, R.E.; Thompson, J.R.; Mamdani, H.; Dowlati, A.; Aggarwal, R.R.; Gramza, A.W.; et al. Impact of Brain Metastases on Safety and Efficacy of MK-6070, a DLL3-Targeting T Cell Engager, in Small Cell Lung Cancer. J. Thorac. Oncol. 2024, 19, 32–33. Available online: https://www.jto.org/article/S1556-0864(24)00934-1/abstract (accessed on 19 April 2025). [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04885998 (accessed on 13 April 2025).
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT05361395 (accessed on 13 April 2025).
- Perol, M.; Ahn, M.J.; Cheng, Y.; Clarke, J.; Dingemans, A.M.; Gay, C.; Navarro, A.; Schuler, M.; Yoshida, T.; Martinez, P.; et al. Tarlatamab Plus Durvalumab as First-Line Maintenance in Extensive-Stage Small Cell Lung Cancer: DeLLphi-305 Phase 3 Trial. J. Thorac. Oncol. 2024, 19, 206–207. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06117774 (accessed on 13 April 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06502977 (accessed on 13 April 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06598306 (accessed on 13 April 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06745323 (accessed on 13 April 2025).
- Dowlati, A.; Chiang, A.C.; Cervantes, A.; Babu, S.; Hamilton, E.; Wong, S.F.; Tazbirkova, A.; Sullivan, I.G.; van Marcke, C.; Italiano, A.; et al. Phase 2 Open-Label Study of Sacituzumab Govitecan as Second-Line Therapy in Patients with Extensive-Stage SCLC: Results From TROPiCS-03. J. Thorac. Oncol. 2025, S1556-0864(24)02549-8. [Google Scholar] [CrossRef]
- Wang, J.; Wu, L.; Li, X.; Xing, N.; Zhang, S.; Song, Z.; Chen, L.; Dang, Q.; Liu, C.; Li, Y.; et al. OA04.05 SHR-A1921, A TROP-2 Targeted Antibody-Drug Conjugate (ADC), In Patients (Pts) with Advanced Small-Cell Lung Cancer (SCLC). J. Thorac. Oncol. 2024, 19, S16–S17. [Google Scholar] [CrossRef]
- Malapelle, U.; Parente, P.; Pepe, F.; Di Micco, M.C.; Russo, A.; Clemente, C.; Graziano, P.; Rossi, A. B7-H3/CD276 Inhibitors: Is There Room for the Treatment of Metastatic Non-Small Cell Lung Cancer? Int. J. Mol. Sci. 2022, 23, 16077. [Google Scholar] [CrossRef]
- Belluomini, L.; Sposito, M.; Avancini, A.; Insolda, J.; Milella, M.; Rossi, A.; Pilotto, S. Unlocking New Horizons in Small-Cell Lung Cancer Treatment: The Onset of Antibody-Drug Conjugates. Cancers 2023, 15, 5368. [Google Scholar] [CrossRef]
- Rudin, C.M.; Ahn, M.-J.; Johnson, M.; Hann, C.L.; Girard, N.; Nishio, M.; Cheng, Y.; Hayashi, H.; Jung Kim, Y.; Navarro, A.; et al. OA04.03 Ifinatamab Deruxtecan (I-DXd) in Extensive-Stage Small Cell Lung Cancer (ES-SCLC): Interim Analysis of Ideate-Lung01. J. Thorac. Oncol. 2024, 19, S15–S16. [Google Scholar] [CrossRef]
- Wang, J.; Duan, J.; Wu, L.; Wang, Q.; Xing, L.; Sun, Y.; Lu, P.; Ning, F.; Yang, H.; Su, H.; et al. OA04.06 Efficacy and Safety of HS-20093 in Extensive Stage Small Cell Lung Cancer in A Multicenter, Phase 1 Study (ARTEMIS-001). J. Thorac. Oncol. 2024, 19, S17. [Google Scholar] [CrossRef]
- Morgensztern, D.; Ready, N.; Johnson, M.L.; Dowlati, A.; Choudhury, N.; Carbone, D.P.; Schaefer, E.; Arnold, S.M.; Puri, S.; Piotrowska, Z.; et al. A Phase I First-in-Human Study of ABBV-011, a Seizure-Related Homolog Protein 6-Targeting Antibody-Drug Conjugate, in Patients with Small Cell Lung Cancer. Clin. Cancer Res. 2024, 30, 5042–5052. [Google Scholar] [CrossRef]

| Drug | Target | Trial Name | Setting | n | ORR (%) | mPFS (mos) | mOS (mos) | AEs G3-G5 | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| I-DXd | B7-H3 | Phase 2, Ideate-Lung01 | ES-SCLC | 88 | −12 mg/kg, 54.8% (95% CI, 38.7–70.2) −8 mg/kg, 26.1% (95% CI, 14.3–41.1) | −8 mg/kg, 4.2 mos (2.8–5.6) −12 mg/kg, 5.5 m (4.2–6.7) | −8 mg/kg, 9.4 mos (7.8–15.9) −12 mg/kg, 11.8 mos (8.9–15.3) | −43.5% (8 mg/kg) −50% (12 mg/kg) | [57] |
| HS-20093 | B7-H3 | Phase 1, ARTEMIS-001 | ES-SCLC | 56 | −8.0 mg/kg, 61.3% (CI 95%, 42.2–78.2) −10.0 mg/kg 50.0% (CI 95%, 28.2–71.8) | −8 mg/kg, 5.9 mos −10.0 mg/kg, 7.3 | −8 mg/kg, 9.8 mos −10.0 mg/kg, NR | N/A | [58] |
| SACITUZUMAB-GOVITECAN | TROP 2 | TROPICS-03 | ES-SCLC | 43 | 41.9% (95% CI, 27.0–57.9) | 4.40 mos (95% CI, 3.81–6.11) | 12.2 mos | 51.2% | [53] |
| SHR-A1921 | TROP-2 | Wang J et al., 2024 | ES-SCLC | 17 | 33.3% (95% CI 15.2-58.3) | 3.8 mos (95% CI 1.4-NR) | 35.3% | [54] | |
| ABBV-011 | SEZ6 | (NCT03639194) | ES-SCLC | dose escalation, n 36; dose expansion, n 60 | 1-mg/kg dose-expansion cohort (n 40) 25% | 3.5 mos (95% CI, 1.5–4.2) | N/A | 34% | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muscolino, P.; Omero, F.; Speranza, D.; Infurna, C.; Parisi, S.; Cianci, V.; Berretta, M.; Russo, A.; Santarpia, M. ADCs and TCE in SCLC Therapy: The Beginning of a New Era? Curr. Oncol. 2025, 32, 261. https://doi.org/10.3390/curroncol32050261
Muscolino P, Omero F, Speranza D, Infurna C, Parisi S, Cianci V, Berretta M, Russo A, Santarpia M. ADCs and TCE in SCLC Therapy: The Beginning of a New Era? Current Oncology. 2025; 32(5):261. https://doi.org/10.3390/curroncol32050261
Chicago/Turabian StyleMuscolino, Paola, Fausto Omero, Desirèe Speranza, Carla Infurna, Silvana Parisi, Vincenzo Cianci, Massimiliano Berretta, Alessandro Russo, and Mariacarmela Santarpia. 2025. "ADCs and TCE in SCLC Therapy: The Beginning of a New Era?" Current Oncology 32, no. 5: 261. https://doi.org/10.3390/curroncol32050261
APA StyleMuscolino, P., Omero, F., Speranza, D., Infurna, C., Parisi, S., Cianci, V., Berretta, M., Russo, A., & Santarpia, M. (2025). ADCs and TCE in SCLC Therapy: The Beginning of a New Era? Current Oncology, 32(5), 261. https://doi.org/10.3390/curroncol32050261






