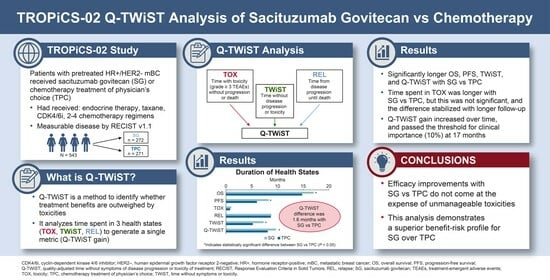
Q-TWiST Analysis of Sacituzumab Govitecan vs. Chemotherapy in Previously Treated Patients with HR+/HER2− Metastatic Breast Cancer †
Abstract
1. Introduction
2. Methods
2.1. TROPiCS-02
2.2. Health States
2.3. Q-TWiST Calculation
2.4. Sensitivity Analyses
3. Results
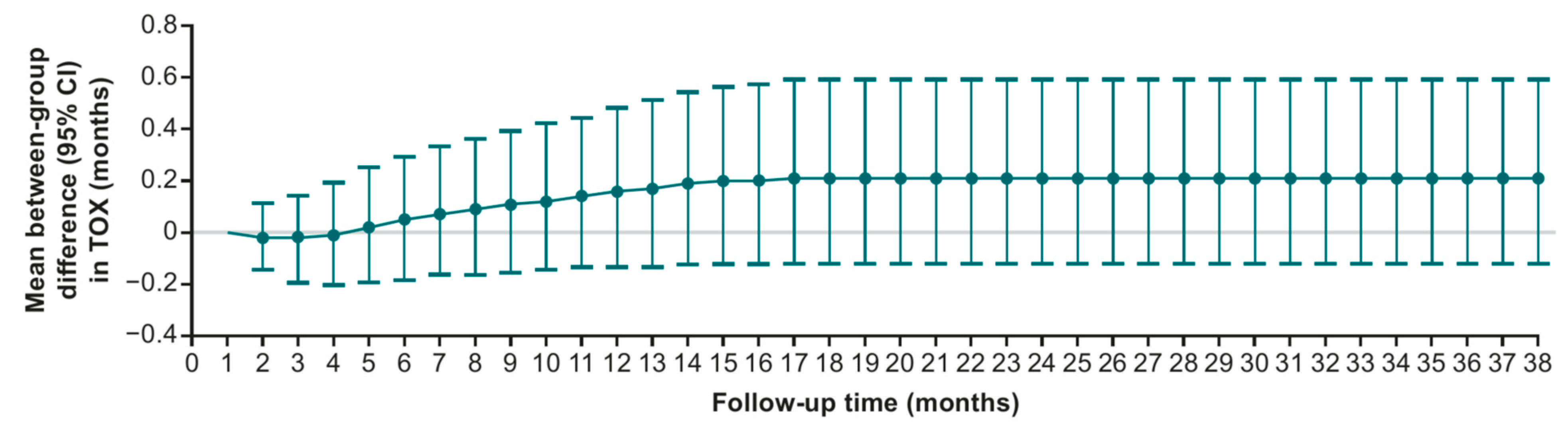
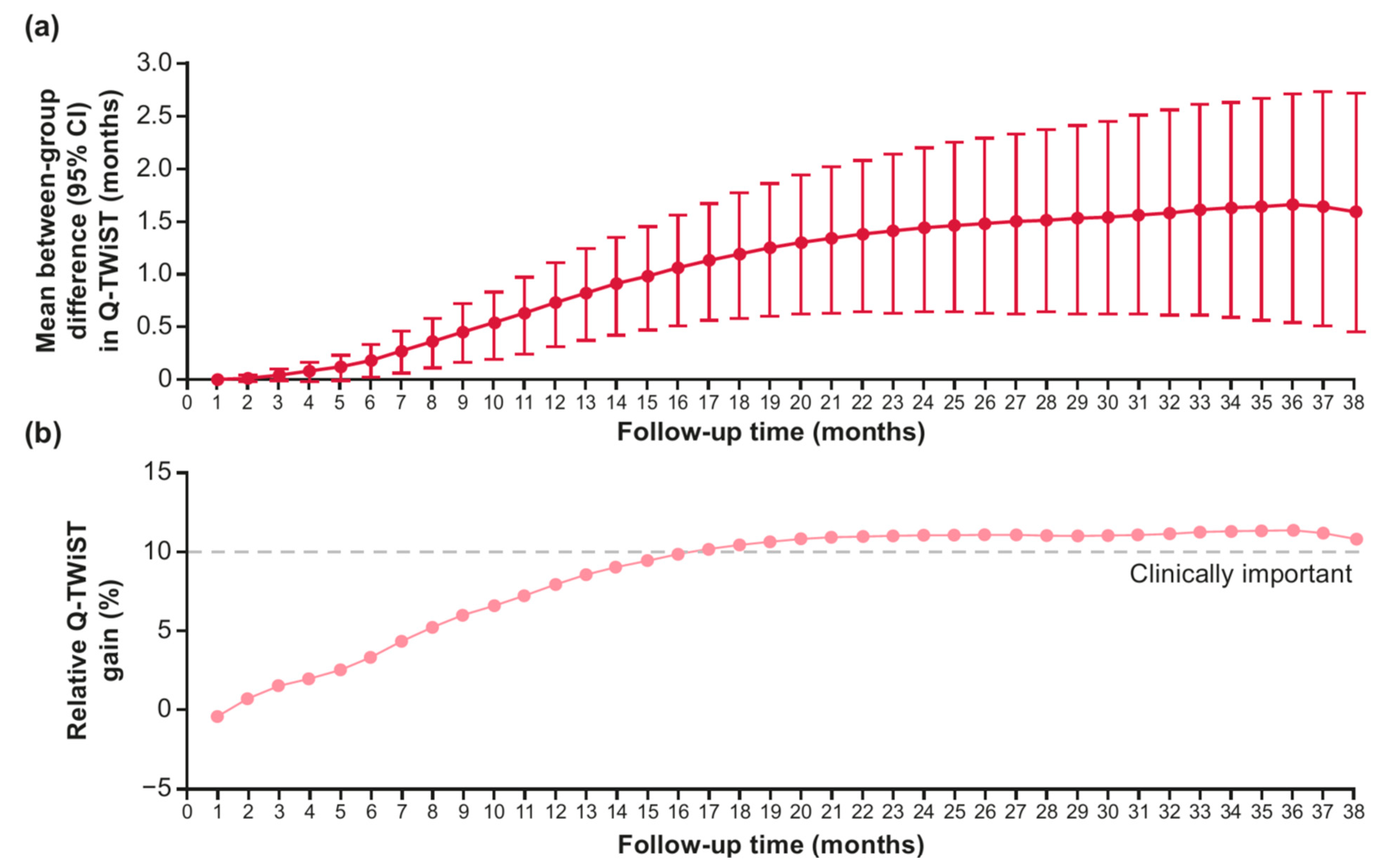
3.1. Sensitivity Analysis: Variation in Follow-Up
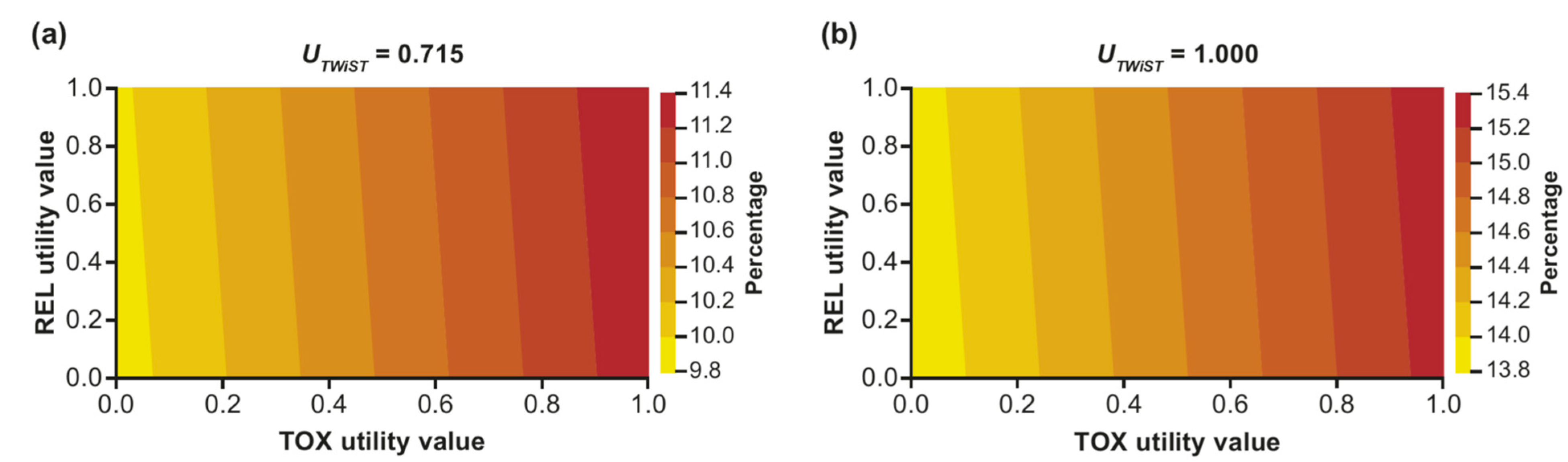
3.2. Sensitivity Analysis: Two-Way Utility Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer. World Health Organization. Global Cancer Observatory. Breast; International Agency for Research on Cancer: Lyon, France, 2022; Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/20-breast-fact-sheet.pdf (accessed on 17 September 2024).
- American Cancer Society. Female Breast Cancer Subtypes. Available online: https://seer.cancer.gov/statfacts/html/breast-subtypes.html (accessed on 17 September 2024).
- Hammond, M.E.H.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline focused update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Cardillo, T.M.; Govindan, S.V.; Rossi, E.A.; Sharkey, R.M. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 2015, 6, 22496–22512. [Google Scholar] [CrossRef]
- TRODELVY® (sacituzumab govitecan-hziy); Gilead Sciences, Inc.: Foster City, CA, USA, 2023.
- TRODELVY® (sacituzumab govitecan-hziy). Summary of Product Characteristics; Gilead Sciences Ireland UC: Dublin, Ireland, 2023.
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortes, J.; Schmid, P.; Loirat, D.; Trédan, O.; Ciruelos, E.; Dalenc, F.; Pardo, P.G.; et al. Sacituzumab govitecan in hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. 2022, 40, 3365–3376. [Google Scholar] [CrossRef]
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortés, J.; Schmid, P.; Loirat, D.; Trédan, O.; Ciruelos, E.; Dalenc, F.; Pardo, P.G.; et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 402, 1423–1433. [Google Scholar] [CrossRef]
- Rugo, H.S.; Schmid, P.; Tolaney, S.M.; Dalenc, F.; Marmé, F.; Shi, L.; Verret, W.; Shah, A.; Gharaibeh, M.; Bardia, A.; et al. Health-related quality of life with sacituzumab govitecan in HR+/HER2- metastatic breast cancer in the phase III TROPiCS-02 trial. Oncologist 2024, 29, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Clarijs, M.E.; Thurell, J.; Kühn, F.; Groot, C.A.U.-D.; Hedayati, E.; Karsten, M.M.; Jager, A.; Koppert, L.B. Measuring quality of life using patient-reported outcomes in real-world metastatic breast cancer patients: The need for a standardized approach. Cancers 2021, 13, 2308. [Google Scholar] [CrossRef]
- Michael, Y.L.; Kawachi, I.; Berkman, L.F.; Holmes, M.D.; Colditz, G.A. The persistent impact of breast carcinoma on functional health status: Prospective evidence from the Nurses’ Health Study. Cancer 2000, 89, 2176–2186. [Google Scholar] [CrossRef] [PubMed]
- Glasziou, P.P.; Simes, R.J.; Gelber, R.D. Quality adjusted survival analysis. Stat. Med. 1990, 9, 1259–1276. [Google Scholar] [CrossRef]
- Revicki, D.A.; Feeny, D.; Hunt, T.L.; Cole, B.F. Analyzing oncology clinical trial data using the Q-TWiST method: Clinical importance and sources for health state preference data. Qual. Life Res. 2006, 15, 411–423. [Google Scholar] [CrossRef]
- Gelber, R.D.; Cole, B.F.; Goldhirsch, A.; Bonadonna, G.; Howell, A.; McArdle, C.S.; Mouridsen, H.T.; Rubens, R.D.; Welvaart, K. Adjuvant chemotherapy for premenopausal breast cancer: A meta-analysis using quality-adjusted survival. Cancer J. Sci. Am. 1995, 1, 114–121. [Google Scholar] [PubMed]
- Gelber, R.; Cole, B.; Goldhirsch, A.; Rose, C.; Fisher, B.; Osborne, C.; Boccardo, F.; Gray, R.; Gordon, N.; Bengtsson, N.-O.; et al. Adjuvant chemotherapy plus tamoxifen compared with tamoxifen alone for postmenopausal breast cancer: Meta-analysis of quality-adjusted survival. Lancet 1996, 347, 1066–1071. [Google Scholar] [CrossRef]
- Bardia, A.; Rugo, H.S.; Sedrak, M.S.; Loibl, S.; Tolaney, S.M.; Punie, K.; Hurvitz, S.A.; Kalinsky, K.M.; Cortés, J.; O’Shaughnessy, J.A.; et al. Q-TWiST analysis to assess benefit-risk of sacituzumab govitecan in previously treated patients with metastatic triple-negative breast cancer. JCO Oncol. Pract. 2025, in press. [Google Scholar]
- Huang, M.; O’Shaughnessy, J.; Haiderali, A.; Pan, W.; Hu, P.; Chaudhuri, M.; Tilleghem, C.L.B.D.; Cappoen, N.; Fasching, P.A. Q-TWiST analysis of pembrolizumab combined with chemotherapy as first-line treatment of metastatic triple-negative breast cancer that expresses PD-L1. Eur. J. Cancer 2022, 177, 45–52. [Google Scholar] [CrossRef]
- Sherrill, B.; Amonkar, M.M.; Stein, S.; Walker, M.; Geyer, C.; Cameron, D. Q-TWiST analysis of lapatinib combined with capecitabine for the treatment of metastatic breast cancer. Br. J. Cancer 2008, 99, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Tarhini, A.; Corman, S.; Rao, S.; Margolin, K.; Ji, X.; Mehta, S.; Botteman, M. Healthcare Resource Utilization and Associated Costs in Patients with Advanced Melanoma Receiving First-Line Ipilimumab. J. Cancer Ther. 2015, 6, 833–840. [Google Scholar] [CrossRef]
- McGregor, B.; Geynisman, D.M.; Burotto, M.; Porta, C.; Suarez, C.; Bourlon, M.T.; Del Tejo, V.; Du, E.X.; Yang, X.; Sendhil, S.R.; et al. Grade 3/4 Adverse Event Costs of Immuno-oncology Combination Therapies for Previously Untreated Advanced Renal Cell Carcinoma. Oncologist 2023, 28, 72–79. [Google Scholar] [CrossRef]
- Lloyd, A.; Nafees, B.; Narewska, J.; Dewilde, S.; Watkins, J. Health state utilities for metastatic breast cancer. Br. J. Cancer 2006, 95, 683–690. [Google Scholar] [CrossRef]
- Wolowacz, S.E.; Briggs, A.; Belozeroff, V.; Clarke, P.; Doward, L.; Goeree, R.; Lloyd, A.; Norman, R. Estimating Health-State Utility for Economic Models in Clinical Studies: An ISPOR Good Research Practices Task Force Report. Value Health 2016, 19, 704–719. [Google Scholar] [CrossRef]
- Huang, M.; Pietanza, M.C.; Samkari, A.; Pellissier, J.; Burke, T.; Chandwani, S.; Kong, F.; Pickard, A.S. Q-TWiST Analysis to Assess Benefit–Risk of Pembrolizumab in Patients with PD-L1–Positive Advanced or Metastatic Non-small Cell Lung Cancer. PharmacoEconomics 2019, 37, 105–116. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Solem, C.T.; Kwon, Y.; Shah, R.M.; Aly, A.; Botteman, M.F. Systematic review and benchmarking of Quality-Adjusted Time Without Symptoms or Toxicity (Q-TWiST) in oncology. Expert Rev. Pharmacoeconomics Outcomes Res. 2018, 18, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Brufsky, A.; Bardia, A.; Sedrak, M.; Loibl, S.; Tolaney, S.; Punie, K.; Hurvitz, S.; Kalinsky, K.; Cortés, J.; O’Shaughnessy, J.; et al. 392P Quality-adjusted time without symptoms of disease progression or toxicity of treatment (Q-TWiST) analysis to assess benefit-risk of sacituzumab govitecan (SG) in previously treated patients (pts) with metastatic triple-negative breast cancer (mTNBC). Ann. Oncol. 2024, 35, S382–S383. [Google Scholar] [CrossRef]
- Corey-Lisle, P.K.; Peck, R.; Mukhopadhyay, P.; Orsini, L.; Safikhani, S.; Bell, J.A.; Hortobagyi, G.; Roche, H.; Conte, P.; Revicki, D.A. Q-TWiST analysis of ixabepilone in combination with capecitabine on quality of life in patients with metastatic breast cancer. Cancer 2012, 118, 461–468. [Google Scholar] [CrossRef]
- Cortes, J.; Pérez-García, J.; Whiting, S.; Wan, Y.; Solem, C.; Tai, M.-H.; Margunato-Debay, S.; Ko, A.; Fandi, A.; Botteman, M. Quality-Adjusted Survival With nab-Paclitaxel Versus Standard Paclitaxel in Metastatic Breast Cancer: A Q-TWiST Analysis. Clin. Breast Cancer 2018, 18, e919–e926. [Google Scholar] [CrossRef]
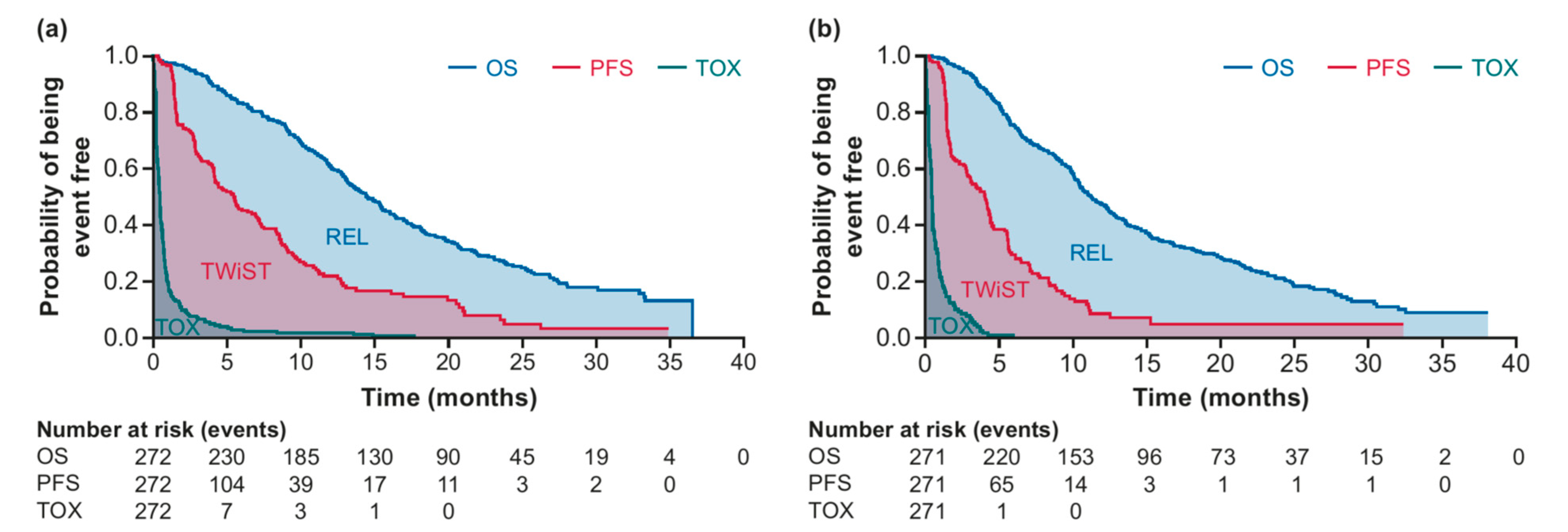
| Duration (Mo) | SG (n = 272) | TPC (n = 271) | Difference | p-Value |
|---|---|---|---|---|
| OS (95% CI) | 17.0 (15.8–18.4) | 14.8 (13.4–16.2) | 2.3 (0.5–4.3) | 0.0168 |
| PFS (95% CI) | 8.3 (7.0–9.7) | 6.0 (4.6–7.7) | 2.3 (0.1–4.2) | 0.0289 |
| TOX (95% CI) | 1.0 (0.8–1.4) | 0.8 (0.7–1.0) | 0.2 (−0.1–0.6) | 0.2590 |
| REL (95% CI) | 8.8 (7.5–10.1) | 8.7 (7.1–10.4) | <0.1 (−2.0–2.3) | 0.9937 |
| TWiST (95% CI) | 7.3 (6.1–8.6) | 5.2 (3.8–6.8) | 2.1 (0.1–3.9) | 0.0417 |
| Q-TWiST (95% CI) | 9.7 (8.9–10.5) | 8.1 (7.3–9.0) | 1.6 (0.5–2.7) | 0.0067 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rugo, H.S.; Bardia, A.; Schmid, P.; Tolaney, S.M.; Dasgupta, A.; Kaushik, A.; Verret, W.; Gosset, M.; Brufsky, A.; Cortés, J.; et al. Q-TWiST Analysis of Sacituzumab Govitecan vs. Chemotherapy in Previously Treated Patients with HR+/HER2− Metastatic Breast Cancer. Curr. Oncol. 2025, 32, 169. https://doi.org/10.3390/curroncol32030169
Rugo HS, Bardia A, Schmid P, Tolaney SM, Dasgupta A, Kaushik A, Verret W, Gosset M, Brufsky A, Cortés J, et al. Q-TWiST Analysis of Sacituzumab Govitecan vs. Chemotherapy in Previously Treated Patients with HR+/HER2− Metastatic Breast Cancer. Current Oncology. 2025; 32(3):169. https://doi.org/10.3390/curroncol32030169
Chicago/Turabian StyleRugo, Hope S., Aditya Bardia, Peter Schmid, Sara M. Tolaney, Anandaroop Dasgupta, Ankita Kaushik, Wendy Verret, Marine Gosset, Adam Brufsky, Javier Cortés, and et al. 2025. "Q-TWiST Analysis of Sacituzumab Govitecan vs. Chemotherapy in Previously Treated Patients with HR+/HER2− Metastatic Breast Cancer" Current Oncology 32, no. 3: 169. https://doi.org/10.3390/curroncol32030169
APA StyleRugo, H. S., Bardia, A., Schmid, P., Tolaney, S. M., Dasgupta, A., Kaushik, A., Verret, W., Gosset, M., Brufsky, A., Cortés, J., & Marmé, F. (2025). Q-TWiST Analysis of Sacituzumab Govitecan vs. Chemotherapy in Previously Treated Patients with HR+/HER2− Metastatic Breast Cancer. Current Oncology, 32(3), 169. https://doi.org/10.3390/curroncol32030169