Evaluation of Clinical Parameters Associated with Response and Resistance to Cemiplimab in Locally Advanced and Metastatic Cutaneous Squamous Cell Carcinoma: A Multi-Institutional Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Treatment
2.2. Statistics
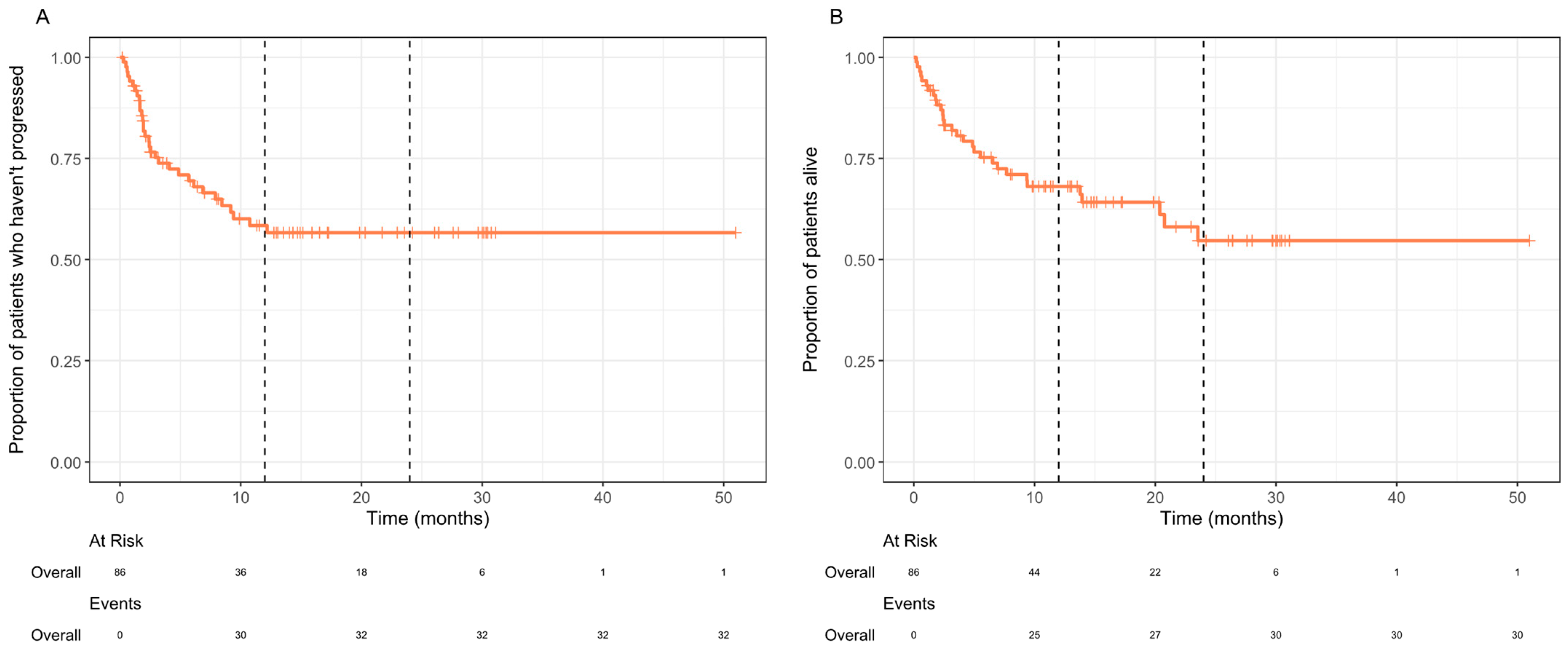
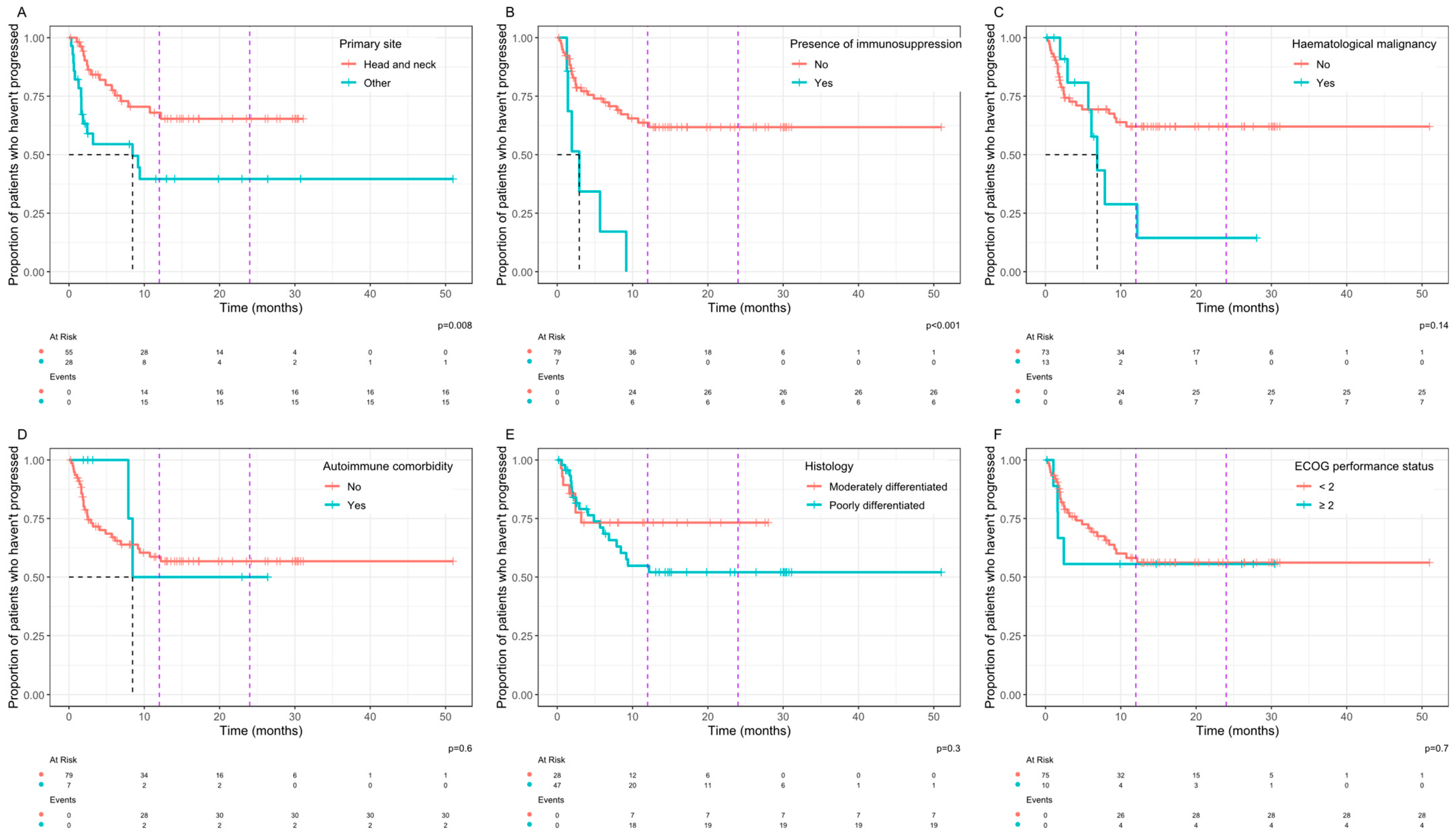
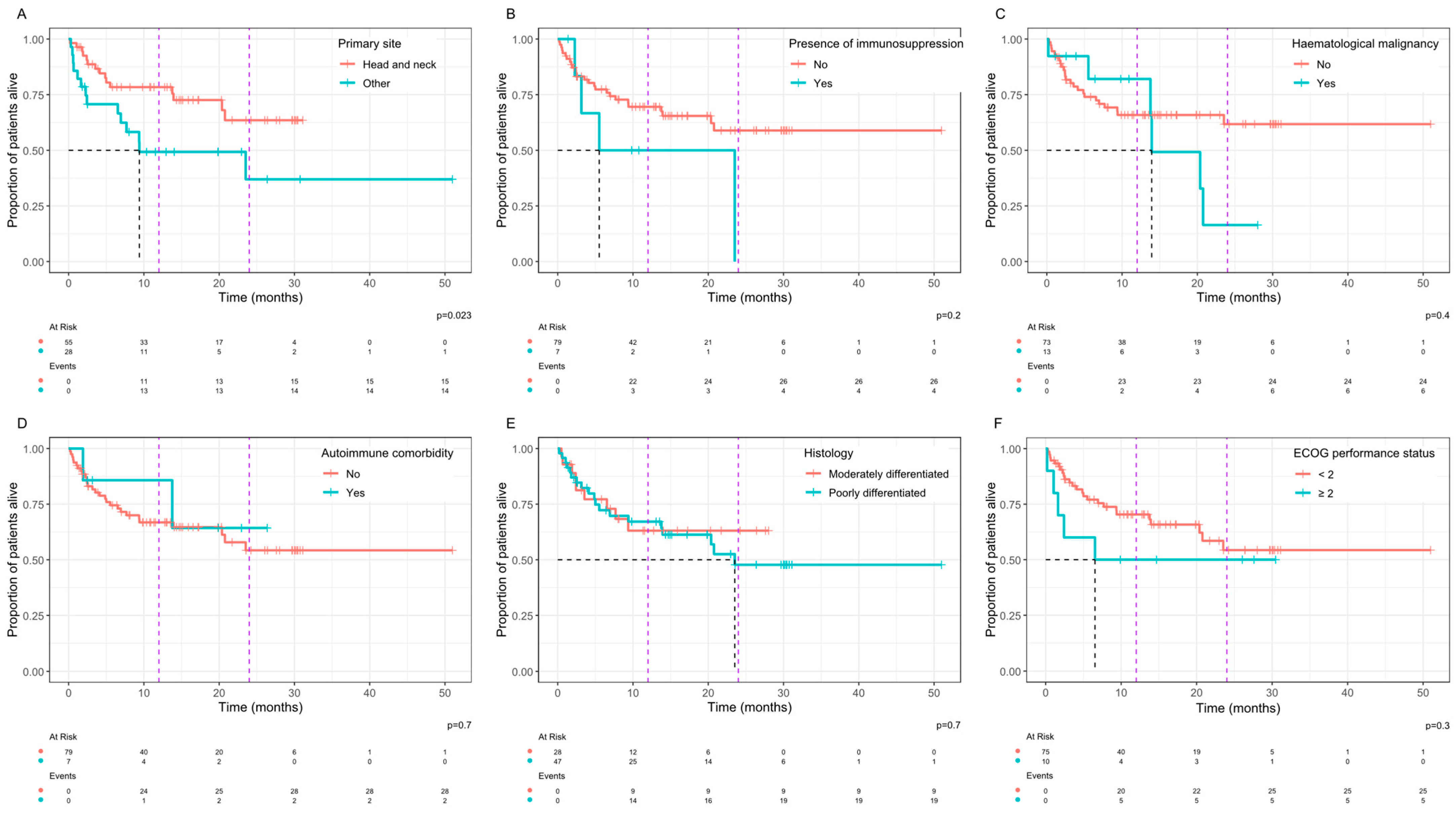
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- New Data Shows a Record 224,000 Skin Cancers in England in 2019. Available online: https://www.skinhealthinfo.org.uk/new-data-shows-a-record-224000-skin-cancers-in-england-in-2019 (accessed on 12 September 2023).
- Waldman, A.; Schmults, C. Cutaneous Squamous Cell Carcinoma. Hematol./Oncol. Clin. N. Am. 2019, 33, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schmults, C.D.; Karia, P.S.; Carter, J.B.; Han, J.; Qureshi, A.A. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: A 10-year, single-institution cohort study. JAMA Dermatol. 2013, 149, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Alberti, A.; Bossi, P. Immunotherapy for Cutaneous Squamous Cell Carcinoma: Results and Perspectives. Front. Oncol. 2021, 11, 727027. [Google Scholar] [CrossRef] [PubMed]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. NEJM 2018, 379, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Rischin, D.; Khushalani, N.I.; Schmults, C.D.; Guminski, A.; Chang, A.L.S.; Lewis, K.D.; Lim, A.M.; Hernandez-Aya, L.; Hughes, B.G.M.; Schadendorf, D.; et al. Integrated analysis of a phase 2 study of cemiplimab in advanced cutaneous squamous cell carcinoma: Extended follow-up of outcomes and quality of life analysis. J. Immunother. Cancer 2021, 9, e002757. [Google Scholar] [CrossRef] [PubMed]
- Migden, M.R.; Khushalani, N.I.; Chang, A.L.S.; Lewis, K.D.; Schmults, C.D.; Hernandez-Aya, L.; Meier, F.; Schadendorf, D.; Guminski, A.; Hauschild, A.; et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: Results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020, 21, 294–305. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org (accessed on 19 September 2024).
- Sjoberg, D.; Baillie, M.; Fruechtenicht, C.; Haesendonckx, S.; Treis, T. ggsurvfit: Flexible Time-to-Event Figures. R package Version 1.0.0. 2023. Available online: https://CRAN.R-project.org/package=ggsurvfit (accessed on 19 September 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 19 September 2024).
- Denaro, N.; Passoni, E.; Indini, A.; Nazzaro, G.; Beltramini, G.A.; Benzecry, V.; Colombo, G.; Cauchi, C.; Solinas, C.; Scartozzi, M.; et al. Cemiplimab in Ultra-Octogenarian Patients with Cutaneous Squamous Cell Carcinoma: The Real-Life Experience of a Tertiary Referral Center. Vaccines 2023, 11, 1500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hober, C.; Fredeau, L.; Pham-Ledard, A.; Boubaya, M.; Herms, F.; Celerier, P.; Aubin, F.; Beneton, N.; Dinulescu, M.; Jannic, A.; et al. Cemiplimab for Locally Advanced and Metastatic Cutaneous Squamous-Cell Carcinomas: Real-Life Experience from the French CAREPI Study Group. Cancers 2021, 13, 3547. [Google Scholar] [CrossRef] [PubMed]
- Baggi, A.; Quaglino, P.; Rubatto, M.; Depenni, R.; Guida, M.; Ascierto, P.A.; Trojaniello, C.; Queirolo, P.; Saponara, M.; Peris, K.; et al. Real world data of cemiplimab in locally advanced and metastatic cutaneous squamous cell carcinoma. Eur. J. Cancer 2021, 157, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Samstein, R.M.; Lee, C.H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, G.; Orlando, G.; Rumore, M.R.; Morrone, A.; Fruttero, E.; Caliendo, V.; Picciotto, F.; Sciarrillo, A.; Quaglino, P.; Cassoni, P.; et al. Predictors of Recurrence and Progression in Poorly Differentiated Cutaneous Squamous Cell Carcinomas: Insights from a Real-Life Experience. Dermatology 2024, 240, 329–336. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | n | % |
|---|---|---|
| Total patients | 86 | 100 |
| Age | 71 (median) | Range 34–93 |
| Male | 62 | 72 |
| Smoking status | ||
| Current | 16 | 19 |
| Ex-smoker | 30 | 35 |
| Never smoked | 30 | 35 |
| Not documented | 10 | 12 |
| Previous treatment | ||
| Surgery | 58 | 69 |
| Radiotherapy | 49 | 58 |
| SACT | 5 | 6 |
| Performance status at treatment initiation | ||
| 0 | 29 | 34 |
| 1 | 46 | 53 |
| 2 | 9 | 10 |
| 3 | 1 | 1 |
| Not documented | 1 | 1 |
| Level of de-differentiation | ||
| Moderate | 28 | 33 |
| Poor | 48 | 55 |
| Not documented | 11 | 13 |
| Site of primary disease | ||
| Head and neck | 55 | 64 |
| Torso | 6 | 7 |
| Lower limb | 16 | 19 |
| Upper limb | 6 | 7 |
| Not documented | 3 | 3 |
| Site of recurrence/metastasis | ||
| Local recurrence | 59 | 69 |
| Metastasis (any site) | 82 | 95 |
| Lymph node | 64 | 74 |
| Lung | 28 | 33 |
| Bone | 16 | 19 |
| Liver | 5 | 6 |
| Locally advanced only without recurrence | 3 | 3 |
| Haematological malignancy | ||
| Yes | 13 | 15 |
| No | 73 | 85 |
| Immunosuppression | ||
| Yes | 7 | 8 |
| Solid organ transplant | ||
| Yes | 3 | 3 |
| Response Rate | n | % | 95% Confidence Interval |
|---|---|---|---|
| Complete response | 22 | 29.7 | 20–41% |
| Partial response | 23 | 31.1 | 21–43% |
| Stable disease | 10 | 13.5 | 7–24% |
| Progressive disease | 19 | 25.7 | 17–37% |
| Overall response rate | 45 | 60.8 | 49–71% |
| Clinical benefit rate | 55 | 74.3 | 63–83% |
| Clinical Parameter | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Primary site | 0.4 | 0.13–1.12 | 0.07 |
| Immunosuppression | 1.58 | 0.25–11.53 | 0.70 |
| Haematological malignancy | 2.03 | 0.53–8.70 | 0.37 |
| Autoimmune comorbidity | 1.58 | 0.25–11.53 | 0.70 |
| Histology | 1.13 | 0.40–3.21 | 0.82 |
| Performance status | 1.14 | 0.24–5.41 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haigh, J.E.; Rack, S.; Yan, R.; Babu, S.; Donnelly, O.; Walter, H.; Faust, G.; Bhagani, S.; Isola, P.; Metcalf, R. Evaluation of Clinical Parameters Associated with Response and Resistance to Cemiplimab in Locally Advanced and Metastatic Cutaneous Squamous Cell Carcinoma: A Multi-Institutional Retrospective Cohort Study. Curr. Oncol. 2025, 32, 168. https://doi.org/10.3390/curroncol32030168
Haigh JE, Rack S, Yan R, Babu S, Donnelly O, Walter H, Faust G, Bhagani S, Isola P, Metcalf R. Evaluation of Clinical Parameters Associated with Response and Resistance to Cemiplimab in Locally Advanced and Metastatic Cutaneous Squamous Cell Carcinoma: A Multi-Institutional Retrospective Cohort Study. Current Oncology. 2025; 32(3):168. https://doi.org/10.3390/curroncol32030168
Chicago/Turabian StyleHaigh, Joseph Edward, Sam Rack, Ruiyang Yan, Sherin Babu, Olly Donnelly, Harriet Walter, Guy Faust, Shradha Bhagani, Patrick Isola, and Robert Metcalf. 2025. "Evaluation of Clinical Parameters Associated with Response and Resistance to Cemiplimab in Locally Advanced and Metastatic Cutaneous Squamous Cell Carcinoma: A Multi-Institutional Retrospective Cohort Study" Current Oncology 32, no. 3: 168. https://doi.org/10.3390/curroncol32030168
APA StyleHaigh, J. E., Rack, S., Yan, R., Babu, S., Donnelly, O., Walter, H., Faust, G., Bhagani, S., Isola, P., & Metcalf, R. (2025). Evaluation of Clinical Parameters Associated with Response and Resistance to Cemiplimab in Locally Advanced and Metastatic Cutaneous Squamous Cell Carcinoma: A Multi-Institutional Retrospective Cohort Study. Current Oncology, 32(3), 168. https://doi.org/10.3390/curroncol32030168





