Abstract
Stereotactic body radiation therapy has emerged as a promising alternative to brachytherapy, delivering high doses to tumors with precision while sparing surrounding organs. This systematic review evaluates the role of SBRT as a boost for patients who are ineligible for brachytherapy. A total of 17 studies, involving 288 patients, were analyzed, focusing on dosimetric parameters and toxicity. The radiation regimens varied in dose and fractionation schedules, with external beam doses ranging from 44 to 61.6 Gy, and SBRT boost doses ranging from 5 to 30 Gy. The total EQD2 doses were between 50.5 and 92.4 Gy. The results indicate adequate tumor control with SBRT, with local control rates ranging from 57% to 95.5%. The acute genitourinary and gastrointestinal toxicities were mostly grade 1 or 2, while late toxicities were less common. The overall survival rates varied between 34% and 96%. These results suggest that SBRT boost offers a viable option for cervical cancer patients ineligible for brachytherapy, with acceptable toxicity and promising survival outcomes. Nevertheless, the scarcity of data, which mainly originate from small studies with patients having varied stages of disease, as well as the lack of long-term follow up with SBRT, should encourage clinicians to utilize brachytherapy whenever suitable as a boost in these patient cohorts.
1. Introduction
According to the World Health Organization (WHO) and the World Cancer Research Fund (WCRF) statistics, cervical cancer is the fourth most common malignancy in women worldwide [1]. Low- and middle-income countries from South-East Asia, Central America and sub-Saharan Africa have the highest incidence and death rates from cervical cancer. Inequalities in access to vaccination; screening and treatment facilities; risk factors, such as HIV incidence; and social and economic determinants, such as gender, gender stigma and poverty are all associated with regional differences in the burden of cervical cancer [2].
To achieve high tumor control in cervical cancer therapy, brachytherapy (internal radiotherapy) is an essential treatment component along with external beam radiotherapy, delivering combined doses of more than 90 Gy to the tumor volume. The basic principle behind internal radiotherapy consists of the rapid reduction in peripheral doses while delivering high-dose conformal radiation to the tumor, thereby reducing normal tissue toxicity and optimizing tumor control [3]. Although brachytherapy provides superior protection of neighboring organs at risk, there are several factors that may render a patient ineligible for brachytherapy, such as medical comorbidities, anatomic abnormalities, contraindications to anesthesia or an obstructing tumor mass [4].
The introduction of specialized external radiotherapy techniques has led to a significant reduction in normal tissue toxicities, providing the possibility of administering the whole treatment, including the boost dose, with the same external beam radiotherapy technique, thus justifying the replacement of a brachytherapy boost in ineligible patients [4,5,6,7].
In view of the information above, stereotactic body radiation therapy (SBRT) can deliver a high dose to a tumor volume over a few fractions and can be used for multiple localizations with high accuracy using either on-board imaging or fiducial markers to track both the movement of the tumor volume and the position of surrounding organs [7]. SBRT was found to be suitable for the treatment of several types of cancer, including prostate [8,9], lung [10] and kidney [11]. Additionally, SBRT is now commonly used to treat liver, spine and lung metastases [12].
The aim of this systematic review is to summarize the current literature on the employment of SBRT as a boost for patients with cervical cancer that are either ineligible for or cannot benefit from brachytherapy due to varied reasons. The dosimetric results for tumor control and organs at risk, acute and late toxicities occurring during treatment and overall survival of patients are presented and discussed.
2. Materials and Methods
All articles published between 2005 and 2024 that had a reference to SBRT for cervical cancer were included in this review. The databases of Pubmed and the National Library of Medicine were searched using the following keywords: “cervical cancer” OR “gynecologic malignancy” AND “SBRT” OR “SABR” OR “Stereotactic Body Radiation Therapy” OR “Stereotactic Ablative Radiotherapy”.
The eligibility criteria for the selected articles were the following: (1) presents dosimetric parameters pertaining to treatment delivery; (2) reports acute and late toxicities and/or overall survival of cervical cancer patients treated with SBRT/SABR boost. Articles were selected if they presented either separate dosimetric values from boost treatment alone or doses summed with the initial treatment. Articles reporting dosimetric data related to the target volume, as well as for the organs at risk were considered eligible, including articles reporting on acute and/or late toxicities and overall survival. Publications that were not in English, did not report dosimetric values and/or did not report toxicities or overall survival were excluded, as shown in Figure 1. Using the linear quadratic formula with an = 10 for the tumor and = 3 for late effects, the prescribed SBRT dose was converted to a 2 Gy-equivalent dose (EQD2) whenever this was not reported by the analyzed study.
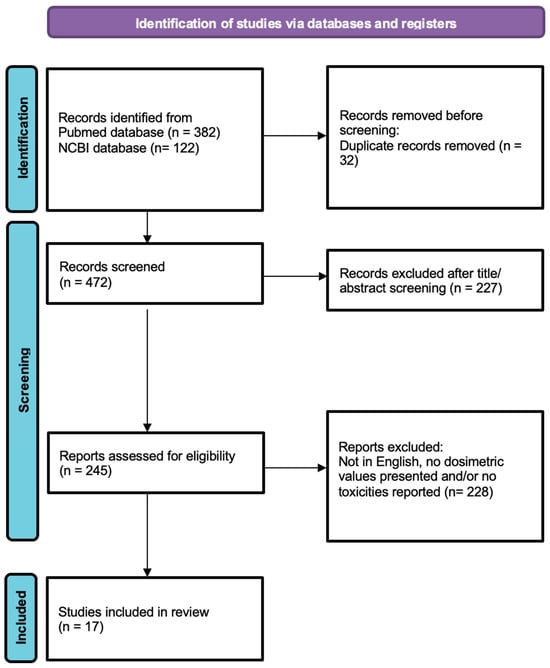
Figure 1.
Prisma chart. Study selection diagram.
The following data were collected from the reviewed articles: number of patients, external dose for primary treatment, SBRT boost dose, equivalent dose, median follow-up time, overall survival, acute and late toxicities (genitourinary and gastrointestinal), tumor volume coverage and doses received by organs at risk.
3. Results
A total of 472 studies were screened, of which 17 articles met the eligibility criteria. These studies included an overall number of 288 patients with cervical cancer treated with external beam radiotherapy (EBRT), followed by an SBRT boost with various fractionation regimens.
The studies presented in Table 1 included patients with a wide variety of stages, from early-stage tumors (IA, IB, IIA) to more advanced disease (IIIB, IVA, IVB). The clinical target volume (CTV) and planning target volume (PTV) are determined based on the extent of the disease, with margins ranging from 0 to 15 mm beyond the gross tumor volume (GTV). The GTV is normally determined using a combination of MRI, CT scans and physical examination. The CTV is defined differently according to each individual study: some cover the entire pelvic region or certain regions, such as the vaginal vault, while others concentrate on the cervix, uterus and lymph nodes. Variations in tumor size are reflected in the median tumor and target volumes, which range from 9.163 cm3 to 120 cm3. Target volume definitions and median tumor volumes, as shown in Table 1, vary owing to different reporting systems used by the evaluated studies. In addition, the information offered by most articles did not differentiate between volume designations for EBRT and SBRT, thus this aspect is reflected in a limited number of studies.

Table 1.
Summary of studies with patient/tumor characteristics and motivations for ineligibility for brachytherapy (N/A = data not available).
Ineligibility for brachytherapy can occur for a variety of reasons, including anatomical, medical, technical and patient-related factors. Some of the contributing factors are tumor size and location and proximity to vital organs, such as the bladder and rectum, as well as difficulties in positioning the required devices, such as sleeves. Patients with certain medical conditions (cardiovascular, other co-morbidities or poor general health) are ineligible. Finally, patient refusal, combined with factors such as unfavorable anatomy or inability to undergo anesthesia, are other contributors to ineligibility for brachytherapy.
3.1. Prescribed Dose and Fractionation: Tumor Volume Dosimetry
The doses prescribed for external beam treatment ranged from 44 Gy to 61.6 Gy, with the predominant fractionation schedule being 45 Gy in 25 fractions, as shown in Figure 2. The most common fractionation for the boost volume was 25 Gy in 5 fractions (fr), ranging from 5 Gy/1 fr to 30 Gy/5 fr, with total equivalent doses (i.e., summed with the external dose) ranging from 50.5 Gy to 92.4 Gy for = 10 and from 51.2 Gy to 106.6 Gy for = 3, as shown in Table 2. The median follow-up time varied from 6 to 47 months, and the overall survival ranged from 34% to 96%, depending on years of follow-up. Local control varied from 57% to 95.5%. A Pearson correlation between the total equivalent dose and local control was performed, leading to a weak correlation only, which might be due to the large variations in patient selection, data reporting and follow-up times among the studies.
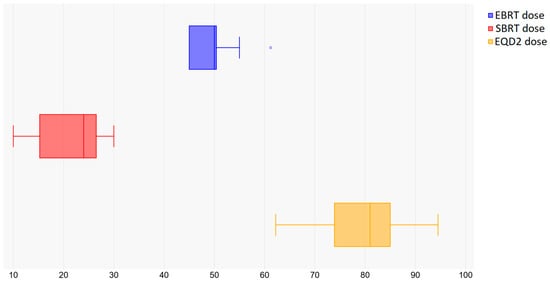
Figure 2.
Dose prescriptions for EBRT, SBRT and EQD2.

Table 2.
Summary of studies with SBRT boost reporting radiation schedule, EQD2, overall survival and toxicities (N/A = data not available).
A dosimetric comparison of the evaluated studies showed satisfactory tumor volume coverage, as illustrated by the collated data of most representative dosimetric parameters (D90 (Gy) and D95 (%)) presented in Figure 3.
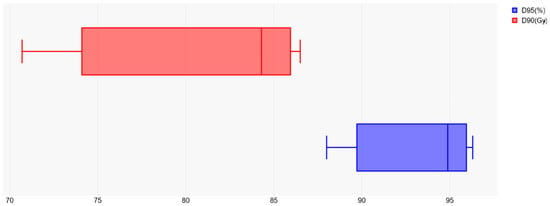
Figure 3.
Target volume coverage reflected by D95 (%) and D90 (Gy) dosimetric parameters.
3.2. Organ at Risk Dosimetry
Acute genitourinary toxicities were reported by most studies and ranged from grades 0 to 3, as shown in Figure 4. Grades 4 and 5 acute toxicities were not reported. Grade 0 toxicities were reported in 4/17 studies, grade 1 toxicities were reported in 13/17 studies, grade 2 toxicities in 12/17 studies, and grade 3 toxicities in 2/17 studies. Late toxicities were presented as grade 0 in 3/17 studies, grade 1 in 4/17 studies and grade 2 in 6/17 studies.
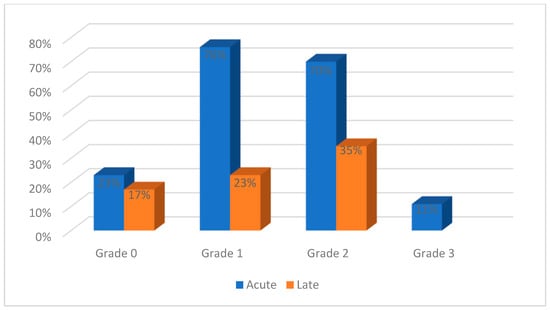
Figure 4.
Genitourinary toxicities reported in the evaluated studies.
Acute gastrointestinal toxicities were reported in all the studies and ranged from grades 0 to 3, as shown in Figure 5. Grades 4 and 5 acute toxicities were not reported. Grade 0 toxicities were reported in 4/17 studies, grade 1 in 14/17 studies, grade 2 in 13/17 studies, and grade 3 in 6/17 studies. Late toxicities were evaluated in 10 studies, ranging from grade 0 to 4: grade 0 in 3/17 studies, grade 1 in 4/17 studies, grade 2 in 6/17 studies, grade 3 in 4/17 studies, and grade 4 in 1/17 studies.
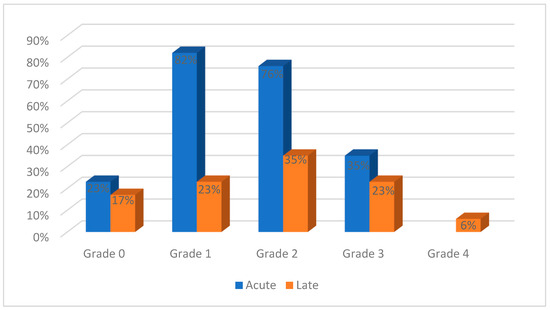
Figure 5.
Gastrointestinal toxicities reported in the evaluated studies.
Regarding bladder dosimetry, only the values presented for the boost volume were different, with Facondo et al. [14] reporting 16.92 Gy for the maximum dose and Pontoriero et al. [15] reporting 17.57 Gy, while the highest value was reported by Kubicek et al. [13] at 25.7 Gy. The lowest value for D2cc was achieved by Pontoriero et al. (3 Gy) [15], and the highest value (19 Gy) was reported by Kubicek et al. [13]. For D1cc, Kubicek et al. [13] also reported the highest value (20.7 Gy), whereas Cengiz et al. [29] achieved the lowest dose (6.78 Gy).
The boost dose values summed with the corresponding external beam dose for the bladder are varied. For D2cc, the lowest dose was reported by Cheng et al. [19] at a value of 71.97 Gy, and Turna et al. [16] obtained 80.86 Gy. The highest dose was presented by Dahbi et al. [30] at 84.7 Gy.
The rectal doses, according to plans delivered with an SBRT boost only, are diverse. The maximum dose was reported by three studies with very dissimilar values: 8.68 Gy [15], 15.62 Gy [14] and 23.8 Gy [13]. For D2cc, the lowest doses were obtained by Pontoriero et al. [15] (4 Gy) and Cengiz et al. [29] (4.56 Gy), while Facondo et al. [14] and Ito et al. [31] delivered larger doses of 13.07 Gy and 16.3 Gy, respectively. The largest dose was again administered by Kubicek et al. [13] (19.3 Gy). Only two studies reported the D1cc parameter: 5.09 Gy [29] and 19.6 Gy [13], respectively.
The combined boost and external beam SBRT resulted in similar doses for the D2cc parameter, ranging from 62.49 Gy [16] to 74.7 Gy [30].
The sigmoid was an under-reported OAR, with a limited number of studies presenting dosimetric values for D2cc: 57.65 Gy [16], 62.53 Gy [19] and 75.75 Gy [30].
4. Discussion
A number of guidelines recommend that the standard treatment for a patient diagnosed with any type of cervical cancer should be external beam radiotherapy (with or without chemotherapy), followed by 3–4 fractions of brachytherapy with a dose between 5–7 Gy per fraction [32,33,34]. The brachytherapy boost allows the target volume to receive an elevated radiation dose for better tumor control, while sparing the surrounding organs. However, the procedure is invasive and discomforting, usually requiring anesthesia, and employs completely different equipment than external beam radiotherapy, operated by personnel specializing in brachytherapy. It is documented that a small percentage of individuals are unable to receive brachytherapy due to medical comorbidity, anatomical abnormalities or obstructing tumor mass [5].
With the development of specialized radiotherapy techniques, brachytherapy was considered by some to be replaced with SBRT or other external beam techniques. This led to a decline in the utilization of brachytherapy over the first decade of this century (by about 10%), compensated by the increase in the use of EBRT as a boost in cervical cancer [32].
Compared to conventional EBRT, SBRT allows the delivery of a larger biologically equivalent dose to the target in a highly conformal manner over fewer fractions [21,35].
Prior to the widespread use of intensity-modulated and stereotactic body radiotherapy, there were several attempts to replace a brachytherapy boost with the conventional EBRT boost. While the dose delivered to the tumor was often lower than that of the brachytherapy or EBRT boost using contemporary techniques, earlier EBRT boost trials using the 3D-CRT approach showed satisfactory therapeutic outcomes and toxicity profiles [36,37]. Barraclough et al. [4] delivered 60–65 Gy to the tumor volume with conventional fractionation in 71% of patients, reporting good 3-year survival rates (100%—stage I, 70%—stage II and 42% survival among stage III cancer patients), with late grade 1/2 toxicities reported in 41% of patients. These lower doses for target volume coverage were most likely due to the use of fixed fields without intensity modulation by fixed angles, which hindered the reduction in toxicities to surrounding organs at risk.
Ito et al. [38] suggested that an EQD2 between 54.2 and 75.3 Gy at point A (the point at which the uterine artery and ureter cross) might result in a 76% pelvic control rate treated with brachytherapy. According to the University of Wisconsin’s experience, a 3-year pelvic control rate of 71% may be attained, with the EQD2 = 70.8–84.1 Gy at point A [39]. Likewise, EQD2 ranging between 58.3 and 66.7 Gy at point A could offer a remarkable 3-year pelvic control rate (76%) for an advanced stage, according to Toita et al. [40].
Hsieh et al. [18] suggested that an EQD2 ranging from 64.6 Gy to 82.7 Gy led to 78% locoregional control with SBRT over a 3-year follow-up, but at the same time, they mentioned that these numbers should be treated cautiously owing to the small number of patients included in their study, as well as the outcome bias induced by the concurrent chemotherapy received by some patients.
Regarding the target volume coverage, Molla et al. [6] used margins of 6–9 mm for PTV in their study but stated that margins of up to 10 mm were more adequate to cover the tumor volume. Jorcano et al. [24] confirmed the need for using a 10 mm margin; they also employed a marker system to compare the CT-based plan with the delivered dose.
Concerning normal tissue toxicity, according to Pourquier et al. [41], the incidence of grade 2 and 3 rectal complications was less than 5% when doses below 75–80 Gy were administered to small-volume tumors; however, the incidence of complications increased to 10–15% with higher doses. To avoid a higher incidence of grade 2+ severe rectal complications, Cheng et al. [42] suggested a proximal rectal dose of less than 62 Gy.
As shown in Table 2, the summed equivalent doses for EBRT and SBRT boost range from 50.5 to 92.4 Gy, but higher doses do not necessarily result in higher toxicities. Pontoriero et al. [15] delivered equivalent doses of 81.5–88.8 Gy and reported both genitourinary and gastrointestinal grade 2 toxicities. On the other hand, Marnitz et al. [22] delivered an equivalent dose of 90 Gy and reported only second-degree toxicities in a similar patient cohort. Dalwadi et al. [25] used equivalent doses up to 89.56 Gy that led to grade 2 late toxicities in only one patient, whereas Park et al. [43] administered doses above 110 Gy and reported higher grade (>2) late genitourinary and gastrointestinal toxicities.
The variability in toxicity outcomes observed across different radiation doses and treatment methods highlights the importance of understanding how different techniques impact both acute and late toxicities. While higher doses in EBRT and SBRT do not always lead to more severe toxicities [44], other factors, such as the proximity of the radiation source to critical organs, play a significant role. For instance, in brachytherapy, where the radioactive source is placed close to the tumor, radiation exposure to surrounding tissues is minimized, potentially reducing toxicity. In contrast, SBRT involves delivering radiation that passes through larger volumes of healthy tissue, which may increase the risk of both acute and late toxicities, particularly in the gastrointestinal and genitourinary systems. This distinction between the radiation delivery methods, along with the dose ranges, contributes to the differing patterns of toxicities observed in the studies discussed.
Table 2 shows that the most commonly reported acute toxicities after SBRT are grades 1 (76%) and 2 (70%) genitourinary toxicities and grades 1 (82%) and 2 (76%) gastrointestinal toxicities. While grade 3+ toxicities are less common, they usually affect the gastrointestinal system. Late toxicities are more likely to be grade 2, both genitourinary and gastrointestinal. Gadda et al. [45] reported one grade 1 acute gastrointestinal toxicity and one late gastrointestinal toxicity of grade 2/3 with brachytherapy, while Chopra et al. [46] reported late grade 2/3 genitourinary (11%) and gastrointestinal toxicity (12.5%) and one patient with grade 4 bowel toxicity from brachytherapy with an EQD2 between 80 and 85 Gy.
When comparing the use of SBRT vs. brachytherapy in terms of patient cohorts, Table 3 shows that the number of patients reported in clinical studies employing a brachytherapy boost is larger, owing to the fact that brachytherapy is still the main treatment approach for these patients. However, the staging of disease is comparable between the SBRT and brachytherapy studies, ranging from early stages to advanced ones, with most studies incorporating patients of all stages. Although brachytherapy was implemented in clinics long before SBRT, thus providing more follow-up data, which makes a direct comparison between treatment outcomes more difficult, the evidence to date shows that local control in cervical cancer patients is superior with a brachytherapy boost. Toxicities are generally comparable to an SBRT boost, though in some cases, they may be lower with brachytherapy.
By calculating the equivalent doses for both tumor (α/β = 10) and late effects (α/β = 3) for the two techniques, it can be seen (Table 2 and Table 3) that they are generally within the same ranges. Pötter et al. [47] reported genitourinary and gastrointestinal toxicities in 5.1% of patients after administering equivalent doses of up to 104.4 Gy3 with EBRT + brachytherapy boost, with similar outcomes reported by Dalwadi et al. [25] in terms of late toxicities (4%) caused by comparable biological doses (102.4 Gy3) delivered via EBRT + SBRT boost. Banerjee et al. [48] concluded that late genitourinary and gastrointestinal toxicities of grade 3 or higher occurring with brachytherapy are less than 10%, while with SBRT, these values are similar for genitourinary toxicities but can go up to 29% for gastrointestinal effects. In the studies shown in Table 3, the percentages of grades 1 and 2 genitourinary and gastrointestinal toxicities are similar to those observed with SBRT, while grade 3 toxicities are lower. This may be due to the fact that with brachytherapy, the radioactive source is placed near the tumor volume, and the radiation does not reach the entire organ at risk, whereas with SBRT, the radiation passes through most of the organs at risk to offer adequate coverage to the tumor volume. The fact that the clinical outcome is generally better with a brachytherapy boost as compared to SBRT suggests that the former remains the main treatment for cervical cancer in patients who are eligible for this type of treatment.

Table 3.
Clinical outcome after EBRT + brachytherapy boost as reported by the literature.
Table 3.
Clinical outcome after EBRT + brachytherapy boost as reported by the literature.
| Study | Number of Patients/Staging | EBRT Dose/Boost Dose Gy (Fr) | EQD2 α/β = 10/ EQD2 α/β = 3 (Gy) | Median Follow-Up (Months) | Overall Survival | Local Control | Toxicities |
|---|---|---|---|---|---|---|---|
| Kang et al. [49] | 97 IB–IVB | 45–50.4 (25–28)/25–30 (5–6) | 75.5–87.1/83.2–96.1 | 50 | Progression-free survival: 80% at 3 years | 93% at 3 years | 2 patients with late rectal bleeding |
| Castelnaud-Marchand et al. [50] | 225 IB1–IVA | 44–50.4 (25–28)/15 (3) | 62.8–68.3/ 68–72.4 | 38.8 | OS: 76.1% at 3 years | 86.4% at 3 years | 18 late genitourinary and gastrointestinal toxicities in 14 patients |
| Simpson et al. [51] | 76 IB1–IVA | 43.2–50.4 (24–28)/25–30 (3–5) | 78.5–89.6/94.3–102.4 | 17 | OS: 75% at 2 years | 94.2% at 2 years | 2 patients with intractable nausea and vomiting |
| Charra–Brunaud et al. [52] | 117 IB1–IVA | 45 (25)/10–20 (2–4) | 56.8–69.3/59.2–75.2 | 24.3 | OS: 74% at 2 years | 78.5% at 2 years | 1.2% of patients with genitourinary and gastrointestinal toxicities |
| Pötter et al. [47] | 156 IB1–IVA | 45–50.4 (25–28)/ 28 (4) | 83.9–89.2/99.2–104.4 | 42 | OS: 68% at 3 years | 95% at 3 years | 3 patients with genitourinary and 5 patients with gastrointestinal toxicities |
| Tan et al. [53] | 28 IB1–IIIB | 45 (25)/21 (3) | 74/85.2 | 23 | N/A | 93% at 3 years | 2 patients with rectal bleeding |
In a study conducted by the Gynecologic Oncology Group, patients treated with chemoradiotherapy (including intracavitary brachytherapy) had a late toxicity rate (grades 3 or 4) of 1.7%, with 22% of patients presenting local progression and an overall survival (OS) of 60% at 5 years [54]. In the study by Chen et al. [55], the use of image-guided brachytherapy demonstrated a 2-year local recurrence-free survival and overall survival of 89% and 85%, respectively. According to O’Donnell et al. [33], patients who received an SBRT boost had OS rates that were equal to those receiving intracavitary brachytherapy. In the articles reviewed, the OS showed a wide variation from 53.3% to 96%, as indicated by studies with a 2-year follow-up, and from 34% to 95% in studies reporting 3-year outcome results. The main reasons for the lower OS are deaths due to multiple metastases and comorbidities. Compared to brachytherapy, those who received an SBRT boost had the same survival rate, while those who received an IMRT boost had a lower survival rate, according to a propensity-matched analysis [33]. Yet, recent results of a phase II trial of an SBRT boost for locally advanced cervical cancer show suboptimal outcomes (53.3% 2-year overall survival), which was justified by patient selection (comorbidities, advanced stage disease) and very large tumors (median PTV size = 139 cm3) [23]. The authors only recommend this approach in patients with smaller tumors that are ineligible for brachytherapy.
An important clinical aspect pertaining to SBRT as highlighted by the evaluated studies is the spontaneous organ motion in this anatomical region, leading to interfractional dose variations [56]. For instance, Turna et al. [16] found that for many patients, it was necessary to reposition 2–3 times to achieve the most optimal scenario, including organ filling after performing the position checks. Dincer et al. [57] suggested that a feasible solution to this problem would be the implementation of online adaptive radiotherapy with online contouring and planning. The safety and efficacy of SBRT could be further improved by incorporating tracking techniques, especially for devices with longer treatment times, such as CyberKnife or MR-linac [16].
Despite the disputes regarding the use of SBRT as a boost in cervical cancer management, SBRT is gaining popularity due to accessibility and convenience in use rather than owing to its dosimetric advantage over brachytherapy [32]. The latest investigations across radiotherapy centers, particularly in the United States, point towards underutilization of brachytherapy in cervical cancer patients [58,59]. A survey designed to understand the underutilization of brachytherapy has targeted members of the American Brachytherapy Society (ABS) and showed that the main responsible factors are insufficient training in this field during residency and a lack of professional development to maintain the necessary skills [58].
Nevertheless, brachytherapy is here to stay, and radiotherapy societies and organizations are advised to add more weight to brachytherapy training among radiation oncologists during their residency and to encourage senior clinicians to keep using this technique to improve patients’ outcomes [58]. According to the latest guidelines of the National Comprehensive Cancer Network, brachytherapy is considered a key constituent of cervical cancer management, being pronounced as a critical component of oncological care in patients with unresectable cervical cancers [60]. In view of this, a consensus statement was released by the ABS and the Society of Gynecologic Oncology, whereby external beam radiotherapy, such as IMRT and/or SBRT, should not replace brachytherapy in this patient group [61].
The aim of this review was to evaluate the clinical results of an SBRT boost in cervical cancer in terms of outcome and toxicity and to compare them to conventional brachytherapy boost. This goal was guided by the fact that the number of therapeutic options for patients who cannot or do not wish to receive standard brachytherapy is limited.
This review has a number of limitations, including the large variability among the studies regarding patient-related factors, such as patient selection (particularly variations in staging and tumor size) and patient cohorts (usually small); radiation delivery-related factors, such as different total doses and fractionation schedules (either as a boost or combined techniques); and clinical factors, such as short follow-up times and different reporting of outcomes. Furthermore, the rather limited number of eligible studies and their varied reporting systems renders it difficult to conduct a clinically relevant analysis in order to conclude on definitive judgments about either local control rates or side effect profiles when using SBRT in this patient group.
5. Conclusions
An SBRT boost following external beam radiotherapy can be an alternative to brachytherapy in cervical cancer patients who are not eligible for this treatment or refuse it. Although there is a tendency to associate an SBRT boost with high toxicities and a low dose to the target, this technique can achieve target volume doses comparable to those offered by brachytherapy, with acceptable toxicities. It is to be reiterated that the current recommendations towards the use of SBRT in patients with locally advanced cervical cancer include only those situations when patients are ineligible for brachytherapy and should not be employed as a replacement therapy for the latter.
6. Future Directions
For situations when SBRT is the only treatment option as boost radiotherapy in cervical cancer, there should be focus on optimizing the dose and fractionation schemes to maximize tumor control while minimizing organ toxicity. Specifically, studies should aim to refine the biologically equivalent dose and EQD2 in SBRT, exploring the optimal range for different cervical cancer stages and patient subgroups. To improve treatment protocols, it will be essential to investigate how dose changes in SBRT affects local control, while monitoring acute and late toxicities, particularly in vital organs, such as the bladder, rectum and sigmoid.
Long-term toxicities associated with SBRT compared with conventional brachytherapy, including those affecting the genitourinary and gastrointestinal systems, require further investigation. In addition, research into certain patient risk factors (such as tumor size, HIV infection or other comorbidities) that may confer higher susceptibility to late toxicities will assist in customizing SBRT procedures to reduce these risks.
To address the variability shown in the current review and improve generalizability, international collaborations could standardize procedures and treatment plans in different contexts. These collaborations could potentially enroll larger groups of patients, which would also provide more robust data to support clinical decisions about the use of SBRT in cervical cancer, while still recommending brachytherapy as a key component of cervical cancer management.
Author Contributions
All the authors contributed to the study conception and design. Data collection and analysis were performed by I.G., the original draft was written by I.G. and L.G.M. and review and editing were performed by I.G. and L.G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
The authors would like to thank the hospital staff for their support in the completion of this work.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| WHO | World Health Organization |
| WCRF | World Cancer Research Fund |
| SBRT | Stereotactic body radiation therapy |
| SABR | Stereotactic ablative radiotherapy |
| HIV | Human immunodeficiency viruses |
| EQD2 | Equivalent dose in 2 Gy fractions |
| EBRT | External beam radiotherapy |
| Fr | Fraction |
| D95 | The dose received by 95% of the tumor volume |
| D90 | The dose received by 90% of the tumor volume |
| D2cc | Minimum dose to the most irradiated contiguous volume of 2cc |
| D1cc | Minimum dose to the most irradiated contiguous volume of 1cc |
| OS | Overall survival |
| 3D-CRT | Three-dimensional conformal radiation therapy |
References
- World Health Organization. Cervical Cancer. Available online: www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 25 November 2024).
- World Cancer Research Fund. Cervical Cancer Statistics, 15 November 2024. Available online: www.wcrf.org/preventing-cancer/cancer-statistics/cervical-cancer-statistics/#:~:text=Related%20content-,Latest%20cervical%20cancer%20data,ASR%20%3D%20age%2Dstandardised%20rates (accessed on 12 January 2024).
- Skowronek, J. Current status of brachytherapy in cancer treatment—Short overview. J. Contemp. Brachytherapy 2017, 9, 581–589. [Google Scholar] [CrossRef]
- Barraclough, L.H.; Swindell, R.; Livsey, J.E.; Hunter, R.D.; Davidson, S.E. External Beam Boost for Cancer of the Cervix Uteri When Intracavitary Therapy Cannot Be Performed. Int. J. Radiat. Oncol. 2008, 71, 772–778. [Google Scholar] [CrossRef]
- Logsdon, M.D.; Eifel, P.J. FIGO IIIB squamous cell carcinoma of the cervix: An analysis of prognostic factors emphasizing the balance between external beam and intracavitary radiation therapy. Int. J. Radiat. Oncol. 1999, 43, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Mollà, M.; Escude, L.; Nouet, P.; Popowski, Y.; Hidalgo, A.; Rouzaud, M.; Linero, D.; Miralbell, R. Fractionated stereotactic radiotherapy boost for gynecologic tumors: An alternative to brachytherapy? Int. J. Radiat. Oncol. 2005, 62, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Higginson, D.S.; Morris, D.E.; Jones, E.L.; Clarke-Pearson, D.; Varia, M.A. Stereotactic body radiotherapy (SBRT): Technological innovation and application in gynecologic oncology. Gynecol. Oncol. 2011, 120, 404–412. [Google Scholar] [CrossRef]
- Seo, Y.S.; Kim, M.; Yoo, S.Y.; Cho, C.K.; Choi, C.W.; Kim, J.H.; Han, C.J.; Park, S.C.; Lee, B.H.; Kim, Y.H.; et al. Preliminary result of stereotactic body radiotherapy as a local salvage treatment for inoperable hepatocellular carcinoma. J. Surg. Oncol. 2010, 102, 209–214. [Google Scholar] [CrossRef]
- King, C.R.; Brooks, J.D.; Gill, H.; Pawlicki, T.; Cotrutz, C.; Presti, J.C., Jr. Stereotactic body radiotherapy for localized prostate cancer: Interim results of a prospective phase II clinical trial. Int. J. Radiat. Oncol. 2009, 73, 1043–1048. [Google Scholar] [CrossRef]
- Timmerman, R.; Paulus, R.; Galvin, J.; Michalski, J.; Straube, W.; Bradley, J.; Fakiris, A.; Bezjak, A.; Videtic, G.; Johnstone, D.; et al. Stereotactic Body Radiation Therapy for Inoperable Early Stage Lung Cancer. JAMA 2010, 303, 1070–1076. [Google Scholar] [CrossRef]
- Svedman, C.; Sandström, P.; Pisa, P.; Blomgren, H.; Lax, I.; Kälkner, K.-M.; Nilsson, S.; Wersäll, P. A prospective Phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol. 2006, 45, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.S.; Fakiris, A.J.; Chang, E.L.; Mayr, N.A.; Wang, J.Z.; Papiez, L.; Teh, B.S.; McGarry, R.C.; Cardenes, H.R.; Timmerman, R.D. Stereotactic body radiation therapy: A novel treatment modality. Nat. Rev. Clin. Oncol. 2009, 7, 44–54. [Google Scholar] [CrossRef]
- Kubicek, G.J.; Xue, J.; Xu, Q.; Asbell, S.O.; Hughes, L.; Kramer, N.; Youssef, A.; Chen, Y.; Aikens, J.; Saul, H.; et al. Stereotactic Body Radiotherapy as an Alternative to Brachytherapy in Gynecologic Cancer. BioMed. Res. Int. 2013, 2013, 898953. [Google Scholar] [CrossRef]
- Facondo, G.; Vullo, G.; DE Sanctis, V.; Valeriani, M.; Ascolese, A.M.; Massaro, M.; Anzellini, D.; Osti, M.F. Stereotactic Body Radiation Therapy Boost in Patients With Cervical Cancer Ineligible for Brachytherapy. Cancer Diagn. Progn. 2021, 1, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Pontoriero, A.; Iatì, G.; Aiello, D.; Pergolizzi, S. Stereotactic Radiotherapy in the Retreatment of Recurrent Cervical Cancers, Assessment of Toxicity, and Treatment Response. Technol. Cancer Res. Treat. 2016, 15, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Turna, M.; Rzazade, R.; Küçükmorkoç, E.; Küçük, N.; Canoğlu, M.D.; Çağlar, H.B. Dose escalation with stereotactic body radiotherapy for cervical cancer treatment. BMC Cancer 2024, 24, 1281. [Google Scholar] [CrossRef]
- Haas, J.A.; Witten, M.R.; Clancey, O.; Episcopia, K.; Accordino, D.; Chalas, E. CyberKnife Boost for Patients with Cervical Cancer Unable to Undergo Brachytherapy. Front. Oncol. 2012, 2, 19296. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-H.; Tien, H.-J.; Hsiao, S.-M.; Wei, M.-C.; Wu, W.-Y.; Sun, H.-D.; Wang, L.-Y.; Hsieh; Chen, Y.-J.; Shueng, P.-W.; et al. Stereotactic body radiation therapy via helical tomotherapy to replace brachytherapy for brachytherapy-unsuitable cervical cancer patients—A preliminary result. OncoTargets Ther. 2013, 6, 59–66. [Google Scholar] [CrossRef]
- Cheng, H.-Y.; Liang, J.-A.; Hung, Y.-C.; Yeh, L.-S.; Chang, W.-C.; Lin, W.-C.; Chen, S.-W. Stereotactic body radiotherapy for pelvic boost in gynecological cancer patients with local recurrence or unsuitable for intracavitary brachytherapy. Taiwan. J. Obstet. Gynecol. 2021, 60, 111–118. [Google Scholar] [CrossRef]
- Guckenberger, M.; Bachmann, J.; Wulf, J.; Mueller, G.; Krieger, T.; Baier, K.; Richter, A.; Wilbert, J.; Flentje, M. Stereotactic body radiotherapy for local boost irradiation in unfavourable locally recurrent gynaecological cancer. Radiother. Oncol. 2010, 94, 53–59. [Google Scholar] [CrossRef]
- Lee, T.H.; Song, C.; Kim, I.A.; Kim, J.-S.; Kim, Y.B.; Kim, K.; No, J.H.; Suh, D.H.; Chung, J.-B.; Eom, K.-Y. Stereotactic ablative body radiotherapy boost for cervical cancer when brachytherapy boost is not feasible. Radiat. Oncol. 2021, 16, 148. [Google Scholar] [CrossRef]
- Marnitz, S.; Köhler, C.; Budach, V.; Neumann, O.; Kluge, A.; Wlodarczyk, W.; Jahn, U.; Gebauer, B.; Kufeld, M. Brachytherapy-emulating robotic radiosurgery in patients with cervical carcinoma. Radiat. Oncol. 2013, 8, 109. [Google Scholar] [CrossRef]
- Albuquerque, K.; Tumati, V.; Lea, J.; Ahn, C.; Richardson, D.; Miller, D.; Timmerman, R. A Phase II Trial of Stereotactic Ablative Radiation Therapy as a Boost for Locally Advanced Cervical Cancer. Int. J. Radiat. Oncol. 2019, 106, 464–471. [Google Scholar] [CrossRef]
- Jorcano, S.; Mollà, M.; Escudé, L.; Sanz, S.; Hidalgo, A.; Toscas, J.I.; Linero, D.; Miralbell, R. Hypofractionated Extracranial Stereotactic Radiotherapy Boost for Gynecologic Tumors: A Promising Alternative to High-Dose Rate Brachytherapy. Technol. Cancer Res. Treat. 2010, 9, 509–514. [Google Scholar] [CrossRef]
- Dalwadi, S.; Echeverria, A.; Jhaveri, P.; Bui, T.; Waheed, N.; Tran, D.; Bonnen, M.; Ludwig, M. Non-invasive stereotactic ablative boost in patients with locally advanced cervical cancer. Int. J. Gynecol. Cancer 2020, 30, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Hadi, I.; Eze, C.; Schönecker, S.; von Bestenbostel, R.; Rogowski, P.; Nierer, L.; Bodensohn, R.; Reiner, M.; Landry, G.; Belka, C.; et al. MR-guided SBRT boost for patients with locally advanced or recurrent gynecological cancers ineligible for brachytherapy: Feasibility and early clinical experience. Radiat. Oncol. 2022, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Kemmerer, E.; Hernandez, E.; Ferriss, J.S.; Valakh, V.; Miyamoto, C.; Li, S.; Micaily, B. Use of Image-Guided Stereotactic Body Radiation Therapy in Lieu of Intracavitary Brachytherapy for the Treatment of Inoperable Endometrial Neoplasia. Int. J. Radiat. Oncol. 2012, 85, 129–135. [Google Scholar] [CrossRef]
- Lazzari, R.; Riva, G.; Augugliaro, M.; Vavassori, A.; Dicuonzo, S.; Cattani, F.; Comi, S.; Colombo, N.; Jereczek-Fossa, B.A. Intensity modulated radiation therapy boost in locally-advanced cervical cancer in the absence of brachytherapy. Int. J. Gynecol. Cancer 2020, 30, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, M.; Dogan, A.; Ozyigit, G.; Erturk, E.; Yildiz, F.; Selek, U.; Ulger, S.; Colak, F.; Zorlu, F. Comparison of intracavitary brachytherapy and stereotactic body radiotherapy dose distribution for cervical cancer. Brachytherapy 2011, 11, 125–129. [Google Scholar] [CrossRef]
- Dahbi, Z.; Fadila, K.; Vinh-Hung, V. Brachytherapy Versus Stereotactic Body Radiotherapy for Cervical Cancer Boost: A Dosimetric Comparison. Cureus 2023, 15, e37235. [Google Scholar] [CrossRef]
- Ito, K.; Nakajima, Y.; Ogawa, H.; Furusawa, A.; Murofushi, K.N.; Kito, S.; Kino, N.; Yasugi, T.; Uno, T.; Karasawa, K. Phase I/II study of stereotactic body radiotherapy boost in patients with cervical cancer ineligible for intracavitary brachytherapy. Jpn. J. Radiol. 2024, 42, 909–917. [Google Scholar] [CrossRef]
- Gill, B.S.; Lin, J.F.; Krivak, T.C.; Sukumvanich, P.; Laskey, R.A.; Ross, M.S.; Lesnock, J.L.; Beriwal, S. National Cancer Data Base Analysis of Radiation Therapy Consolidation Modality for Cervical Cancer: The Impact of New Technological Advancements. Int. J. Radiat. Oncol. 2014, 90, 1083–1090. [Google Scholar] [CrossRef]
- O’donnell, B.; Shiao, J.C.; Pezzi, T.A.; Waheed, N.; Sharma, S.; Bonnen, M.D.; Ludwig, M.S. Stereotactic Body Radiation Therapy, Intensity-Modulated Radiation Therapy, and Brachytherapy Boost Modalities in Invasive Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Major, T.; Fröhlich, G.; Ágoston, P.; Polgár, C.; Takácsi-Nagy, Z. The value of brachytherapy in the age of advanced external beam radiotherapy: A review of the literature in terms of dosimetry. Strahlenther. Onkol. 2021, 198, 93–109. [Google Scholar] [CrossRef]
- Ito, K.; Kito, S.; Nakajima, Y.; Shimizuguchi, T.; Ogawa, H.; Nihei, K.; Tanaka, H.; Kino, N.; Yasugi, T.; Karasawa, K. Determining the recommended dose of stereotactic body radiotherapy boost in patients with cervical cancer who are unsuitable for intracavitary brachytherapy: A phase I dose-escalation study. Ultrasound Med. Biol. 2019, 49, 856–861. [Google Scholar] [CrossRef]
- Chan, P.; Yeo, I.; Perkins, G.; Fyles, A.; Milosevic, M. Dosimetric comparison of intensity-modulated, conformal, and four-field pelvic radiotherapy boost plans for gynecologic cancer: A retrospective planning study. Radiat. Oncol. 2006, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Tanimoto, H.; Fujita, K.; Hashimoto, Y.; Murakami, Y.; Kenjo, M.; Kaneyasu, Y.; Wadasaki, K.; Ito, K. Early clinical outcomes of 3D-conformal radiotherapy using accelerated hyperfractionation without intracavitary brachytherapy for cervical cancer. Gynecol. Oncol. 2007, 104, 11–14. [Google Scholar] [CrossRef]
- Ito, H.; Kutuki, S.; Nishiguchi, I.; Shigematsu, N.; Kuribayashi, T.; Uematsu, M.; Nakayama, T.; Ka, W.-J.; Takemasa, K.; Ando, Y.; et al. Radiotherapy for cervical cancer with high-dose rate brachytherapy—Correlation between tumor size, dose and failure. Radiother. Oncol. 1994, 31, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Petereit, D.G.; Sarkaria, J.N.; Potter, D.M.; Schink, J.C. High-dose-rate versus low-dose-rate brachytherapy in the treatment of cervical cancer: Analysis of tumor recurrence—The University of Wisconsin experience. Int. J. Radiat. Oncol. 1999, 45, 1267–1274. [Google Scholar] [CrossRef]
- Toita, T.; Kakinohana, Y.; Ogawa, K.; Adachi, G.; Moromizato, H.; Nagai, Y.; Maehama, T.; Sakumoto, K.; Kanazawa, K.; Murayama, S. Combination external beam radiotherapy and high-dose-rate intracavitary brachytherapy for uterine cervical cancer: Analysis of dose and fractionation schedule. Int. J. Radiat. Oncol. 2003, 56, 1344–1353. [Google Scholar] [CrossRef]
- Pourquier, H.; Dubois, J.B.; Delard, R. Cancer of the uterine cervix: Dosimetric guidelines for prevention of late rectal and rectosigmoid complications as a result of radiotherapeutic treatment. Int. J. Radiat. Oncol. 1982, 8, 1887–1895. [Google Scholar] [CrossRef]
- Cheng, J.C.-H.; Peng, L.-C.; Chen, Y.-H.; Huang, D.Y.; Wu, J.-K.; Jian, J.J.-M. Unique role of proximal rectal dose in late rectal complications for patients with cervical cancer undergoing high-dose-rate intracavitary brachytherapy. Int. J. Radiat. Oncol. 2003, 57, 1010–1018. [Google Scholar] [CrossRef]
- Park, H.J.; Chang, A.R.; Seo, Y.; Cho, C.K.; Jang, W.-I.; Kim, M.S.; Choi, C. Stereotactic Body Radiotherapy for Recurrent or Oligometastatic Uterine Cervix Cancer: A Cooperative Study of the Korean Radiation Oncology Group (KROG 14-11). Anticancer Res. 2015, 35, 5103–5110. [Google Scholar]
- Gultekin, M.; Yilmaz, M.T.; Sari, S.Y.; Yildiz, D.; Ozyigit, G.; Yildiz, F. Stereotactic body radiotherapy boost in patients with cervical cancer. J. Obstet. Gynaecol. 2022, 42, 3033–3040. [Google Scholar] [CrossRef] [PubMed]
- Gadda, I.R.; Khan, N.A.; Wani, S.Q.; Baba, M.H. To evaluate the use of tandem and cylinder as an intracavitary brachytherapy device for carcinoma of the cervix with regard to local control and toxicities. J. Cancer Res. Ther. 2022, 18, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Mulani, J.; Mittal, P.; Singh, M.; Shinde, A.; Gurram, L.; Scaria, L.; Aravindakshan, D.; Kohle, S.; Rane, P.; et al. Early outcomes of abbreviated multi-fractionated brachytherapy schedule for cervix cancer during COVID-19 pandemic. Brachytherapy 2022, 22, 125–131. [Google Scholar] [CrossRef]
- Pötter, R.; Georg, P.; Dimopoulos, J.C.A.; Grimm, M.; Berger, D.; Nesvacil, N.; Georg, D.; Schmid, M.P.; Reinthaller, A.; Sturdza, A.; et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother. Oncol. 2011, 100, 116–123. [Google Scholar] [CrossRef]
- Kamrava, M.; Banerjee, R. Brachytherapy in the treatment of cervical cancer: A review. Int. J. Women’s Health 2014, 6, 555–564. [Google Scholar] [CrossRef]
- Kang, H.-C.; Shin, K.H.; Park, S.-Y.; Kim, J.-Y. 3D CT-based high-dose-rate brachytherapy for cervical cancer: Clinical impact on late rectal bleeding and local control. Radiother. Oncol. 2010, 97, 507–513. [Google Scholar] [CrossRef]
- Castelnau-Marchand, P.; Chargari, C.; Maroun, P.; Dumas, I.; Del Campo, E.R.; Cao, K.; Petit, C.; Martinetti, F.; Tafo-Guemnie, A.; Lefkopoulos, D.; et al. Clinical outcomes of definitive chemoradiation followed by intracavitary pulsed-dose rate image-guided adaptive brachytherapy in locally advanced cervical cancer. Gynecol. Oncol. 2015, 139, 288–294. [Google Scholar] [CrossRef]
- Simpson, D.R.; Scanderbeg, D.J.; Carmona, R.; McMurtrie, R.M.; Einck, J.; Mell, L.K.; McHale, M.T.; Saenz, C.C.; Plaxe, S.C.; Harrison, T.; et al. Clinical Outcomes of Computed Tomography-Based Volumetric Brachytherapy Planning for Cervical Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 150–157. [Google Scholar] [CrossRef]
- Charra-Brunaud, C.; Harter, V.; Delannes, M.; Haie-Meder, C.; Quetin, P.; Kerr, C.; Castelain, B.; Thomas, L.; Peiffert, D. Impact of 3D image-based PDR brachytherapy on outcome of patients treated for cervix carcinoma in France: Results of the French STIC prospective study. Radiother. Oncol. 2012, 103, 305–313. [Google Scholar] [CrossRef]
- Tan, L.T.; Coles, C.E.; Hart, C.; Tait, E. Clinical impact of computed tomography-based image-guided brachytherapy for cervix cancer using the tandem-ring applicator—The Addenbrooke’s experience. Clin. Oncol. (R. Coll. Radiol.) 2009, 21, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.; Eifel, P.J.; Lu, J.; Grigsby, P.W.; Levenback, C.; Stevens, R.E.; Rotman, M.; Gershenson, D.M.; Mutch, D.G. Pelvic Radiation with Concurrent Chemotherapy Compared with Pelvic and Para-Aortic Radiation for High-Risk Cervical Cancer. N. Engl. J. Med. 1999, 340, 1137–1143. [Google Scholar] [CrossRef]
- Chen, S.-W.; Liang, J.-A.; Hung, Y.-C.; Yeh, L.-S.; Chang, W.-C.; Lin, W.-C.; Chang, Y.-Y. Effectiveness of Image-guided Brachytherapy in Patients With Locally Advanced Cervical Squamous Cell Carcinoma Receiving Concurrent Chemoradiotherapy. Anticancer Res. 2019, 39, 3015–3024. [Google Scholar] [CrossRef] [PubMed]
- Panizza, D.; Faccenda, V.; Lucchini, R.; Daniotti, M.C.; Trivellato, S.; Caricato, P.; Pisoni, V.; De Ponti, E.; Arcangeli, S. Intrafraction Prostate Motion Management During Dose-Escalated Linac-Based Stereotactic Body Radiation Therapy. Front. Oncol. 2022, 12, 883725. [Google Scholar] [CrossRef]
- Dincer, N.; Ugurluer, G.; Mustafayev, T.Z.; Serkizyan, A.; Aydin, G.; Güngör, G.; Yapici, B.; Atalar, B.; Özyar, E. Dosimetric comparison of stereotactic MR-guided radiation therapy (SMART) and HDR brachytherapy boost in cervical cancer. Brachytherapy 2023, 23, 18–24. [Google Scholar] [CrossRef]
- Ma, T.M.; Harkenrider, M.M.; Yashar, C.M.; Viswanathan, A.N.; Mayadev, J.S. Understanding the underutilization of cervical brachytherapy for locally advanced cervical cancer. Brachytherapy 2019, 18, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.W.; Mayadev, J. Underutilization of brachytherapy for cervical cancer in the United States. Brachytherapy 2023, 22, 15–20. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology. Cervical Cancer, Version 1. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (accessed on 1 March 2025).
- Holschneider, C.H.; Petereit, D.G.; Chu, C.; Hsu, I.C.; Ioffe, Y.J.; Klopp, A.H.; Pothuri, B.; Chen, L.M.; Yashar, C. Brachytherapy: A critical component of primary radiation therapy for cervical cancer: From the Society of Gynecologic Oncology (SGO) and the American Brachytherapy Society (ABS). Gynecol. Oncol. 2019, 152, 540–547. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).