A Comprehensive Review of Margin Identification Methods in Soft Tissue Sarcoma
Simple Summary
Abstract
1. Introduction
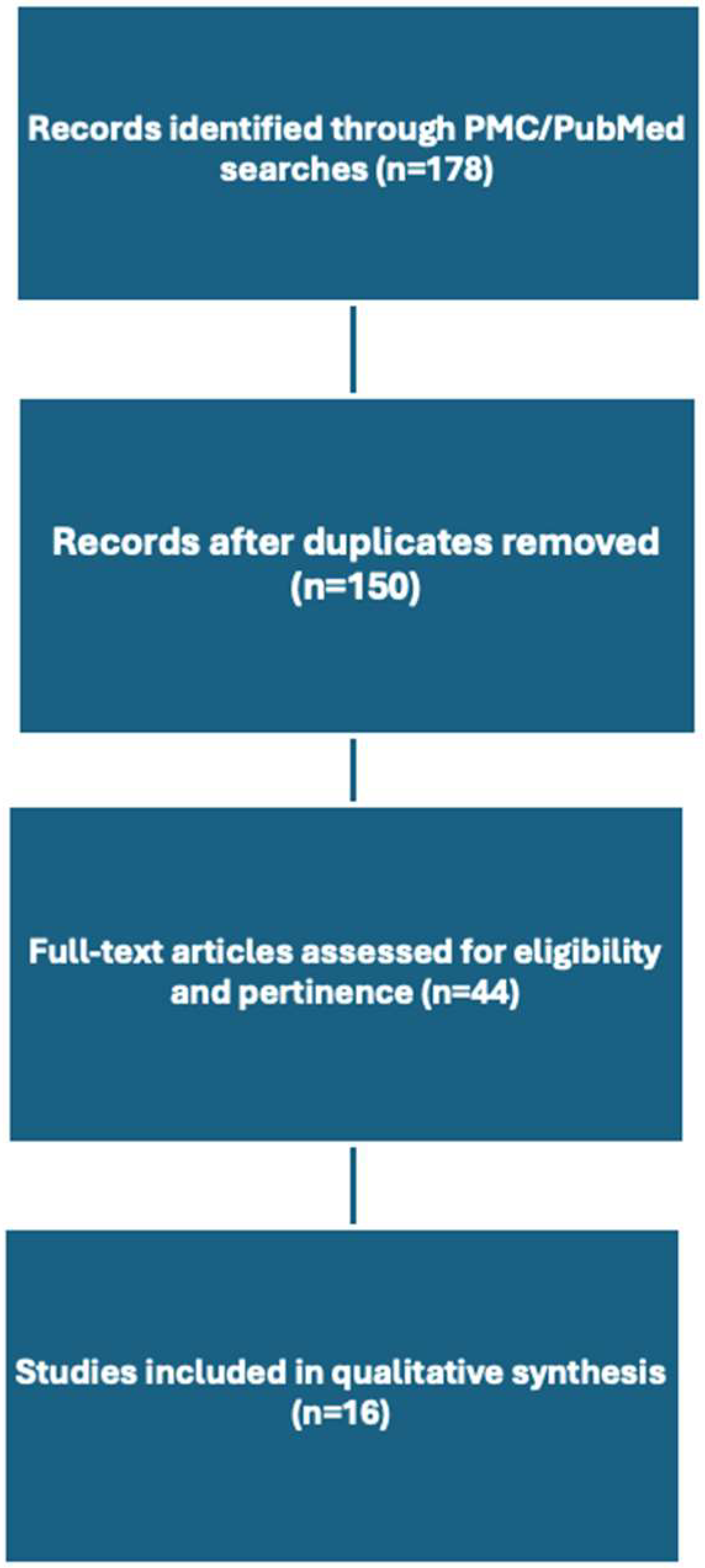
2. Materials and Methods
3. Results: Margin Assessment Techniques
3.1. Frozen Section and Histopathology
3.2. Fluorescence-Guided Surgery
3.2.1. Indocyanine Green
3.2.2. Acridine Orange
3.3. Spectroscopy
3.3.1. Raman
3.3.2. Diffuse Reflectance
3.3.3. Rapid Evaporative Ionization Mass Spectrometry (REIMS)
3.4. Optical Coherence Tomography (OCT)
3.5. Intraoperative Ultrasound
3.6. Artificial Intelligence, Augmented Reality, and Future Multimodal Approaches
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Demetri, G.D.; Antonia, S.; Benjamin, R.S.; Bui, M.M.; Casper, E.S.; Conrad, E.U.; DeLaney, T.F.; Ganjoo, K.N.; Heslin, M.J.; Hutchinson, R.J.; et al. Soft tissue sarcoma. J. Natl. Compr. Cancer Netw. 2010, 8, 630–674. [Google Scholar] [CrossRef]
- Key Statistics for Soft Tissue Sarcomas; American Cancer Society: Hagerstown, MD, USA, 2025.
- Soft Tissue Sarcoma Statistics; Canadian Cancer Society: Toronto, ON, Canada, 2025.
- Alkazemi, B.; Ghazawi, F.M.; Lagace, F.; Nechaev, V.; Zubarev, A.; Litvinov, I.V. Investigation of the Incidence and Geographic Distribution of Bone and Soft Tissue Sarcomas in Canada: A National Population-Based Study. Curr. Oncol. 2023, 30, 5631–5651. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Tao, P.; Wang, J.; Fan, P.; Luo, W.; Liu, W.; Lu, W.; Ma, L.; Zhang, Y.; Tong, H. An Update on the Global Disparities in Sarcoma Disease Burden and Research Across World Countries and Regions; Social Science Research Network: Rochester, NY, USA, 2025. [Google Scholar]
- Nedea, E.A.; DeLaney, T.F. Sarcoma and skin radiation oncology. Hematol. Oncol. Clin. N. Am. 2006, 20, 401–429. [Google Scholar] [CrossRef]
- Wittenberg, S.; Paraskevaidis, M.; Jarosch, A.; Flörcken, A.; Brandes, F.; Striefler, J.; Kaul, D.; Roohani, S.; Khakzad, T.; Märdian, S.; et al. Surgical Margins in Soft Tissue Sarcoma Management and Corresponding Local and Systemic Recurrence Rates: A Retrospective Study Covering 11 Years and 169 Patients in a Single Institution. Life 2022, 12, 1694. [Google Scholar] [CrossRef]
- Pasquali, S.; Moura, D.S.; Danks, M.R.; Manasterski, P.J.; Zaffaroni, N.; Stacchiotti, S.; Mondaza-Hernandez, J.L.; Kerrison, W.G.J.; Martin-Broto, J.; Huang, P.H.; et al. Preclinical models of soft tissue sarcomas—Generation and applications to enhance translational research. Crit. Rev. Oncol./Hematol. 2025, 207, 104621. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Glehen, O.; Zivanovic, O.; Brennan, D.; Nadeau, C.; Van Driel, W.; Bakrin, N. The 2022 PSOGI International Consensus on HIPEC Regimens for Peritoneal Malignancies: Epithelial Ovarian Cancer. Ann. Surg. Oncol. 2023, 30, 8115–8137. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.D.; Manning, M.A.; Al-Refaie, W.B.; Miettinen, M.M. Soft-Tissue Sarcomas of the Abdomen and Pelvis: Radiologic-Pathologic Features, Part 1—Common Sarcomas: From the Radiologic Pathology Archives. Radiographics 2017, 37, 462–483. [Google Scholar] [CrossRef]
- Abaricia, S.; Van Tine, B.A. Management of localized extremity and retroperitoneal soft tissue sarcoma. Curr. Probl. Cancer 2019, 43, 273–282. [Google Scholar] [CrossRef]
- Dominguez, D.A.; Sampath, S.; Agulnik, M.; Liang, Y.; Nguyen, B.; Trisal, V.; Melstrom, L.G.; Lewis, A.G.; Paz, I.B.; Roberts, R.F.; et al. Surgical Management of Retroperitoneal Sarcoma. Curr. Oncol. 2023, 30, 4618–4631. [Google Scholar] [CrossRef]
- Swallow, C.J.; Strauss, D.C.; Bonvalot, S.; Rutkowski, P.; Desai, A.; Gladdy, R.A.; Gonzalez, R.; Gyorki, D.E.; Fairweather, M.; van Houdt, W.J.; et al. Management of Primary Retroperitoneal Sarcoma (RPS) in the Adult: An Updated Consensus Approach from the Transatlantic Australasian RPS Working Group. Ann. Surg. Oncol. 2021, 28, 7873–7888. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network Soft Tissue Sarcoma P. NCCN Clinical Practice Guidelines in Oncology: Soft Tissue Sarcoma. Version 2.2025 Plymouth Meeting (PA): NCCN. 2025. Available online: https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf (accessed on 8 September 2025).
- Hayes, A.J.; Nixon, I.F.; Strauss, D.C.; Seddon, B.M.; Desai, A.; Benson, C.; Judson, I.R.; Dangoor, A. UK guidelines for the management of soft tissue sarcomas. Br. J. Cancer 2025, 132, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Trans-Atlantic RPSWG. Management of Recurrent Retroperitoneal Sarcoma (RPS) in the Adult: A Consensus Approach from the Trans-Atlantic RPS Working Group. Ann. Surg. Oncol. 2016, 23, 3531–3540. [Google Scholar] [CrossRef] [PubMed]
- Gyorki, D.E.; Brennan, M.F. Management of recurrent retroperitoneal sarcoma. J. Surg. Oncol. 2014, 109, 53–59. [Google Scholar] [CrossRef]
- Gronchi, A.; Strauss, D.C.; Miceli, R.; Bonvalot, S.; Swallow, C.J.; Hohenberger, P.; Van Coevorden, F.; Rutkowski, P.; Callegaro, D.; Hayes, A.J.; et al. Variability in Patterns of Recurrence After Resection of Primary Retroperitoneal Sarcoma (RPS): A Report on 1007 Patients from the Multi-institutional Collaborative RPS Working Group. Ann. Surg. 2016, 263, 1002–1009. [Google Scholar] [CrossRef]
- Bonvalot, S.; Roland, C.; Raut, C.; Le Pechoux, C.; Tzanis, D.; Frezza, A.M.; Gronchi, A. Histology-tailored multidisciplinary management of primary retroperitoneal sarcomas. Eur. J. Surg. Oncol. 2023, 49, 1061–1067. [Google Scholar] [CrossRef]
- Biau, D.J.; Ferguson, P.C.; Chung, P.; Griffin, A.M.; Catton, C.N.; O’Sullivan, B.; Wunder, J.S. Local recurrence of localized soft tissue sarcoma: A new look at old predictors. Cancer 2012, 118, 5867–5877. [Google Scholar] [CrossRef]
- Gronchi, A.; Casali, P.G.; Fiore, M.; Mariani, L.; Lo Vullo, S.; Bertulli, R.; Colecchia, M.; Lozza, L.; Olmi, P.; Santinami, M.; et al. Retroperitoneal soft tissue sarcomas: Patterns of recurrence in 167 patients treated at a single institution. Cancer 2004, 100, 2448–2455. [Google Scholar] [CrossRef] [PubMed]
- Novais, E.N.; Demiralp, B.; Alderete, J.; Larson, M.C.; Rose, P.S.; Sim, F.H. Do surgical margin and local recurrence influence survival in soft tissue sarcomas? Clin. Orthop. Relat. Res. 2010, 468, 3003–3011. [Google Scholar] [CrossRef]
- Zagars, G.K.; Ballo, M.T.; Pisters, P.W.T.; Pollock, R.E.; Patel, S.R.; Benjamin, R.S.; Evans, H.L. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: An analysis of 1225 patients. Cancer 2003, 97, 2530–2543. [Google Scholar] [CrossRef]
- Voss, R.K.; Callegaro, D.; Chiang, Y.J.; Fiore, M.; Miceli, R.; Keung, E.Z.; Feig, B.W.; Torres, K.E.; Scally, C.P.; Hunt, K.K.; et al. Sarculator is a Good Model to Predict Survival in Resected Extremity and Trunk Sarcomas in US Patients. Ann. Surg. Oncol. 2022. [Google Scholar] [CrossRef]
- Sambri, A.; Caldari, E.; Fiore, M.; Zucchini, R.; Giannini, C.; Pirini, M.G.; Spinnato, P.; Cappelli, A.; Donati, D.M.; De Paolis, M. Margin Assessment in Soft Tissue Sarcomas: Review of the Literature. Cancers 2021, 13, 1687. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, A.; Leung, D.H.; Hoos, A.; Jaques, D.P.; Lewis, J.J.; Brennan, M.F. Analysis of the prognostic significance of microscopic margins in 2084 localized primary adult soft tissue sarcomas. Ann. Surg. 2002, 235, 424–434. [Google Scholar] [CrossRef]
- Trovik, C.S.; Scanadinavian Sarcoma Group Project. Local recurrence of soft tissue sarcoma. A Scandinavian Sarcoma Group Project. Acta Orthop. Scand. Suppl. 2001, 72, 1–31. [Google Scholar]
- Acidi, B.; Faron, M.; Mir, O.; Levy, A.; Ghallab, M.; Haddag-Miliani, L.; Ngo, C.; Kasraoui, I.; Kanaan, C.; Verret, B.; et al. Intraoperative motive for incomplete resection in primary retroperitoneal sarcoma. Progrès Urol. 2023, 33, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Goertz, O.; Pieper, A.; von der Lohe, L.; Stricker, I.; Dadras, M.; Behr, B.; Lehnhardt, M.; Harati, K. The Impact of Surgical Margins and Adjuvant Radiotherapy in Patients with Undifferentiated Pleomorphic Sarcomas of the Extremities: A Single-Institutional Analysis of 192 Patients. Cancers 2020, 12, 362. [Google Scholar] [CrossRef]
- Olson, C.R.; Suarez-Kelly, L.P.; Ethun, C.G.; Shelby, R.D.; Yu, P.Y.; Hughes, T.M.; Palettas, M.; Tran, T.B.; Poultsides, G.; Tseng, J.; et al. Resection Status Does Not Impact Recurrence in Well-Differentiated Liposarcoma of the Extremity. Am. Surg. 2021, 87, 1752–1759. [Google Scholar] [CrossRef]
- Guidelines Detail; NCCN National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2025.
- Bourgeois, P.; Veys, I.; Noterman, D.; De Neubourg, F.; Chintinne, M.; Vankerckhove, S.; Nogaret, J.-M. Near-Infrared Fluorescence Imaging of Breast Cancer and Axillary Lymph Nodes After Intravenous Injection of Free Indocyanine Green. Front. Oncol. 2021, 11, 602906. [Google Scholar] [CrossRef]
- Reinhart, M.B.; Huntington, C.R.; Blair, L.J.; Heniford, B.T.; Augenstein, V.A. Indocyanine Green: Historical Context, Current Applications, and Future Considerations. Surg. Innov. 2016, 23, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, N.; Takizawa, H.; Sawada, T.; Matsumoto, D.; Tsuboi, M.; Toba, H.; Yoshida, M.; Kawakami, Y.; Kondo, K.; Tangoku, A. Indocyanine green fluorescence imaging for resection of pulmonary metastasis of hepatocellular carcinoma. J. Thorac. Dis. 2019, 11, 944–949. [Google Scholar] [CrossRef]
- Chan, C.; Brookes, M.; Tanwani, R.; Hope, C.; Pringle, T.; Knight, J.; Rankin, K. Investigating the mechanisms of indocyanine green (ICG) cellular uptake in sarcoma. bioRxiv 2021. [Google Scholar] [CrossRef]
- Zhang, J.; He, J.; Chen, J.; Zhong, Y.; He, J.; Li, S. Application of indocyanine green injection guided by electromagnetic navigation bronchoscopy in localization of pulmonary nodules. Transl. Lung Cancer Res. 2021, 10, 4414–4422. [Google Scholar] [CrossRef]
- Nicoli, F.; Saleh, D.B.; Baljer, B.; Chan, C.D.; Beckingsale, T.; Ghosh, K.M.; Ragbir, M.; Rankin, K.S. Intraoperative Near-infrared Fluorescence (NIR) Imaging with Indocyanine Green (ICG) Can Identify Bone and Soft Tissue Sarcomas Which May Provide Guidance for Oncological Resection. Ann. Surg. 2021, 273, e63–e68. [Google Scholar] [CrossRef]
- Mesa, K.J.; Selmic, L.E.; Pande, P.; Monroy, G.L.; Reagan, J.; Samuelson, J.; Driskell, E.; Li, J.; Marjanovic, M.; Chaney, E.J.; et al. Intraoperative optical coherence tomography for soft tissue sarcoma differentiation and margin identification. Lasers Surg. Med. 2017, 49, 240–248. [Google Scholar] [CrossRef]
- Nguyen, J.Q.; Gowani, Z.S.; O’Connor, M.; Pence, I.J.; Nguyen, T.-Q.; Holt, G.E.; Schwartz, H.S.; Halpern, J.L.; Mahadevan-Jansen, A. Intraoperative Raman Spectroscopy of Soft Tissue Sarcomas. Lasers Surg. Med. 2016, 48, 774–781. [Google Scholar] [CrossRef]
- Li, L.; Mustahsan, V.M.; He, G.; Tavernier, F.B.; Singh, G.; Boyce, B.F.; Khan, F.; Kao, I. Classification of Soft Tissue Sarcoma Specimens with Raman Spectroscopy as Smart Sensing Technology. Cyborg Bionic Syst. 2021, 2021, 9816913. [Google Scholar] [CrossRef] [PubMed]
- Dulude, J.P.; Le Moel, A.; Dallaire, F.; Doyon, J.; Urmey, K.; Marple, E.; Leblanc, G.; Basile, G.; Mottard, S.; Isler, M.; et al. Intraoperative use of high-speed Raman spectroscopy during soft tissue sarcoma resection. Sci. Rep. 2025, 15, 8789. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13 (Suppl. S1), S31–S34. [Google Scholar] [CrossRef]
- Bui, M.M.; Smith, P.; Agresta, S.V.; Cheong, D.; Letson, G.D. Practical issues of intraoperative frozen section diagnosis of bone and soft tissue lesions. Cancer Control 2008, 15, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Zeitlinger, L.; Chavez, G.M.; Wilson, M.D.; Darrow, M.; Canter, R.J.; Randall, R.L.; Thorpe, S.W. Intraoperative Peripheral Frozen Margin Assessment in Soft Tissue Sarcoma. J. Surg. Oncol. 2025, 131, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Hermanek, P.; Wittekind, C. The pathologist and the residual tumor (R) classification. Pathol. Res. Pract. 1994, 190, 115–123. [Google Scholar] [CrossRef]
- Onda, N.; Kimura, M.; Yoshida, T.; Shibutani, M. Preferential tumor cellular uptake and retention of indocyanine green for in vivo tumor imaging. Int. J. Cancer 2016, 139, 673–682. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kawaguchi, Y.; Kobayashi, Y.; Matsumura, M.; Ishizawa, T.; Akamatsu, N.; Kaneko, J.; Arita, J.; Sakamoto, Y.; Kokudo, N.; et al. Identification of liver lesions using fluorescence imaging: Comparison of methods for administering indocyanine green. HPB 2021, 23, 262–269. [Google Scholar] [CrossRef]
- Cho, M.; Kim, K.Y.; Park, S.H.; Kim, Y.M.; Kim, H.I.; Hyung, W.J. Securing Resection Margin Using Indocyanine Green Diffusion Range on Gastric Wall during NIR Fluorescence-Guided Surgery in Early Gastric Cancer Patients. Cancers 2022, 14, 5223. [Google Scholar] [CrossRef]
- Brookes, M.J.; Chan, C.D.; Nicoli, F.; Crowley, T.P.; Ghosh, K.M.; Beckingsale, T.; Saleh, D.; Dildey, P.; Gupta, S.; Ragbir, M.; et al. Intraoperative Near-Infrared Fluorescence Guided Surgery Using Indocyanine Green (ICG) for the Resection of Sarcomas May Reduce the Positive Margin Rate: An Extended Case Series. Cancers 2021, 13, 6284. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.F.; Li, W.T.; Bhogal, S.; Royes, B.; Heim, T.; Silvaggio, M.; Malek, M.; Dhupar, R.; Lee, S.J.; McGough, R.L.; et al. Intraoperative Evaluation of Soft Tissue Sarcoma Surgical Margins with Indocyanine Green Fluorescence Imaging. Cancers 2023, 15, 582. [Google Scholar] [CrossRef] [PubMed]
- Singaravelu, A.; Dalli, J.; Potter, S.; Cahill, R.A. Artificial intelligence for optimum tissue excision with indocyanine green fluorescence angiography for flap reconstructions: Proof of concept. JPRAS Open 2024, 41, 389–393. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, H.; Peng, B.; Huang, X.; Hu, Y.; Zheng, C.; Zhang, Z. Illuminating the future of precision cancer surgery with fluorescence imaging and artificial intelligence convergence. NPJ Precis. Oncol. 2024, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; He, L.; Ma, Q.; Wang, Y.; Li, K.; Wang, Z.; Chen, X.; Zhu, S.; Zhan, Y.; Cao, X. Indocyanine green-based fluorescence imaging improved by deep learning. J. Biophotonics 2023, 16, e202300066. [Google Scholar] [CrossRef]
- Byvaltsev, V.A.; Bardonova, L.A.; Onaka, N.R.; Polkin, R.A.; Ochkal, S.V.; Shepelev, V.V.; Aliyev, M.A.; Potapov, A.A. Acridine Orange: A Review of Novel Applications for Surgical Cancer Imaging and Therapy. Front. Oncol. 2019, 9, 925. [Google Scholar] [CrossRef]
- Kusuzaki, K.; Hosogi, S.; Ashihara, E.; Matsubara, T.; Satonaka, H.; Nakamura, T.; Matsumine, A.; Sudo, A.; Uchida, A.; Murata, H.; et al. Translational Research of Photodynamic Therapy with Acridine Orange which Targets Cancer Acidity. Curr. Pharm. Des. 2012, 18, 1414–1420. [Google Scholar] [CrossRef]
- Tsuchie, H.; Emori, M.; Miyakoshi, N.; Nagasawa, H.; Okada, K.; Murahashi, Y.; Mizushima, E.; Shimizu, J.; Yamashita, T.; Shimada, Y. Impact of Acridine Orange in Patients With Soft Tissue Sarcoma Treated with Marginal Resection. Anticancer Res. 2019, 39, 6365–6372. [Google Scholar] [CrossRef]
- Nakamura, T.; Kusuzaki, K.; Matsubara, T.; Murata, H.; Hagi, T.; Asanuma, K.; Sudo, A. Long-term clinical outcome in patients with high-grade soft tissue sarcoma who were treated with surgical adjuvant therapy using acridine orange after intra-lesional or marginal resection. Photodiagnosis Photodyn. Ther. 2018, 23, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Kusuzaki, K.; Matsumine, A.; Nakamura, T.; Sudo, A. Can a Less Radical Surgery Using Photodynamic Therapy with Acridine Orange Be Equal to a Wide-margin Resection? Clin. Orthop. Relat. Res. 2013, 471, 792–802. [Google Scholar] [CrossRef]
- Ember, K.J.I.; Hoeve, M.A.; McAughtrie, S.L.; Bergholt, M.S.; Dwyer, B.J.; Stevens, M.M.; Faulds, K.; Forbes, S.J.; Campbell, C.J. Raman spectroscopy and regenerative medicine: A review. NPJ Regen. Med. 2017, 2, 12. [Google Scholar] [CrossRef]
- Lim, Z.Y.; Mohan, S.; Balasubramaniam, S.; Ahmed, S.; Siew, C.C.H.; Shelat, V.G. Indocyanine green dye and its application in gastrointestinal surgery: The future is bright green. World J. Gastrointest. Surg. 2023, 15, 1841–1857. [Google Scholar] [CrossRef]
- Ahmed, T.; Pai, M.V.; Mallik, E.; Varghese, G.M.; Ashish, S.; Acharya, A.; Krishna, A. Applications of indocyanine green in surgery: A single center case series. Ann. Med. Surg. 2022, 77, 103602. [Google Scholar] [CrossRef]
- Santos, I.P.; van Doorn, R.; Caspers, P.J.; Bakker Schut, T.C.; Barroso, E.M.; Nijsten, T.E.C.; Noordhoek Hegt, V.; Koljenovic, S.; Puppels, G.J. Improving clinical diagnosis of early-stage cutaneous melanoma based on Raman spectroscopy. Br. J. Cancer 2018, 119, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, W.C.; Jones, V.; Anderson, S.M.; Echevarria, A.; Miller, N.L.; Stashko, C.; Schmolze, D.; Cha, P.D.; Kothari, R.; Fong, Y.; et al. Raman Spectroscopy for Rapid Evaluation of Surgical Margins during Breast Cancer Lumpectomy. Sci. Rep. 2019, 9, 14639. [Google Scholar] [CrossRef] [PubMed]
- Livermore, L.J.; Isabelle, M.; Bell, I.M.; Scott, C.; Walsby-Tickle, J.; Gannon, J.; Plaha, P.; Vallance, C.; Ansorge, O. Rapid intraoperative molecular genetic classification of gliomas using Raman spectroscopy. Neurooncol. Adv. 2019, 1, vdz008. [Google Scholar] [CrossRef]
- Noothalapati, H.; Iwasaki, K.; Yamamoto, T. Non-invasive diagnosis of colorectal cancer by Raman spectroscopy: Recent developments in liquid biopsy and endoscopy approaches. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 258, 119818. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, Q.; Li, B.; Zhao, X. Raman Spectroscopy: A Novel Technology for Gastric Cancer Diagnosis. Front. Bioeng. Biotechnol. 2022, 10, 856591. [Google Scholar] [CrossRef]
- Picot, F.; Shams, R.; Dallaire, F.; Sheehy, G.; Trang, T.; Grajales, D.; Birlea, M.; Trudel, D.; Menard, C.; Kadoury, S.; et al. Image-guided Raman spectroscopy navigation system to improve transperineal prostate cancer detection. Part 1: Raman spectroscopy fiber-optics system and in situ tissue characterization. J. Biomed. Opt. 2022, 27, 095003. [Google Scholar] [CrossRef]
- Ma, X.; Sun, X.; Wang, H.; Wang, Y.; Chen, D.; Li, Q. Raman Spectroscopy for Pharmaceutical Quantitative Analysis by Low-Rank Estimation. Front. Chem. 2018, 6, 400. [Google Scholar] [CrossRef]
- Wills, H.; Kast, R.; Stewart, C.; Rabah, R.; Pandya, A.; Poulik, J.; Auner, G.; Klein, M.D. Raman spectroscopy detects and distinguishes neuroblastoma and related tissues in fresh and (banked) frozen specimens. J. Pediatr. Surg. 2009, 44, 386–391. [Google Scholar] [CrossRef]
- Daoust, F.; Dallaire, F.; Tavera, H.; Ember, K.; Guiot, M.C.; Petrecca, K.; Leblond, F. Preliminary study demonstrating cancer cells detection at the margins of whole glioblastoma specimens with Raman spectroscopy imaging. Sci. Rep. 2025, 15, 6453. [Google Scholar] [CrossRef]
- Evers, D.; Hendriks, B.; Lucassen, G.; Ruers, T. Optical spectroscopy: Current advances and future applications in cancer diagnostics and therapy. Future Oncol. 2012, 8, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Geldof, F.; Witteveen, M.; Sterenborg, H.J.C.M.; Ruers, T.J.M.; Dashtbozorg, B. Diffuse reflection spectroscopy at the fingertip: Design and performance of a compact side-firing probe for tissue discrimination during colorectal cancer surgery. Biomed. Opt. Express 2022, 14, 128–147. [Google Scholar] [CrossRef] [PubMed]
- de Boer, L.L.; Bydlon, T.M.; van Duijnhoven, F.; Vranken Peeters, M.-J.T.F.D.; Loo, C.E.; Winter-Warnars, G.A.O.; Sanders, J.; Sterenborg, H.J.C.M.; Hendriks, B.H.W.; Ruers, T.J.M. Towards the use of diffuse reflectance spectroscopy for real-time in vivo detection of breast cancer during surgery. J. Transl. Med. 2018, 16, 367. [Google Scholar] [CrossRef] [PubMed]
- Geldof, F.; Schrage, Y.M.; van Houdt, W.J.; Sterenborg, H.; Dashtbozorg, B.; Ruers, T.J.M. Toward the use of diffuse reflection spectroscopy for intra-operative tissue discrimination during sarcoma surgery. J. Biomed. Opt. 2024, 29, 027001. [Google Scholar] [CrossRef]
- Das, N.K.; Dai, Y.; Liu, P.; Hu, C.; Tong, L.; Chen, X.; Smith, Z.J. Raman Plus X: Biomedical Applications of Multimodal Raman Spectroscopy. Sensors 2017, 17, 1592. [Google Scholar] [CrossRef]
- Balog, J.; Szaniszlo, T.; Schaefer, K.C.; Denes, J.; Lopata, A.; Godorhazy, L.; Szalay, D.; Balogh, L.; Sasi-Szabo, L.; Toth, M.; et al. Identification of biological tissues by rapid evaporative ionization mass spectrometry. Anal. Chem. 2010, 82, 7343–7350. [Google Scholar] [CrossRef]
- Vaysse, P.-M.; van den Hout, M.F.C.M.; Engelen, S.M.E.; Keymeulen, K.B.M.I.; Bemelmans, M.H.A.; Heeren, R.M.A.; Olde Damink, S.W.M.; Porta Siegel, T. Lipid profiling of electrosurgical vapors for real-time assistance of soft tissue sarcoma resection. J. Surg. Oncol. 2024, 129, 499–508. [Google Scholar] [CrossRef]
- Joo, I. The role of intraoperative ultrasonography in the diagnosis and management of focal hepatic lesions. Ultrasonography 2015, 34, 246–257. [Google Scholar] [CrossRef]
- Bhosale, P.R.; Wei, W.; Ernst, R.D.; Bathala, T.K.; Reading, R.M.; Wood, C.G.; Bedi, D.G. Intraoperative sonography during open partial nephrectomy for renal cell cancer: Does it alter surgical management? AJR Am. J. Roentgenol. 2014, 203, 822–827. [Google Scholar] [CrossRef]
- Coco, D.; Leanza, S. Routine Intraoperative Ultrasound for the Detection of Liver Metastases during Resection of Primary Colorectal Cancer—A Systematic Review. Maedica 2020, 15, 250–252. [Google Scholar] [CrossRef]
- Rahusen, F.D.; Bremers, A.J.A.; Fabry, H.F.J.; van Amerongen, A.H.M.T.; Boom, R.P.A.; Meijer, S. Ultrasound-guided lumpectomy of nonpalpable breast cancer versus wire-guided resection: A randomized clinical trial. Ann. Surg. Oncol. 2002, 9, 994–998. [Google Scholar] [CrossRef]
- Lubner, M.G.; Mankowski Gettle, L.; Kim, D.H.; Ziemlewicz, T.J.; Dahiya, N.; Pickhardt, P. Diagnostic and procedural intraoperative ultrasound: Technique, tips and tricks for optimizing results. Br. J. Radiol. 2021, 94, 20201406. [Google Scholar] [CrossRef]
- Jagadeesan, J.; Davies, J.A.; Raurell, A.; Ashford, R.U. Intraoperative portable ultrasonography localization of clinically impalpable soft-tissue tumors. World J. Surg. Oncol. 2012, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Farfalli, G.L.; Aponte, T.L.A.; Rasumoff, A.; Ayerza, M.A.; Muscolo, D.L. Intraoperative Ultrasound Assistance for Excision of Impalpable Musculoskeletal Soft Tissue Tumors. Orthopedics 2011, 34, e570–e573. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, A.; Yamamoto, N.; Hayashi, K.; Miwa, S.; Igarashi, K.; Yonezawa, H.; Morinaga, S.; Araki, Y.; Asano, Y.; Ikeda, H.; et al. Intraoperative ultrasonography-guided surgery for malignant soft tissue tumor. J. Surg. Oncol. 2020, 122, 1791–1801. [Google Scholar] [CrossRef] [PubMed]
- Giannotti, G.D.; Ailabouni, L.D.; Salti, G.I. Ultrasound-guided excision of nonpalpable malignant soft tissue tumors of the abdomen. Future Oncol. 2010, 6, 1513–1515. [Google Scholar] [CrossRef] [PubMed]
- Michot, A.; Le, V.L.; Coindre, J.M.; Velasco, V.; Soussi, M.; Mesli, N.; Italiano, A.; Toulmonde, M.; Le Cesne, A.; Bonvalot, S.; et al. Prognostic prediction in soft-tissue sarcomas using deep learning and digital pathology of tumor and margin areas. Sci. Rep. 2025, 15, 38534. [Google Scholar] [CrossRef] [PubMed]
- Jeremiasse, B.; Hulsker, C.C.C.; van den Bosch, C.H.; Buser, M.A.D.; van der Ven, C.P.; Bökkerink, G.M.J.; Wijnen, M.H.W.A.; Van der Steeg, A.F.W. Fluorescence guided surgery using indocyanine green for pulmonary osteosarcoma metastasectomy in pediatric patients: A feasibility study. EJC Paediatr. Oncol. 2023, 2, 100019. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, D.; Fang, T.; Chen, X.; Jia, H.; Tian, X.; Hao, C.; Yue, S. Rapid and Precise Diagnosis of Retroperitoneal Liposarcoma with Deep-Learned Label-Free Molecular Microscopy. Anal. Chem. 2024, 96, 9353–9361. [Google Scholar] [CrossRef] [PubMed]
| Author | Publication Year | Technique | Key Results |
|---|---|---|---|
| Nicoli et al. [37] | 2021 | ICG Fluorescence |
|
| Brookes et al. [49] | 2021 | ICG Fluorescence |
|
| Gong et al. [50] | 2023 | ICG Fluorescence |
|
| Matsubara et al. [58] | 2013 | Acridine Orange |
|
| Tsuchie et al. [56] | 2019 | Acridine Orange |
|
| Nguyen et al. [39] | 2017 | Raman spectroscopy |
|
| Dulude et al. [41] | 2025 | Raman spectroscopy |
|
| Geldof et al. [72] | 2024 | Diffuse reflectance spectroscopy |
|
| Vaysse et al. [77] | 2024 | Rapid Evaporative Ionization Mass Spectrometry |
|
| Mesa et al. [38] | 2017 | Optical Coherence Tomography |
|
| Farfalli et al. [84] | 2011 | Intraoperative ultrasound |
|
| Takeuchi et al. [85] | 2020 | Intraoperative ultrasound |
|
| Method | Technique/Principle | Main Advantages | Main Limitations |
|---|---|---|---|
| Frozen Section |
|
|
|
| ICG Fluorescence |
|
|
|
| Acridine Orange (AO) |
|
|
|
| Raman Spectroscopy |
|
|
|
| Diffuse Reflectance (DRS) |
|
|
|
| REIMS |
|
|
|
| OCT |
|
|
|
| Intraoperative Ultrasound (IOUS) |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, Y.; Dulude, J.-P.; Leblond, F.; Gervais, M.-K. A Comprehensive Review of Margin Identification Methods in Soft Tissue Sarcoma. Curr. Oncol. 2025, 32, 703. https://doi.org/10.3390/curroncol32120703
Osman Y, Dulude J-P, Leblond F, Gervais M-K. A Comprehensive Review of Margin Identification Methods in Soft Tissue Sarcoma. Current Oncology. 2025; 32(12):703. https://doi.org/10.3390/curroncol32120703
Chicago/Turabian StyleOsman, Yasmin, Jean-Philippe Dulude, Frédéric Leblond, and Mai-Kim Gervais. 2025. "A Comprehensive Review of Margin Identification Methods in Soft Tissue Sarcoma" Current Oncology 32, no. 12: 703. https://doi.org/10.3390/curroncol32120703
APA StyleOsman, Y., Dulude, J.-P., Leblond, F., & Gervais, M.-K. (2025). A Comprehensive Review of Margin Identification Methods in Soft Tissue Sarcoma. Current Oncology, 32(12), 703. https://doi.org/10.3390/curroncol32120703






