The Surveillance After Extremity Tumor Surgery (SAFETY) Pilot International Multi-Center Randomized Controlled Trial
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Randomization
2.4. Interventions
- (1)
- clinic visit and CXR every three months for two years;
- (2)
- clinic visit and CXR every six months for two years;
- (3)
- clinic visit and chest CT scan every three months for two years; or
- (4)
- clinic visit and chest CT scan every six months for two years.
2.5. Outcomes and Assessment
2.6. Statistical Analysis and Study Sample Size
3. Results
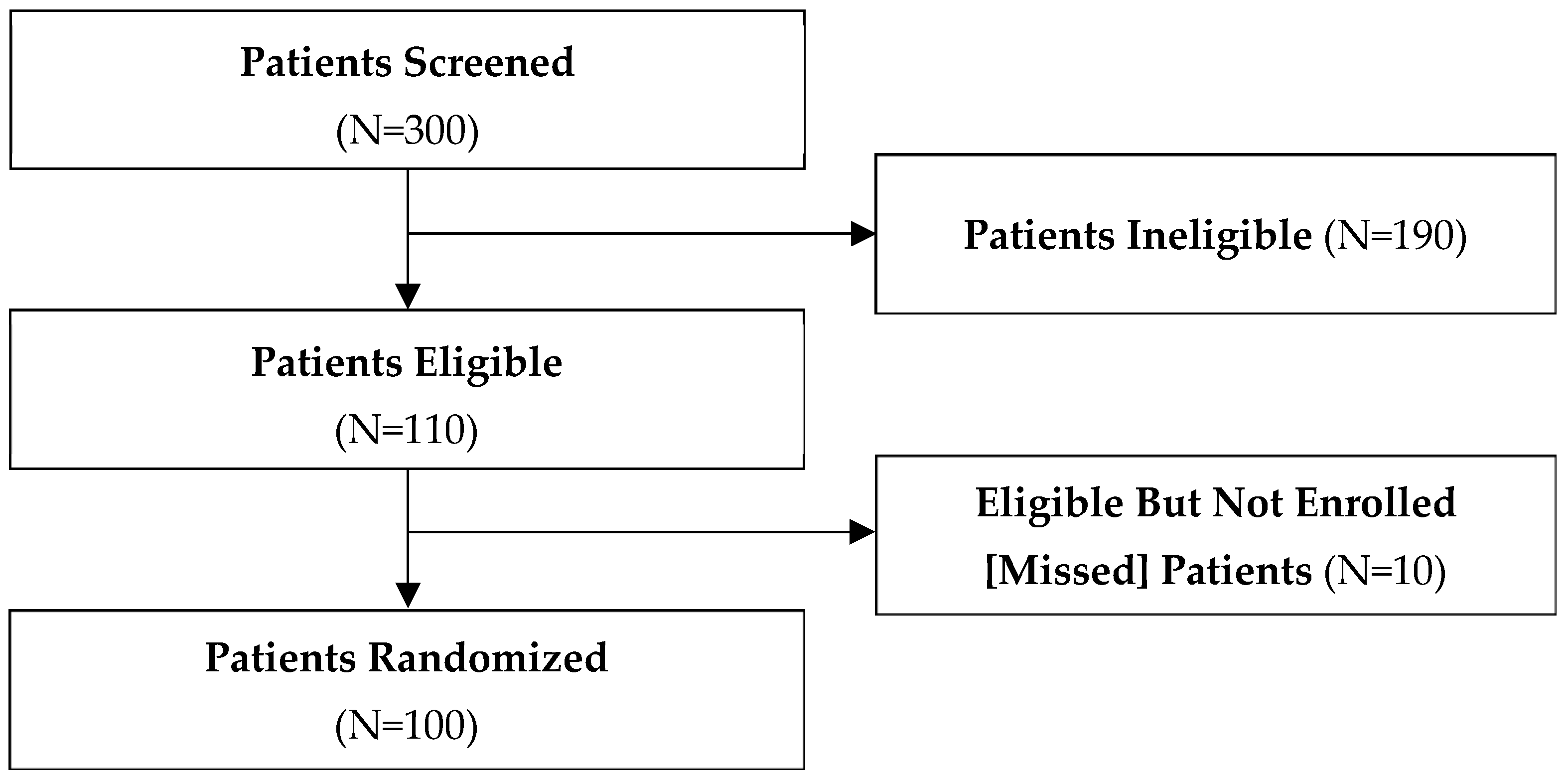
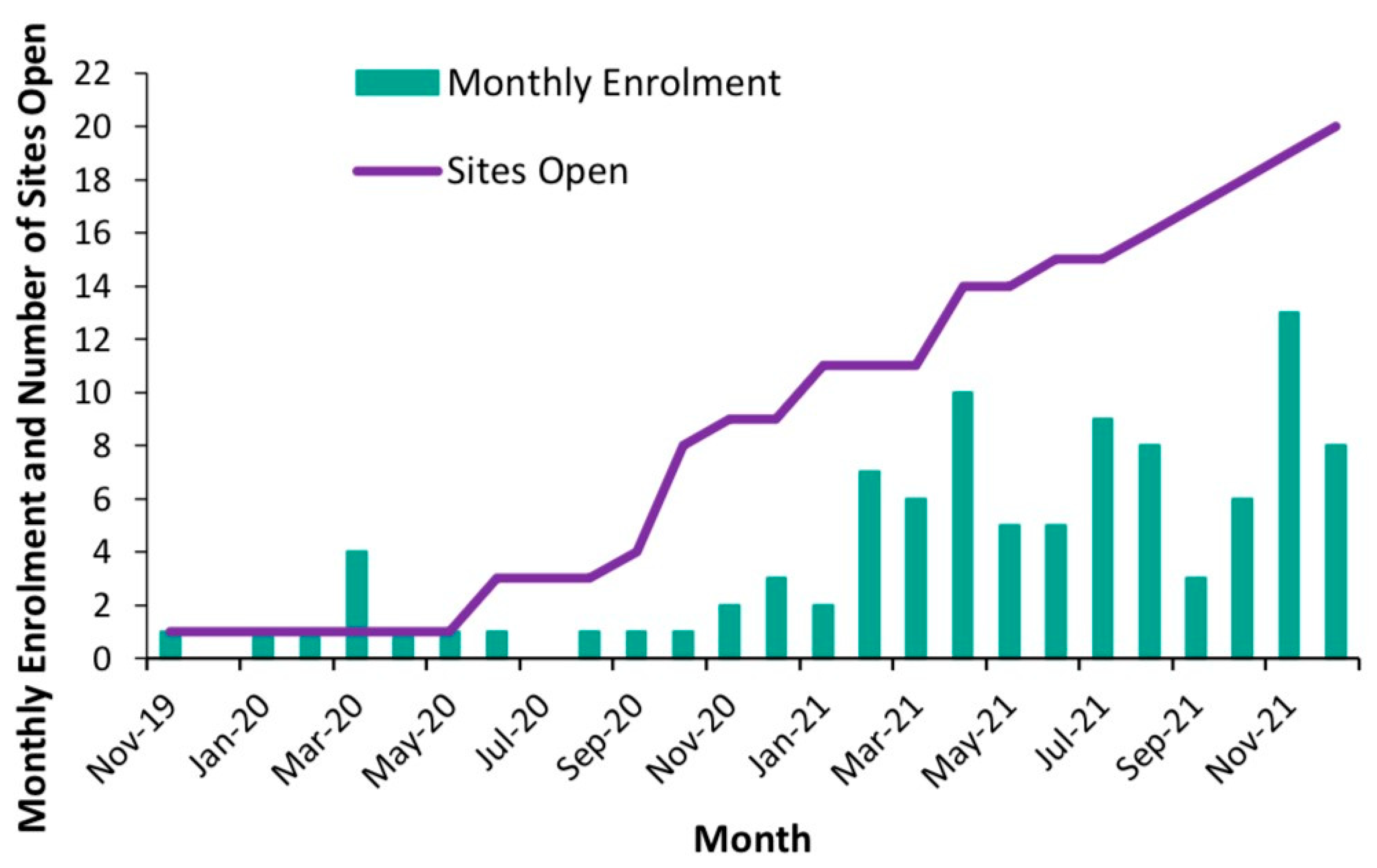
3.1. Enrolment
3.2. Demographics
3.3. Aggregate Clinical Outcomes
3.4. Protocol Adherence
3.5. Follow-Up and Data Quality
4. Discussion
4.1. Summary of Findings
4.2. Feasibility Implications for the Definitive SAFETY Trial
4.3. Aggregate Clinical Outcomes and Implications for the Definitive Trial
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| STS | Soft-tissue sarcoma |
| CXR | Chest x-ray |
| CT | Computed tomography |
| SAFETY | Surveillance AFter Extremity Tumor SurgerY |
| RCT | Randomized controlled trial |
| HiREB | Hamilton integrated Research Ethics Board |
| CRF | Case report form |
| SAE | Serious adverse event |
| PROMIS | Patient-Reported Outcomes Measurement Information System |
| EQ-5D | EuroQol Group-5 Dimensions |
| DSMB | Data and safety monitoring board |
| SD | Standard deviation |
| PROMs | Patient-Reported Outcome Measures |
Appendix A
References
- Pervaiz, N.; Colterjohn, N.; Farrokhyar, F.; Tozer, R.; Figueredo, A.; Ghert, M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008, 113, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Songür, N.; Dinç, M.; Ozdilekcan, C.; Eke, S.; Ok, U.; Oz, M. Analysis of lung metastases in patients with primary extremity sarcoma. Sarcoma 2003, 7, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Savina, M.; Le Cesne, A.; Blay, J.Y.; Ray-Coquard, I.; Mir, O.; Toulmonde, M.; Cousin, S.; Terrier, P.; Ranchere-Vince, D.; Meeus, P.; et al. Patterns of care and outcomes of patients with METAstatic soft tissue SARComa in a real-life setting: The METASARC observational study. BMC Med. 2017, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Mayo, Z.; Kennedy, S.; Gao, Y.; Miller, B.J. What Is the Clinical Importance of Incidental Findings on Staging CT Scans in Patients With Sarcoma? Clin. Orthop. Relat. Res. 2019, 477, 730–737. [Google Scholar] [CrossRef] [PubMed]
- ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25 (Suppl. S3), 1348–1365. [Google Scholar] [CrossRef] [PubMed]
- von Mehren, M.; Kane, J.M.; Agulnik, M.; Bui, M.M.; Carr-Ascher, J.; Choy, E.; Connelly, M.; Dry, S.; Ganjoo, K.N.; Gonzalez, R.J.; et al. Soft Tissue Sarcoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 815–833. [Google Scholar]
- Greenberg, D.D.; Crawford, B. Surveillance Strategies for Sarcoma: Results of a Survey of Members of the Musculoskeletal Tumor Society. Sarcoma 2016, 2016, 8289509. [Google Scholar] [CrossRef] [PubMed]
- Gerrand, C.H.; Billingham, L.J.; Woll, P.J.; Grimer, R.J. Follow up after Primary Treatment of Soft Tissue Sarcoma: A Survey of Current Practice in the United Kingdom. Sarcoma 2007, 2007, 34128. [Google Scholar] [CrossRef] [PubMed]
- Ries, Z.; Gibbs, C.P.; Scarborough, M.T.; Miller, B.J. Pulmonary Surveillance Strategies Following Sarcoma Excision Vary Among Orthopedic Oncologists: A Survey of the Musculoskeletal Tumor Society. Iowa Orthop. J. 2016, 36, 109–116. [Google Scholar] [PubMed]
- Schneider, P.J.; Evaniew, N.; McKay, P.; Ghert, M. Moving Forward Through Consensus: A Modified Delphi Approach to Determine the Top Research Priorities in Orthopaedic Oncology. Clin. Orthop. Relat. Res. 2017, 475, 3044–3055. [Google Scholar] [CrossRef] [PubMed]
- SAFETY Investigators. The Surveillance After Extremity Tumor Surgery (SAFETY) trial: Protocol for a pilot study to determine the feasibility of a multi-centre randomised controlled trial. BMJ Open. 2019, 9, e029054. [Google Scholar] [CrossRef] [PubMed]
- Clover, K.; Lambert, S.D.; Oldmeadow, C.; Britton, B.; Mitchell, A.J.; Carter, G.; King, M.T. Convergent and criterion validity of PROMIS anxiety measures relative to six legacy measures and a structured diagnostic interview for anxiety in cancer patients. J. Patient Rep. Outcomes 2022, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Pickard, A.S.; De Leon, M.C.; Kohlmann, T.; Cella, D.; Rosenbloom, S. Psychometric comparison of the standard EQ-5D to a 5 level version in cancer patient. Med. Care 2007, 45, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Ranganathan, P.; Gulia, A.; Crasto, S.; Hawaldar, R.; Badwe, R.A. Does a less intensive surveillance protocol affect the survival of patients after treatment of a sarcoma of the limb? updated results of the randomized TOSS study. Bone Joint J. 2018, 2, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Gulia, A.; Hawaldar, R.; Ranganathan, P.; Badwe, R.A. Does Intensity of Surveillance Affect Survival After Surgery for Sarcomas? Results of a Randomized Noninferiority Trial. Clin. Orthop. Relat. Res. 2014, 472, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Optimal Sarcoma Surveillance Strategies.pdf. 2018. Available online: https://osf.io/bnfsm (accessed on 27 May 2024).
- Schneider, P.; Giglio, V.; Ghanem, D.; Wilson, D.; Turcotte, R.; Isler, M.; Mottard, S.; Miller, B.; Hayden, J.; Doung, Y.C.; et al. Willingness of patients with sarcoma to participate in cancer surveillance research: A cross-sectional patient survey. BMJ Open 2021, 11, e042742. [Google Scholar] [CrossRef] [PubMed]
- Tepper, S.C.; Holten, A.K.; Jeffe, D.B.; England, P.H.; Hong, Z.L.; Pérez, M.; Ghert, M.; Hirbe, A.C.; Cipriano, C.A. Examining patient perspectives on sarcoma surveillance: The Sarcoma Surveillance Survey. Surg. Oncol. 2022, 45, 101861. [Google Scholar] [CrossRef] [PubMed]
- Jazieh, A.R.; Akbulut, H.; Curigliano, G.; Rogado, A.; Alsharm, A.A.; Razis, E.D.; Mula-Hussain, L.; Errihani, H.; Khattak, A.; De Guzman, R.B.; et al. Impact of the COVID-19 Pandemic on Cancer Care: A Global Collaborative Study. JCO Glob. Oncol. 2020, 6, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, B.; Gan, R.W.; Meyer, C.S.; Wang, J.R.; Zhang, Y.; Hayden, J.; Mahoney, G.; Lund, J.L.; Weberpals, J.; Schneeweiss, S.; et al. Measurement error and bias in real-world oncology endpoints when constructing external control arms. Front. Drug Saf. Regul. 2024, 4, 1423493. [Google Scholar] [CrossRef] [PubMed]
- Mansournia, M.A.; Higgins, J.P.T.; Sterne, J.A.C.; Hernán, M.A. Biases in Randomized Trials: A Conversation Between Trialists and Epidemiologists. Epidemiology 2017, 28, 54–59. [Google Scholar] [CrossRef] [PubMed]
| Feasibility Outcome | Progression Criteria |
|---|---|
| Participant enrolment | Participant retention of at least 85% |
| Participants’ and clinical sites’ adherence to protocol | Protocol deviations below 15% |
| Participant follow-up and data quality | Completed follow-up with accurate data collection of at least 85% of study visits |
| Step | Visit | Relevant Case Report Forms/Study Materials | |||
|---|---|---|---|---|---|
| Surgery | Pre-Baseline Visit | None | |||
| Identification of Patients | Baseline Visit | Screening Log Informed Consent | |||
| Assessment of Patient Eligibility | Screening Form Baseline Forms PROMIS Cancer-Anxiety, PROMIS Satisfaction with Social Roles and Activities, and EQ-5D Questionnaires | ||||
| Randomization to Imaging Modality Frequency (chest CT scan or CXR) | Randomization Form Centralized, 24 h Web-Based Randomization System | ||||
| Randomization to Surveillance Frequency (clinic visit every 3 months or every 6 months) | Randomization Form Centralized, 24 h Web-Based Randomization System | ||||
| Study Intervention | |||||
| Assessment of Study Intervention and Outcomes | Treatment Group #1: CXR every 3 months for 2 years | Treatment Group #2: CXR every 6 months for 2 years | Treatment Group #3: CT every 3 months for 2 years | Treatment Group #1: CT every 6 months for 2 years | |
| 3M CXR + Study Visit | No Imaging or Study Visit | 3M CT + Study Visit | No Imaging or Study Visit | Follow-up Forms | |
| 6M CXR + Study Visit | 6M CXR + Study Visit | 6M CT + Study Visit | 6M CT + Study Visit | Follow-up Forms PROMIS Cancer-Anxiety, PROMIS Satisfaction with Social Roles and Activities, and EQ-5D Questionnaires | |
| 9M CXR + Study Visit | No Imaging or Study Visit | 9M CT + Study Visit | No Imaging or Study Visit | Follow-up Forms | |
| 12M CXR + Study Visit | 12M CXR + Study Visit | 12M CT + Study Visit | 12M CT + Study Visit | Follow-up Forms PROMIS Cancer-Anxiety, PROMIS Satisfaction with Social Roles and Activities, and EQ-5D Questionnaires | |
| 15M CXR + Study Visit | No Imaging or Study Visit | 15M CT + Study Visit | No Imaging or Study Visit | Follow-up Forms | |
| 18M CXR + Study Visit | 18M CXR + Study Visit | 18M CT + Study Visit | 18M CT + Study Visit | Follow-up Forms PROMIS Cancer-Anxiety, PROMIS Satisfaction with Social Roles and Activities, and EQ-5D Questionnaires | |
| 21M CXR + Study Visit | No Imaging or Study Visit | 21M CT + Study Visit | No Imaging or Study Visit | Follow-up Forms | |
| 24M CXR + Study Visit | 24M CXR + Study Visit | 24M CT + Study Visit | 24M CT + Study Visit | Follow-up Forms PROMIS Cancer-Anxiety, PROMIS Satisfaction with Social Roles and Activities, and EQ-5D Questionnaires | |
| Hospital | Institution | Country | Date Opened to Enrolment | Pilot Enrolment | Enrolling in Definitive Study |
|---|---|---|---|---|---|
| Juravinski Hospital and Cancer Centre | McMaster University | Canada | November 2019 | Yes | Yes |
| Albany Medical Center | Albany College | United States | June 2020 | Yes | Yes |
| UC Davis Medical Center | University of California Davis | United States | June 2020 | Yes | Yes |
| Holden Comprehensive Cancer Center | University of Iowa | United States | September 2020 | Yes | Yes |
| Medical University Graz | Medical University Graz | Austria | October 2020 | Yes | Yes |
| NYU Langone Health Perlmutter Cancer Center | New York University | United States | October 2020 | Yes | No |
| Hôpital Maisonneuve-Rosemont | University of Montreal | Canada | October 2020 | Yes | Yes |
| UF Health Shands Hospital | University of Florida | United States | October 2020 | Yes | No |
| McGill University Health Centre | McGill University | Canada | November 2020 | Yes | Yes |
| Montefiore Medical Center | United States | January 2021 | Yes | Yes | |
| L’Hotel-Dieu de Quebec | Laval University | Canada | January 2021 | Yes | Yes |
| Hospital de Clinicas de Porto Alegre | Brazil | March 2021 | Yes | Yes | |
| Centro Traumatologico Ortopedico Hospital | Italy | April 2021 | Yes | Yes | |
| The Cleveland Clinic and Hillcrest Hospital | Cleveland University | United States | May 2021 | Yes | Yes |
| Huntsman Cancer Institute | University of Utah | United States | June 2021 | Yes | Yes |
| Leiden University Medical Centre | Leiden University | The Netherlands | September 2021 | Yes | Yes |
| Oregon Health and Science University Hospital | United States | September 2021 | No | Yes | |
| Hospital Universitario Austral | Argentina | October 2021 | No | Yes | |
| Nova Scotia Health | Dalhousie University | Canada | November 2021 | Yes | Yes |
| The Ottawa Hospital | University of Ottawa | Canada | December 2021 | No | Yes |
| Characteristic | Patients (n = 100) | ||
|---|---|---|---|
| Gender | |||
| Men | 52 | ||
| Women | 48 | ||
| Mean age [in years] (SD) | 59.8 (15) | ||
| Type of tumor | |||
| Undifferentiated Pleiomorphic Sarcoma | 26 | ||
| Myxofibrosarcoma | 20 | ||
| Biphasic Synovial Sarcoma | 7 | ||
| Undifferentiated Spindle Cell Sarcoma | 7 | ||
| Pleiomorphic Liposarcoma | 6 | ||
| Dedifferentiated Liposarcoma | 6 | ||
| Leiomyosarcoma (non-cutaneous) | 5 | ||
| Fibroblastic Sarcoma | 4 | ||
| Spindle Cell Synovial Sarcoma | 3 | ||
| Undifferentiated Sarcoma | 3 | ||
| Other | 13 | ||
| Most common tumor locations | |||
| Thigh | 33 | ||
| Shoulder | 12 | ||
| Hip | 10 | ||
| Groin | 9 | ||
| Knee | 8 | ||
| Other cancer treatment modalities (in addition to surgery) | |||
| No | 1 | ||
| Yes | 99 | ||
| Neoadjuvant chemotherapy | 6 | ||
| Neoadjuvant radiation | 77 | ||
| Adjuvant chemotherapy | 3 | ||
| Adjuvant radiation | 9 | ||
| Protocol Deviation | Patients (n = 55) |
|---|---|
| Potentially ineligible patient enrolled | 2 (4) |
| Unscheduled clinic visit | 33 (60) |
| Missed thoracic imaging | 20 (36) |
| Missed surveillance visit | 15 (27) |
| Unscheduled thoracic imaging | 11 (20) |
| Improper thoracic imaging modality | 3 (5) |
| Eligible for Follow-Up * | Completed CRFs n (%) | Partially Completed CRFs n (%) | Outstanding n (%) | Missed Visit n (%) | Missed PROMs n (%) | |
|---|---|---|---|---|---|---|
| 6 months | 89 | 84 (94) | 5 (6) | 0 (0) | 2 (2) | 7 (9) |
| 12 months | 84 | 81 (96) | 2 (2) | 1 (1) | 3 (4) | 5 (6) |
| 18 months | 81 | 69 (85) | 8 (10) | 4 (5) | 7 (9) | 6 (7) |
| 24 months | 80 | 68 (85) | 2 (3) | 10 (13) | 7 (9) | 9 (11) |
| 30 months | 73 | 53 (73) | 4 (5) | 4 (5) | 1 (1) | N/A |
| 36 months | 33 | 21 (64) | 2 (6) | 4 (12) | 2 (6) | 4 (12) |
| 42 months | 13 | 9 (69) | 0 (0) | 1 (8) | 0 (0) | N/A |
| 48 months | 8 | 7 (88) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 54 months | 0 | - | - | - | - | N/A |
| 60 months | 0 | - | - | - | - | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrukh, H.; Schneider, P.; Hudson, T.; Giglio, V.; Becker, R.G.; Sabharwal, S.; Quan, K.; Francescutti, V.; Goldberg, M.; Sprague, S.; et al. The Surveillance After Extremity Tumor Surgery (SAFETY) Pilot International Multi-Center Randomized Controlled Trial. Curr. Oncol. 2025, 32, 686. https://doi.org/10.3390/curroncol32120686
Farrukh H, Schneider P, Hudson T, Giglio V, Becker RG, Sabharwal S, Quan K, Francescutti V, Goldberg M, Sprague S, et al. The Surveillance After Extremity Tumor Surgery (SAFETY) Pilot International Multi-Center Randomized Controlled Trial. Current Oncology. 2025; 32(12):686. https://doi.org/10.3390/curroncol32120686
Chicago/Turabian StyleFarrukh, Hadia, Patricia Schneider, Tess Hudson, Victoria Giglio, Ricardo Gehrke Becker, Samir Sabharwal, Kimmen Quan, Valerie Francescutti, Mira Goldberg, Sheila Sprague, and et al. 2025. "The Surveillance After Extremity Tumor Surgery (SAFETY) Pilot International Multi-Center Randomized Controlled Trial" Current Oncology 32, no. 12: 686. https://doi.org/10.3390/curroncol32120686
APA StyleFarrukh, H., Schneider, P., Hudson, T., Giglio, V., Becker, R. G., Sabharwal, S., Quan, K., Francescutti, V., Goldberg, M., Sprague, S., & Ghert, M., on behalf of The SAFETY Investigators. (2025). The Surveillance After Extremity Tumor Surgery (SAFETY) Pilot International Multi-Center Randomized Controlled Trial. Current Oncology, 32(12), 686. https://doi.org/10.3390/curroncol32120686





