Zolbetuximab or Immunotherapy as the Initial Targeted Therapy in CLDN18.2-Positive, HER2-Negative Advanced Gastric Cancer: Weighing the Options
Simple Summary
Abstract
1. Introduction
2. Efficacy and Safety Data from the Respective Phase III Studies
2.1. Immune Checkpoint Inhibitors—Efficacy and Toxicity
2.2. Zolbetuximab—Efficacy and Toxicity
3. Biomarker Testing in G/GEJ Adenocarcinoma
4. Considerations Related to Clinical Practicalities
5. Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT3 | 5-hydroxytryptamine type 3 |
| CAPOX | capecitabine+oxaliplatin |
| CAR-T | chimeric antigen receptor T-cell therapy |
| CI | confidence interval |
| CLDN | claudin |
| CPS | combined positive score |
| d/MMR | deficient/mismatch repair |
| FDA | Food and Drug Administration |
| FGFR2b | fibroblast growth factor receptor 2 isoform IIIb |
| FOLFOX | folinic acid+fluorouracil+oxaliplatin |
| FP | fluoropyrimidine |
| G/GEJ | gastric/gastroesophageal |
| HER2 | human epidermal growth factor receptor-2 |
| HR | hazard ratio |
| ICI | immune checkpoint inhibitor |
| IHC | immunohistochemistry |
| irAEs | immune-related adverse events |
| MMR | mismatch repair |
| N/V | nausea and vomiting |
| NK1 | neurokinin-1 |
| PD-L1 | programmed death-ligand 1 |
| PFS | progression-free survival |
| Q2W/Q3W | every 2 weeks/every 3 weeks |
| RxDx | companion diagnostic |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Casamayor, M.; Morlock, R.; Maeda, H.; Anjani, J. Targeted literature review of the global burden of gastric cancer. Ecancermedicalscience 2018, 12, 883. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Rha, S.Y.; Oh, D.-Y.; Yañez, P.; Bai, Y.; Ryu, M.-H.; Lee, J.; Rivera, F.; Alves, G.V.; Garrido, M.; Shiu, K.-K.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1181–1195, https://doi.org/10.1016/s1470-2045(23)00515-6. Erratum in Lancet Oncol. 2024, 25, e26. [Google Scholar] [CrossRef]
- Rohde, C.; Yamaguchi, R.; Mukhina, S.; Sahin, U.; Itoh, K.; Türeci, Ö. Comparison of Claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma. Ultrasound Med. Biol. 2019, 49, 870–876. [Google Scholar] [CrossRef]
- Hato, S.V.; Khong, A.; de Vries, I.J.M.; Lesterhuis, W.J. Molecular Pathways: The Immunogenic Effects of Platinum-Based Chemotherapeutics. Clin. Cancer Res. 2014, 20, 2831–2837. [Google Scholar] [CrossRef]
- Türeci, O.; Sahin, U.; Schulze-Bergkamen, H.; Zvirbule, Z.; Lordick, F.; Koeberle, D.; Thuss-Patience, P.; Ettrich, T.; Arnold, D.; Bassermann, F.; et al. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: The MONO study. Ann. Oncol. 2019, 30, 1487–1495. [Google Scholar] [CrossRef]
- Shitara, K.; Xu, R.-H.; Ajani, J.A.; Moran, D.; Guerrero, A.; Li, R.; Pavese, J.; Matsangou, M.; Bhattacharya, P.; Ueno, Y.; et al. Global prevalence of claudin 18 isoform 2 in tumors of patients with locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma. Gastric Cancer 2024, 27, 1058–1068. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Ajani, J.A.; Moehler, M.; Shen, L.; Garrido, M.; Gallardo, C.; Wyrwicz, L.; Yamaguchi, K.; Cleary, J.M.; Elimova, E.; et al. First-Line Nivolumab Plus Chemotherapy for Advanced Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma: 3-Year Follow-Up of the Phase III CheckMate 649 Trial. J. Clin. Oncol. 2024, 42, 2012–2020. [Google Scholar] [CrossRef]
- Shitara, K.; Van Cutsem, E.; Lordick, F.; Enzinger, P.C.; Ilson, D.H.; Shah, M.A.; Xu, R.-H.; Lonardi, S.; Yamaguchi, K.; Hung, Y.-P.; et al. Final overall survival results from phase 3 SPOTLIGHT study evaluating zolbetuximab + mFOLFOX6 as first-line (1L) treatment for patients (pts) with claudin 18 isoform 2 (CLDN18.2)+, HER2−, locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma. J. Clin. Oncol. 2024, 42, 4036. [Google Scholar] [CrossRef]
- Lordick, F.; Shah, M.; Shitara, K.; Ajani, J.; Bang, Y.-J.; Enzinger, P.; Ilson, D.; van Cutsem, E.; Plazas, J.G.; Huang, J.; et al. 134MO Updated efficacy and safety results from phase III GLOW study evaluating zolbetuximab + CAPOX as first-line (1L) treatment for patients with claudin-18 isoform 2-positive (CLDN18.2+), HER2−, locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma. Ann. Oncol. 2023, 34, S1524. [Google Scholar] [CrossRef]
- Yin, Q.; Wu, L.; Han, L.; Zheng, X.; Tong, R.; Li, L.; Bai, L.; Bian, Y. Immune-related adverse events of immune checkpoint inhibitors: A review. Front. Immunol. 2023, 14, 1167975. [Google Scholar] [CrossRef]
- Pei, W.-G.; Chen, W.-Z.; Wu, Y.-K.; Tan, S.-X.; Jie, Z.-G. Immune-related adverse events associated with immune checkpoint inhibitors for advanced gastric and gastroesophageal junction cancer: A meta-analysis. World J. Gastrointest. Oncol. 2023, 15, 352–367. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126, https://doi.org/10.1200/jco.21.01440. Erratum in J. Clin. Oncol.2022, 40, 315. [Google Scholar] [CrossRef]
- Shitara, K.; Lordick, F.; Bang, Y.-J.; Enzinger, P.; Ilson, D.; AShah, M.; Van Cutsem, E.; Xu, R.-H.; Aprile, G.; Xu, J.; et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2023, 401, 1655–1668, https://doi.org/10.1016/s0140-6736(23)00620-7. Erratum in Lancet2024, 403, 30. [Google Scholar] [CrossRef]
- Shah, M.A.; Shitara, K.; Ajani, J.A.; Bang, Y.-J.; Enzinger, P.; Ilson, D.; Lordick, F.; Van Cutsem, E.; Plazas, J.G.; Huang, J.; et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: The randomized, phase 3 GLOW trial. Nat. Med. 2023, 29, 2133–2141. [Google Scholar] [CrossRef]
- Kang, Y.-K.; Shah, M.; Shitara, K.; Ajani, J.; Lordick, F.; Van Cutsem, E.; Ilson, D.; Klempner, S.; Yamaguchi, K.; Nakajima, T.; et al. 1438P First-line (1L) zolbetuximab + chemotherapy in patients (pts) with claudin 18.2 (CLDN18.2) +, HER2-, locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: A pooled final analysis of SPOTLIGHT + GLOW. Ann. Oncol. 2024, 35, S895. [Google Scholar] [CrossRef]
- Derks, S.; van Laarhoven, H.W. SPOTlight on GLOW. Cell Rep. Med. 2023, 4, 101233. [Google Scholar] [CrossRef] [PubMed]
- VyloyPM. Available online: https://www.vyloypm.com/ (accessed on 23 September 2025).
- Shitara, K.; Shah, M.A.; Lordick, F.; Van Cutsem, E.; Ilson, D.H.; Klempner, S.J.; Kang, Y.-K.; Lonardi, S.; Hung, Y.-P.; Yamaguchi, K.; et al. Zolbetuximab in Gastric or Gastroesophageal Junction Adenocarcinoma. N. Engl. J. Med. 2024, 391, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Pophale, R.; Matsangou, M.; Park, J.W.; Oh, M.; Bhattacharya, P.P.; Ranganath, R. Management of nausea and vomiting (N/V) following first-line (1L) zolbetuximab + chemotherapy treatment in claudin-18.2 (CLDN18.2)+, HER2−, locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: Analysis from the phase 3 SPOTLIGHT and GLOW studies. J. Clin. Oncol. 2024, 42, 372. [Google Scholar] [CrossRef]
- Shimozaki, K.; Ooki, A.; Yamahata, Y.; Aoyama, T.; Yoshino, K.; Tamba, M.; Udagawa, S.; Fukuoka, S.; Osumi, H.; Wakatsuki, T.; et al. Managing zolbetuximab-induced nausea and vomiting: A proposal for a pragmatic approach in clinical practice. ESMO Gastrointest. Oncol. 2025, 7, 100128. [Google Scholar] [CrossRef]
- Klempner, S.; Pazo-Cid, R.; Lonardi, S.; Swanson, L.; Arango, M.; Enzinger, P.; Ko, A.; Vaccaro, G.; Yamaguchi, K.; Saeed, A.; et al. Consensus guidance for prevention and management of nausea and vomiting in patients treated with zolbetuximab + chemotherapy: A RAND/UCLA modified Delphi panel study. ESMO Gastrointest. Oncol. 2025, 7, 100131. [Google Scholar] [CrossRef]
- Khalili-Tanha, G.; Khalili-Tanha, N.; Rouzbahani, A.K.; Mahdieh, R.; Jasemi, K.; Ghaderi, R.; Leylakoohi, F.K.; Ghorbani, E.; Khazaei, M.; Hassanian, S.M.; et al. Diagnostic, prognostic, and predictive biomarkers in gastric cancer: From conventional to novel biomarkers. Transl. Res. 2024, 274, 35–48. [Google Scholar] [CrossRef]
- Brezden-Masley, C.; Fiset, P.O.; Cheung, C.C.; Arnason, T.; Bateman, J.; Borduas, M.; Evaristo, G.; Ionescu, D.N.; Lim, H.J.; Sheffield, B.S.; et al. Canadian Consensus Recommendations for Predictive Biomarker Testing in Gastric and Gastroesophageal Junction Adenocarcinoma. Curr. Oncol. 2024, 31, 7770–7786. [Google Scholar] [CrossRef]
- Snow, S.; Gabrielson, D.; Lim, H.; Tehfe, M.; Brezden-Masley, C. Best Practices for Managing Patients with Unresectable Metastatic Gastric and Gastroesophageal Junction Cancer in Canada. Curr. Oncol. 2024, 31, 2552–2565. [Google Scholar] [CrossRef]
- Sahin, U.; Türeci, Ö.; Manikhas, G.; Lordick, F.; Rusyn, A.; Vynnychenko, I.; Dudov, A.; Bazin, I.; Bondarenko, I.; Melichar, B.; et al. FAST: A randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann. Oncol. 2021, 32, 609–619. [Google Scholar] [CrossRef]
- Covert, W.M.; Rogers, J.E. Future Landscape of Anti-Claudin 18.2 Antibodies in Gastric Adenocarcinoma. Antibodies 2025, 14, 26. [Google Scholar] [CrossRef]
- Jasani, B.; Taniere, P.; Schildhaus, H.-U.; Blighe, K.; Parry, S.; Wilkinson, D.; Atkey, N.; Clare-Antony, S.; McCabe, C.; Quinn, C.; et al. Global Ring Study to Investigate the Comparability of Total Assay Performance of Commercial Claudin 18 Antibodies for Evaluation in Gastric Cancer. Mod. Pathol. 2023, 104, 100284. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.I.; Robbins, C.J.; Gaule, P.; Agostini-Vulaj, D.; Anders, R.A.; Bellizzi, A.M.; Chen, W.; Chen, Z.E.; Gopal, P.; Zhao, L.; et al. Multi-Institutional Study of Pathologist Reading of the Programmed Cell Death Ligand-1 Combined Positive Score Immunohistochemistry Assay for Gastric or Gastroesophageal Junction Cancer. Mod. Pathol. 2023, 36, 100128. [Google Scholar] [CrossRef]
- Robert, M.E.; Rüschoff, J.; Jasani, B.; Graham, R.P.; Badve, S.S.; Rodriguez-Justo, M.; Kodach, L.L.; Srivastava, A.; Wang, H.L.; Tang, L.H.; et al. High Interobserver Variability Among Pathologists Using Combined Positive Score to Evaluate PD-L1 Expression in Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma. Mod. Pathol. 2023, 36, 100154, https://doi.org/10.1016/j.modpat.2023.100154. Erratum in Mod. Pathol. 2023, 36, 100238. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.C.; Barnes, P.; Bigras, G.; Boerner, S.; Butany, J.; Calabrese, F.; Couture, C.; Deschenes, J.; El-Zimaity, H.; Fischer, G.; et al. Fit-For-Purpose PD-L1 Biomarker Testing For Patient Selection in Immuno-Oncology: Guidelines For Clinical Laboratories From the Canadian Association of Pathologists-Association Canadienne Des Pathologistes (CAP-ACP). Appl. Immunohistochem. Mol. Morphol. 2019, 27, 699–714. [Google Scholar] [CrossRef]
- Schoemig-Markiefka, B.; Eschbach, J.; Scheel, A.H.; Pamuk, A.; Rueschoff, J.; Zander, T.; Buettner, R.; Schroeder, W.; Bruns, C.J.; Loeser, H.; et al. Optimized PD-L1 scoring of gastric cancer. Gastric Cancer 2021, 24, 1115–1122. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Q.; Li, Y.-Q.; Li, Z.; Zheng, J.; Hu, W.; Yang, Y.; Wu, D.; Bei, J.-X.; Gu, B.; et al. Comparative single-cell analysis reveals heterogeneous immune landscapes in adenocarcinoma of the esophagogastric junction and gastric adenocarcinoma. Cell Death Dis. 2024, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Nakauchi, M.; Vos, E.L.; Carr, R.A.; Barbetta, A.; Tang, L.H.; Gonen, M.; Russo, A.; Janjigian, Y.Y.; Yoon, S.S.; Sihag, S.; et al. Distinct Differences in Gastroesophageal Junction and Gastric Adenocarcinoma in 2194 Patients. Ann. Surg. 2021, 277, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Panchal, V.; Shah, A.; Vyas, B.; Agrawal, S.; Bharadwaj, S. Immune checkpoint inhibitors in metastatic melanoma therapy (Review). Med. Int. 2024, 4, 13. [Google Scholar] [CrossRef]
- Kawakami, H. New therapeutic target molecules for gastric and gastroesophageal junction cancer. Int. J. Clin. Oncol. 2024, 29, 1228–1236. [Google Scholar] [CrossRef]
- Lordick, F.; Rha, S.Y.; Muro, K.; Yong, W.P.; Obermannová, R.L. Systemic Therapy of Gastric Cancer—State of the Art and Future Perspectives. Cancers 2024, 16, 3337. [Google Scholar] [CrossRef]
- Lim, S.H.; Kuwata, T.; An, M.; Hong, J.Y.; Kim, S.T.; Matsubara, Y.; Shitara, K.; Lee, J. Dynamic modulation of claudin18.2 expression and remodeling of the tumor microenvironment in gastric cancer during chemo-immunotherapy. J. Immunother. Cancer 2025, 13, e012683. [Google Scholar] [CrossRef]
- Klempner, S.J.; Lee, K.-W.; Shitara, K.; Metges, J.-P.; Lonardi, S.; Ilson, D.H.; Fazio, N.; Kim, T.Y.; Bai, L.-Y.; Moran, D.; et al. ILUSTRO: Phase II Multicohort Trial of Zolbetuximab in Patients with Advanced or Metastatic Claudin 18.2–Positive Gastric or Gastroesophageal Junction Adenocarcinoma. Clin. Cancer Res. 2023, 29, 3882–3891. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef]

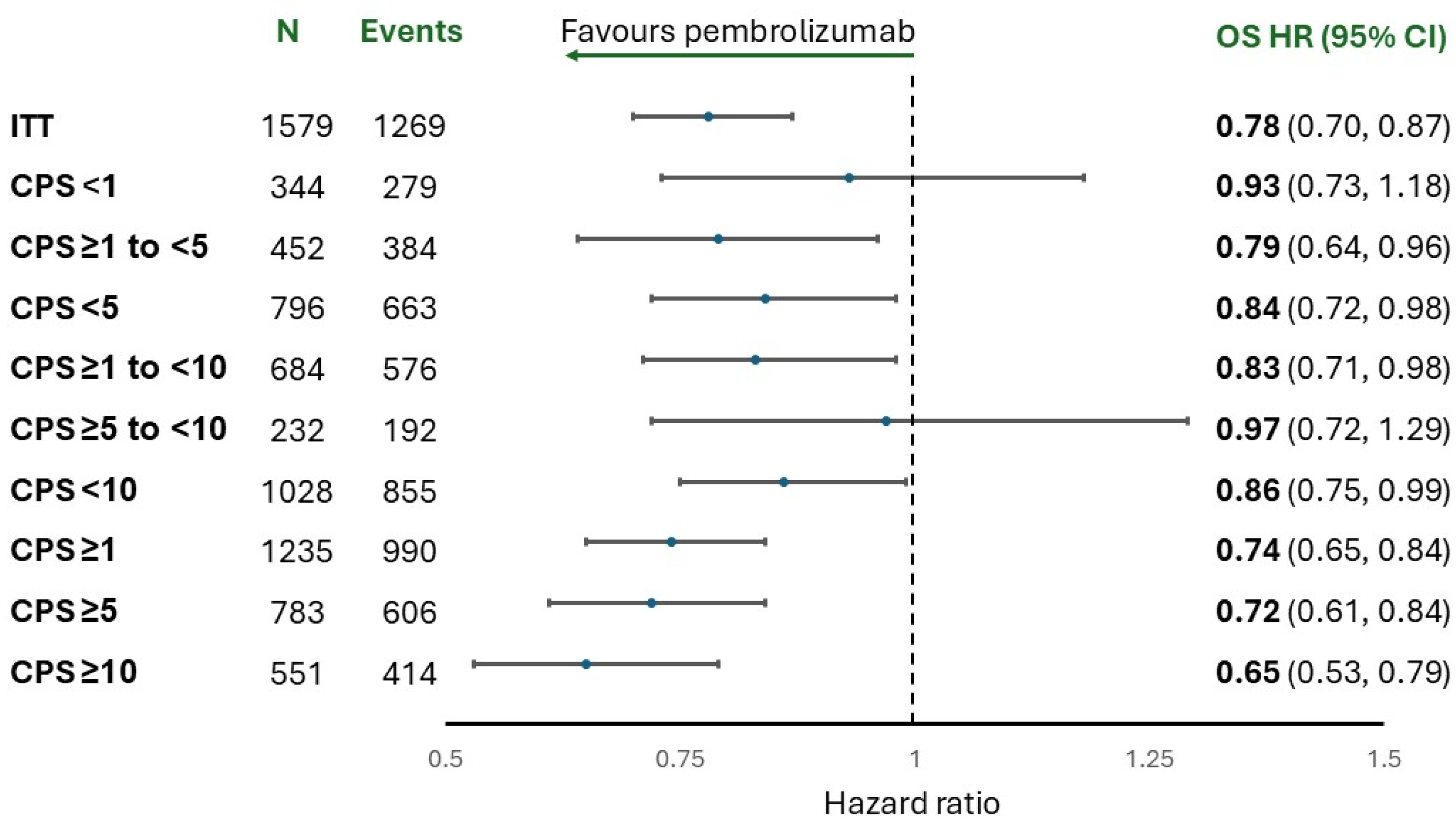
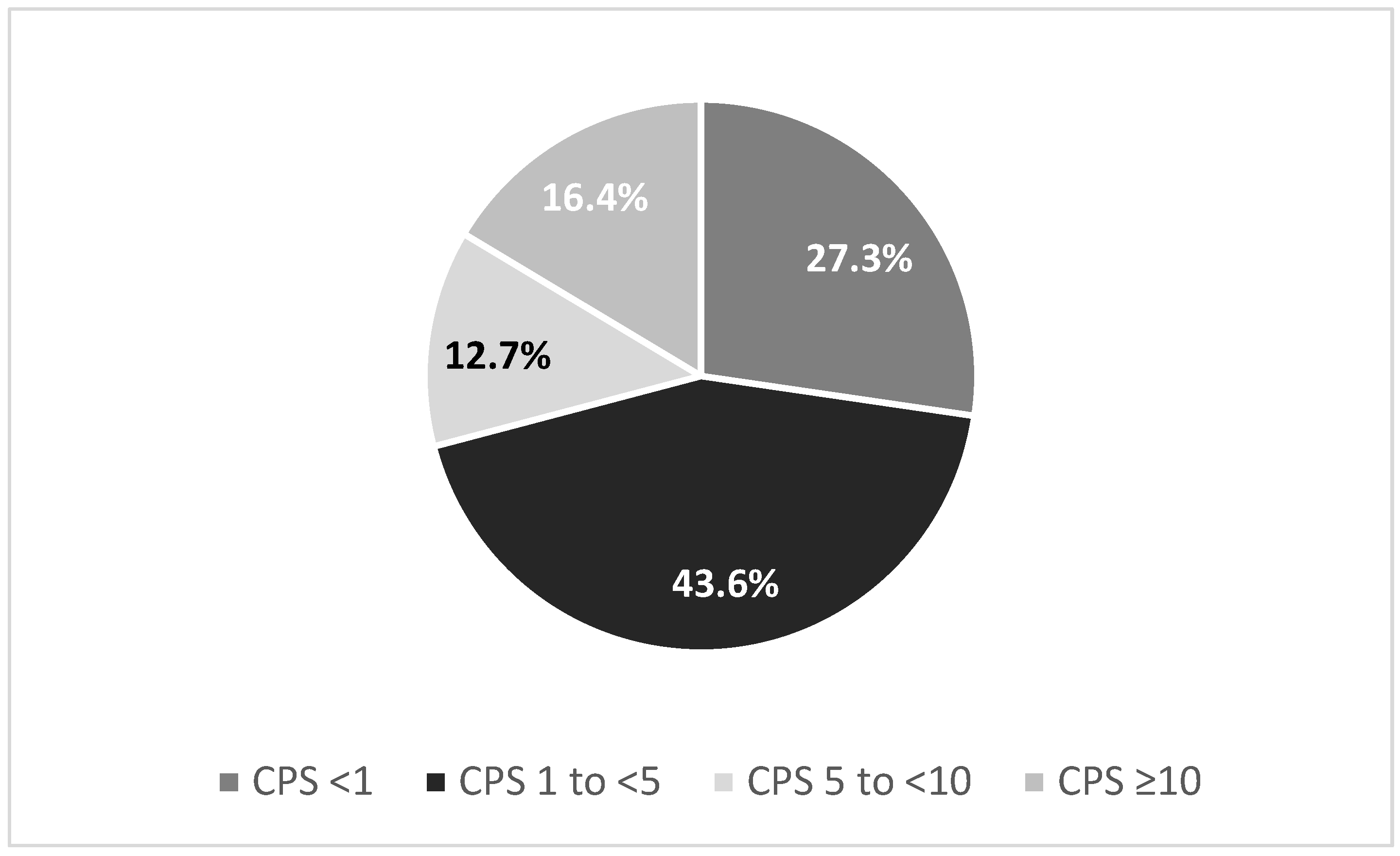

| CheckMate-649 [9] | KEYNOTE-859 [4] | SPOTLIGHT [10] | GLOW [11] | |
|---|---|---|---|---|
| Median follow-up for OS, months | 47.4 | 31.0 | 33.28 | 26.1 |
| Median OS, all patients, months, HR (95% CI) | 13.7 vs. 11.6, | 12.9 vs. 11.5, | 18.23 vs. 15.57, | 14.3 vs. 12.2, |
| 0.79 (0.71, 0.88) | 0.78 (0.70, 0.87) | 0.784 (0.644, 0.954) | 0.77 (0.62, 0.95) | |
| Median OS, patients with PD-L1 CPS <1, months, HR (95% CI) | 13.1 vs. 12.5, | NR | -- | -- |
| 0.95 (0.74, 1.24) | 0.92 (0.73, 1.17) | |||
| Median OS, patients with PD-L1 CPS ≥1, months, HR (95% CI) | 13.8 vs. 11.3, | 13.0 vs. 11.4, | -- | -- |
| 0.75 (0.66, 0.84) | 0.74 (0.65, 0.84) | |||
| Median OS, patients with PD-L1 ≥10, months, HR (95% CI) | 15.0 vs. 10.9, | 15.7 vs. 11.8, | -- | -- |
| 0.66 (0.57, 0.77) | 0.65 (0.53, 0.79) | |||
| Median PFS, all patients, months, HR (95% CI) | 7.7 vs. 6.9, | 6.9 vs. 5.6, | 11.04 vs. 8.94, | 8.3 vs. 6.8, |
| 0.79 (0.71, 0.89) | 0.76 (0.67, 0.85) | 0.734 (0.591, 0.910) | 0.68 (0.55, 0.85) |
| CheckMate-649 [9] | KEYNOTE-859 [4] | |||
|---|---|---|---|---|
| All Grades | Grade 3–4 | All Grades | Grade 3–4 | |
| Gastrointestinal | 34% | 5% | -- | -- |
| Cutaneous | 28% | 4% | -- | -- |
| Hepatic | 27% | 4% | 1% | <1% |
| Endocrine | 14% | <1% | -- | -- |
| Renal | 4% | <1% | 1% | 1% |
| Hypothyroidism | -- | -- | 15% | <1% |
| Hyperthyroidism | -- | -- | 6% | 0% |
| Colitis | -- | -- | 1% | 2% |
| Pneumonitis | 5% | 2% | 2% | 1% 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Easaw, J.C.; Lim, H.J.; Karachiwala, H.; Gill, S.; Zhu, X.; Bateman, J. Zolbetuximab or Immunotherapy as the Initial Targeted Therapy in CLDN18.2-Positive, HER2-Negative Advanced Gastric Cancer: Weighing the Options. Curr. Oncol. 2025, 32, 648. https://doi.org/10.3390/curroncol32110648
Easaw JC, Lim HJ, Karachiwala H, Gill S, Zhu X, Bateman J. Zolbetuximab or Immunotherapy as the Initial Targeted Therapy in CLDN18.2-Positive, HER2-Negative Advanced Gastric Cancer: Weighing the Options. Current Oncology. 2025; 32(11):648. https://doi.org/10.3390/curroncol32110648
Chicago/Turabian StyleEasaw, Jacob C., Howard J. Lim, Hatim Karachiwala, Sharlene Gill, Xiaofu Zhu, and Justin Bateman. 2025. "Zolbetuximab or Immunotherapy as the Initial Targeted Therapy in CLDN18.2-Positive, HER2-Negative Advanced Gastric Cancer: Weighing the Options" Current Oncology 32, no. 11: 648. https://doi.org/10.3390/curroncol32110648
APA StyleEasaw, J. C., Lim, H. J., Karachiwala, H., Gill, S., Zhu, X., & Bateman, J. (2025). Zolbetuximab or Immunotherapy as the Initial Targeted Therapy in CLDN18.2-Positive, HER2-Negative Advanced Gastric Cancer: Weighing the Options. Current Oncology, 32(11), 648. https://doi.org/10.3390/curroncol32110648








