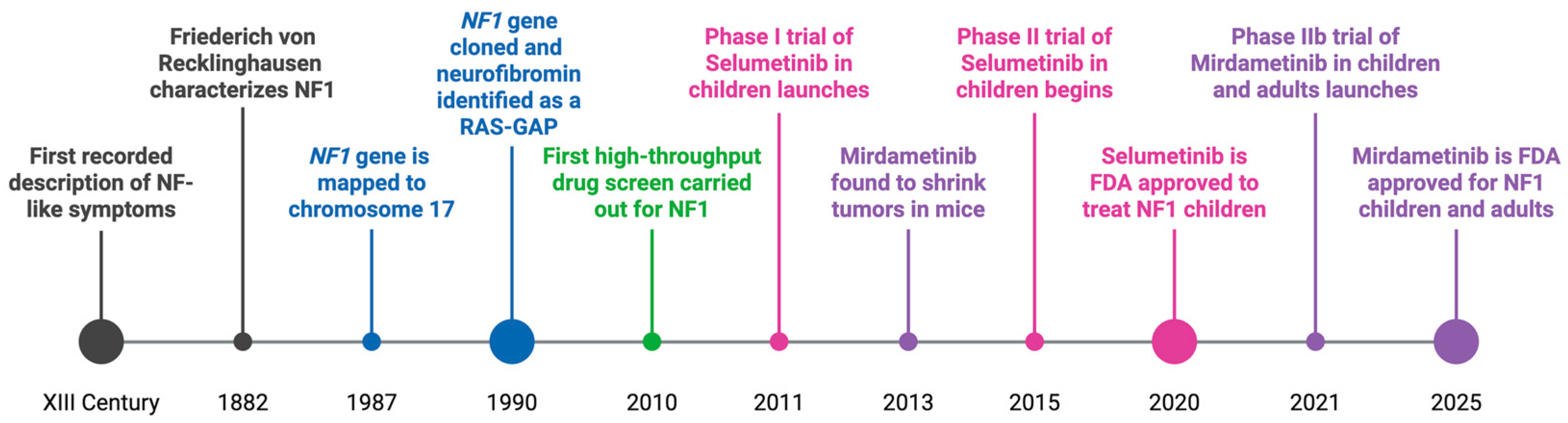
Neurofibromatosis Type 1 and the Search for Effective Tumor Therapies Using High-Throughput Drug Screening
Simple Summary
Abstract
1. Introduction
1.1. Neurofibromatosis Type 1
1.2. Neurofibromin Functions to Regulate the RAS Signaling Pathway
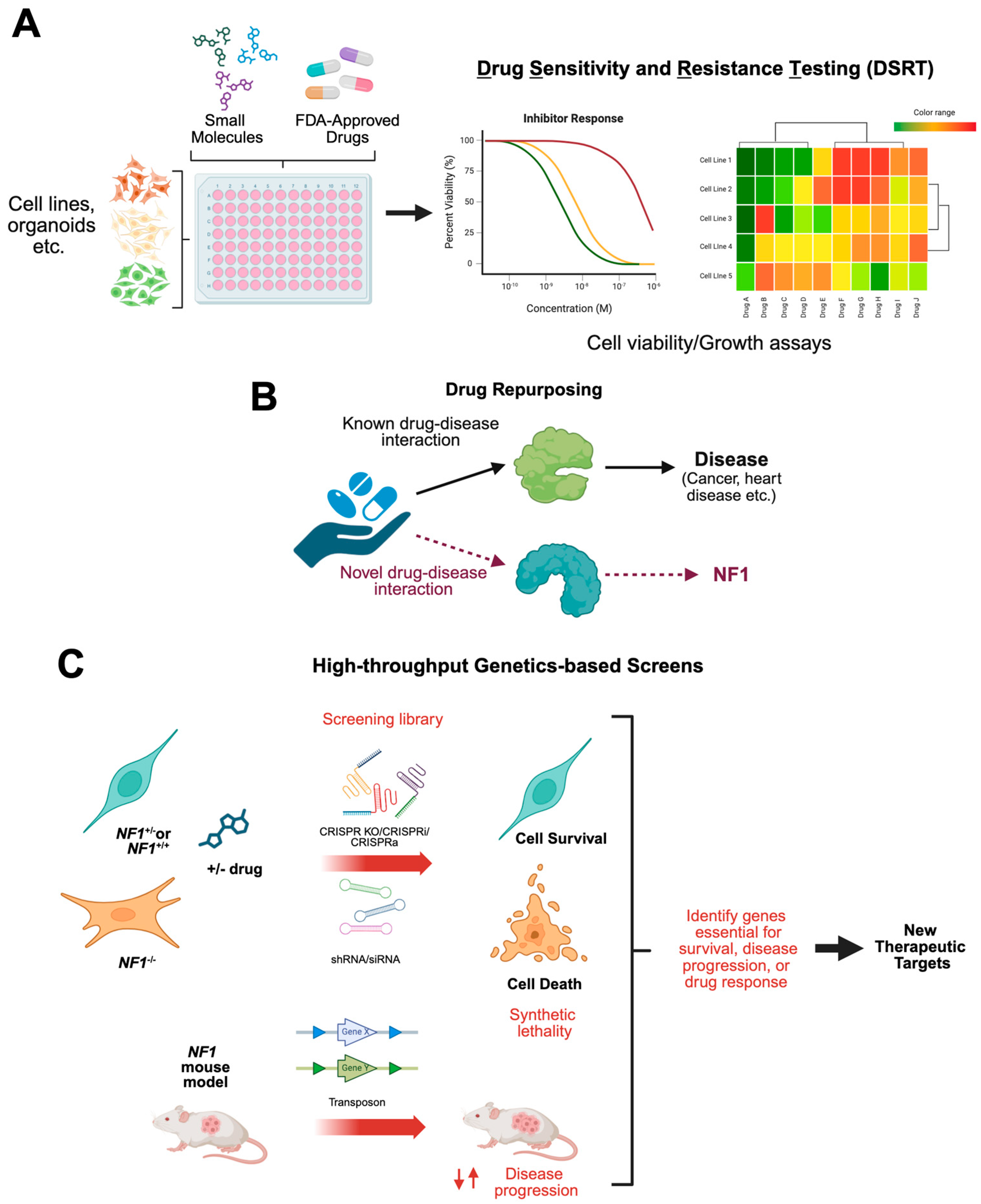
1.3. Drug Screening for New Therapeutic Targets for NF1 Tumors
1.4. Repurposing Existing Drugs for Treating NF1-Deficient Tumors
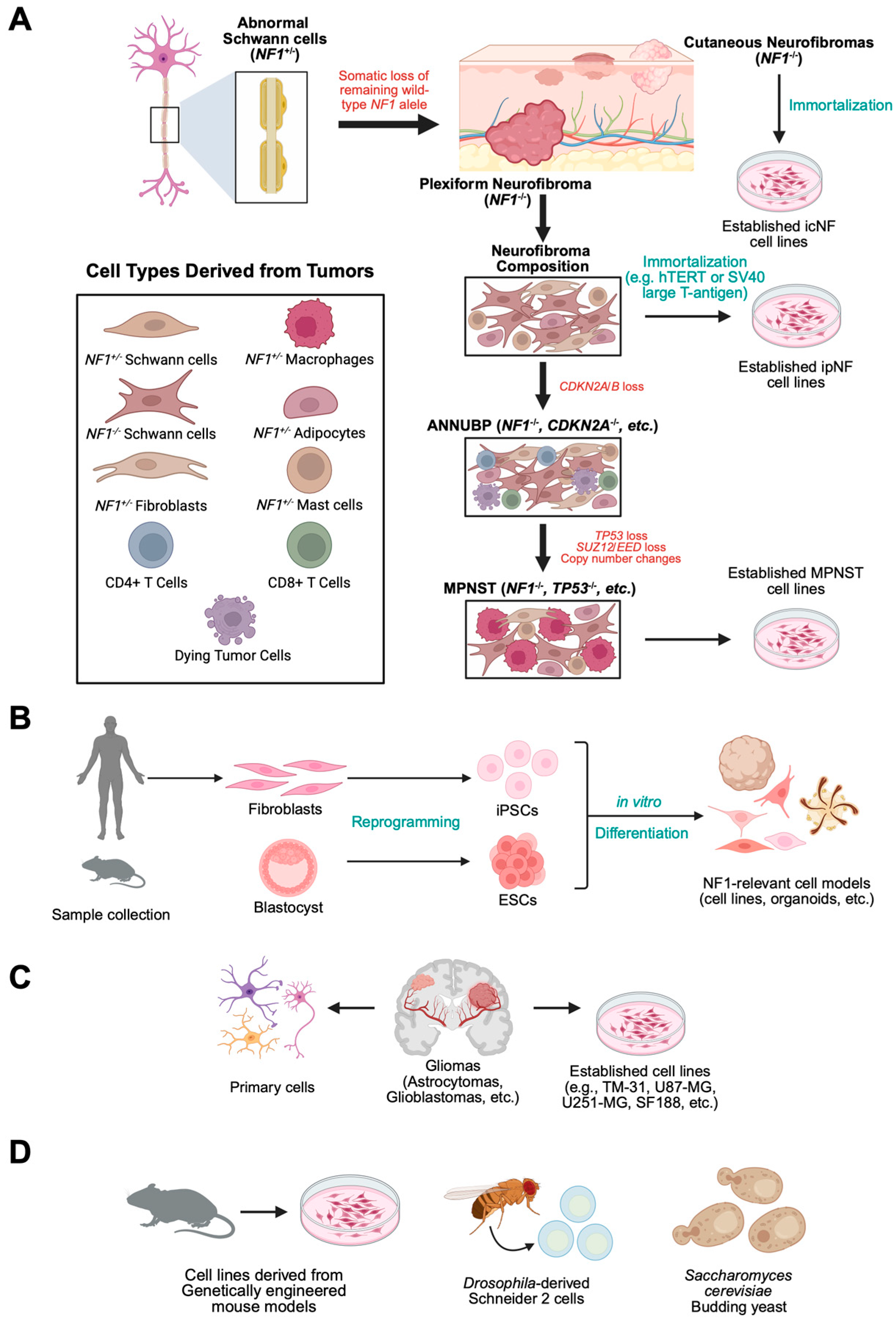
2. In Vitro and In Vivo NF1 Models for High-Throughput and Targeted Drug Screening
2.1. In Vitro 2D NF1 Immortalized Cell Culture Models
2.2. Induced Pluripotent Stem Cells (iPSCs) and Primary NF1 Cell Models
2.3. Isogenic NF1 Cell Culture Model Generation by CRISPR-Cas9 Technology
2.4. In Vitro Primary NF1 Cell Culture Models
2.5. In Vitro 3D NF1 Cell Culture Models
2.6. Single-Celled Organisms and Cell Lines Derived from NF1 Invertebrate Models
2.7. In Vivo Vertebrate Models of NF1 for Testing Therapeutics for NF1-Associated Tumors
3. Targeting the RAS Pathway in NF1
3.1. Understanding the Role of NF1 in RAS Regulation
3.2. RAS Inhibition
3.3. Targeting Downstream RAS Effector Proteins
4. Screening for Novel Treatment Options for Neurofibromas
4.1. Plexiform Neurofibromas
4.2. Cutaneous Neurofibromas
5. MPNST Drug Screens
5.1. The First HTS Investigation in NF1-Derived MPNST Models
5.2. HTS Provides NF1 MPNST Cell Model Profiles
5.3. HTS Focused on Specific MPNST Characteristics
5.4. Combinatorial Inhibition as a Therapeutic Option in MPNSTs
5.5. Pharmacoproteomics Studies to Unveil Novel Targets in MPSNTs
5.6. Rapid In Vitro to In Vivo Small Molecule Screening in NF1-Relevant Models
5.7. HTS in an In Vivo MPNST Model Organism
6. Functional Genomic and Small Molecule Screens to Identify New Therapeutic Targets for NF1-Associated Tumors
6.1. In Vivo Transposon Screen to Validate MPNST-Associated Pathways
6.2. Synthetic Lethal Screens to Identify Novel Therapeutic Targets in NF1-Associated Tumors
7. NF1-Associated Cancers
7.1. Astrocytomas
7.2. Glioblastomas
8. Translational Efforts and Clinical Trials for NF1-Associated Tumors
| Drug Name | Target | Cell Type Efficacy | Status | Monotherapy/ Combinatorial | Screen |
|---|---|---|---|---|---|
| 10-hydroxycamptothecin | TOP1 | GBMs | Further study needed | - | [136] |
| 5,15-DPP | STAT3 | MPNSTs | Further study needed | - | [109] |
| A-443654 | AKT1 | pNFs | Further study needed | - | [104] |
| Actinomycin D | TOP2A/B, TOP1 | MPNSTs | Further study needed | - | [119,127] |
| Afatinib | EGFR, HER2, HER4 | MPNSTs | Further study needed | - | [119] |
| Alvespimycin HCl | HSP90AB1 | pNFs | Further study needed | - | [104] |
| Aminopterin | GBMs | Further study needed | - | [136] | |
| APX3330 | APE1/Ref-1 | MPNSTs | Further study needed | - | [111] |
| Arsenic (III) Oxide | IKBKB, TXNRD1, JUN, CCND1, MAPK3, MAPK1 | MPNSTs | Further study needed | - | [119] |
| AT-7867 | AKT1 | pNFs | Further study needed | - | [104] |
| AT-9283 | AURKA/AURKB | MPNSTs | Further study needed | - | [124] |
| AZ628 | RAF | MPNSTs | Further study needed | - | [113] |
| AZD-8330 | MAP3K1 | GBMs | Further study needed | - | [136] |
| AZD2014 | mTOR | MPNSTs | Further study needed | - | [69] |
| AZD8055 | mTOR | MPNSTs | Further study needed | - | [113,136] |
| Bafilomycin A1 | V-ATPase | pNFs | Further study needed | - | [50] |
| Bardoxolone methyl | NFKBIA | GBMs | Further study needed | - | [136] |
| Belinostat | HDAC | MPNSTs | Further study needed | - | [119,127] |
| Bergapten | NLRP3, Pyroptosis | MPNSTs | Further study needed | - | [119] |
| BI-847325 | MEK/AURKC | MPNSTs | Further study needed | - | [113] |
| BI2536 | PLK1 | Melanoma #, MPNSTs | Phase II Study (NCT00526149- Completed) | Monotherapy | [116] |
| BIIB021 | HSP90AA1 | GBMs | Further study needed | - | [136] |
| Binimetinib/ARRY-162 | MEK | pNFs, MPNSTs | Phase II Study (NCT03231306- Completed) | Monotherapy | [125] |
| BKM120 | PI3K | MPNSTs | Further study needed | - | [123] |
| BMS-186511 | FTI | MPNSTs | Other FTIs tested; Phase II Study (NCT00021541- Completed), Phase II Study (NCT00076102 Completed) | Monotherapy | [78] |
| Bortezomib | Proteosome | pNFs | Further study needed | - | [42,119,127,140] |
| Cabozantinib | VEGFR2 | pNFs, MPNSTs | Phase II Study (NCT02101736- Completed); Phase I Study (NCT06502171-Not Yet Recruiting) | Monotherapy & Combinatorial | [124] |
| Camptothecin | TOP1 | GBMs, MPNSTs | Further study needed | - | [127,136] |
| Cantharidin | PP2A | MPNSTs | Further study needed | - | [118] |
| Carboplatin | Alkylating Agent | pNFs, LGGs, MPNSTs | Phase I Study (NCT00352495- Completed); Phase III Study (NCT03871257 Active, not recruiting); Phase III Study (NCT04166409-Recruiting) | Combinatorial | [119] |
| Carfilzomib | Proteosome | MPNSTs | Further study needed | - | [124] |
| Cephalomannine | HIF1A, APEX1, BCL2L1, MAPK14, SYK, TNF, ADAM17 | MPNSTs | Further study needed | - | [119] |
| Ceritinib | ALK | MPNSTs | Further study needed | - | [127] |
| Chloroquine/ Hydroxychloroquine | Lysosomal Alkalinizing Agent | pNFs, LGGs | Phase I/II Study (NCT04201457-Active, not recruiting) | Combinatorial | [50] |
| CI-1040 | MEK1/2 | GBMs | Further study needed | - | [136] |
| Cladribine | DCK | GBMs, MPNSTs | Further study needed | - | [119,136] |
| Clofarabine | RNR | MPNSTs | Further study needed | - | [119] |
| Clomifene Citrate | ESR1/2 | MPNSTs | Further study needed | - | [119] |
| Clomipramine HCl | Serotonin | pNFs | Further study needed | - | [104] |
| Cobimetinib | BRAF | MPNSTs | Further study needed | - | [123] |
| Copanlisib | PI3K | cNFs | Further study needed | - | [61] |
| Crizotinib | ALK/ROS1 | MPNSTs | Further study needed | - | [113,119,127] |
| Cucurbitacin-I | JAK/STAT3 | pNFs, MPNSTs | Further study needed | - | [106,108,109,111] |
| Dasatinib | BCR-ABL, RTKs | MPNSTs | Further study needed | - | [119] |
| Daunorubicin | TOP2A/B | pNFs, MPNSTs | Phase II Study (NCT00304083- Completed) | Combinatorial | [119,127] |
| Defactinib | RHO, FAK, Pyk2 | MPNSTs | Further study needed | - | [60] |
| Deguelin | AKT | Astrocytomas | Further study needed | - | [134] |
| Dehydrodeguelin | CI | Astrocytomas | Further study needed | - | [134] |
| Deltarasin | PDEδ | MPNSTs | Further study needed | - | [113] |
| Digoxin | Na+/K+ ATPase | cNFs, MPNSTs | Further study needed | - | [49,61] |
| Dinaciclib | CDK1/2/5/9 | pNFs | Further study needed | - | [129,140] |
| Doxorubicin | TOP2A/B, TOP1 | pNFs, MPNSTs | Phase II Study (NCT00304083- Completed) | Combinatorial | [104,119,124,127] |
| Duloxetine HCl | Serotonin | pNFs | Further study needed | - | [104] |
| Econazole Nitrate | Lanosterol 14-alpha Demethylase | cNFs | Further study needed | - | [47] |
| Elesclomol | Copper Chelator | MPNSTs | Further study needed | - | [124] |
| Entrectinib | ROS1/TRK1 | MPNSTs | Further study needed | - | [127] |
| Enzalutamide | AR | MPNSTs | Further study needed | - | [119] |
| Epirubicin | TOP2A/B | pNFs | Further study needed | - | [104,119,127] |
| Erastin | VDAC2/VDAC3 | MPNSTs | Further study needed | - | [113] |
| FLLL31 | JAK2/STAT3 | MPNSTs | Further study needed | - | [109] |
| FLLL32 | JAK2/STAT3 | pNFs | Further study needed | - | [108,110] |
| Fluvastatin | HMGCR, HDAC | pNFs | Further study needed | - | [104] |
| Foretinib | MET/VEGFR | MPNSTs | Further study needed | - | [127] |
| Fostamatinib | SYK | MPNSTs | Further study needed | - | [127] |
| GDC-0941 | PIK3CG | GBMs | Further study needed | - | [136] |
| GDC-0973 | MAP2K1 | GBMs | Further study needed | - | [136] |
| Geldanamycin | HSP90AB1 | pNFs | Further study needed | - | [104] |
| Gemcitabine | DNA Synthesis | Melanoma #, MPNSTs | Phase IB/II Study (NCT01418001- Terminated); Phase II Study (NCT01532687-Completed) | Combinatorial | [116] |
| GGTI-2Z | GGTI | MPNSTs | Further study needed | - | [86] |
| GNE-490 | PI3K | pNFs | Further study needed | - | [104] |
| GSK-2126458 | PI3K/mTOR | GBMs | Further study needed | - | [136] |
| GSK-2636771 | PI3K | pNFs | Further study needed | - | [104] |
| GSK-461364 | PLK1 | Melanoma #, MPNSTs | Further study needed | - | [116] |
| Homoharringtonine | RPL2/3 | MPNSTs | Further study needed | - | [119,127] |
| I-BET151 | BRD2/3/4 | MPNSTs | Further study needed | - | [125] |
| Idarubicin | TOP2A/B | pNFs | Further study needed | - | [104,119,127] |
| IKK-2 Inhibitor VII | IKK | pNFs | Further study needed | - | [104] |
| Imatinib | TRK | pNFs, MPNSTs | Phase I/II Study (NCT01140360- Completed); Phase II Study (NCT02177825- Terminated); Phase II Study (NCT01673009-Completed); Phase II Study (NCT03688568-Withdrawn); Phase II/III Study (NCT00427583- Terminated) | Monotherapy & Combinatorial | [108] |
| INK128 | mTOR | MPNSTs | Further study needed | - | [69,123] |
| Irinotecan | TOP1 | MPNSTs | Further study needed | - | [69] |
| Isradipine | Calcium Channels | pNFs | Further study needed | - | [104] |
| JNK inhibitor IX | JNK | MPNSTs | Further study needed | - | [113] |
| JQ1 | BRD4 | MPNSTs | Further study needed | - | [113,140] |
| Ketorolac | COX | pNFs | Further study needed | - | [104] |
| Lamotrigine | Sodium Channels | pNFs, MPNSTs | Phase II Study (NCT03504501- Terminated); Phase II/III Study (NCT02256124- Terminated) | Monotherapy | [119] |
| LDN-193189 | ALK | pNFs | Further study needed | - | [103] |
| Linagliptin | DPP4 | MPNSTs | Further study needed | - | [119] |
| Linsitinib | IGF1R | cNFs | Further study needed | - | [61] |
| Lomitapide | Cytochrome P450 3A4 | GBMs | Further study needed | - | [48] |
| Lovastatin | HMG-CoA | MPNSTs | Tested for NF1-related cognitive deficits (NCT00352599, NCT00853580), autism spectrum disorder (NCT03826940), and reading disabilities (NCT02964884) | Monotherapy | [84,85,86] |
| LY2606368 | CHEK1 | HGGs | Further study needed | - | [140] |
| LY3009120 | RAF | MPNSTs | Further study needed | - | [113] |
| Marizomib | Proteasome | GBMs | Further study needed | - | [136] |
| Mirdametinib/PD0325901 | MEK | pNFs, GBMs, MPNSTs | FDA-approved for pNFs | Monotherapy | [113,136] |
| Mitomycin C | Alkylating Agent | MPNSTs | Further study needed | - | [119,127] |
| Mitoxantrone | TOP2A/B | pNFs | Further study needed | - | [104,127] |
| MK-1775 | WEE1 | MPNSTs | Further study needed | - | [124] |
| MLN8237 | AURKA | MPNSTs | Further study needed | - | [117] |
| Mycophenolic Acid | IMPDH | pNFs | Further study needed | - | [104] |
| Napabucasin | STAT3 | MPNSTs | Further study needed | - | [111] |
| Neratinib | EGFR | MPNSTs | Further study needed | - | [124] |
| Niclosamide | STAT | MPNSTs | Further study needed | - | [113] |
| Nifedipine | Calcium Channels | MPNSTs | Further study needed | [118] | |
| NVP-BGT226 | PI3K | MPNSTs | Further study needed | - | [124] |
| Onalespib | HSP90 | cNFs | Further study needed | - | [61] |
| Osimertinib | EGFR | MPNSTs | Further study needed | - | [127] |
| Panobinostat | HDAC | MPNSTs | Further study needed | - | [124] |
| PD-318088 | MEK | GBMs | Further study needed | - | [136] |
| PF-04217903 | c-Met | MPNSTs | Further study needed | - | [109] |
| PF-3758309 | PAK1/2/3/4/5/6 | MPNSTs | Further study needed | - | [113] |
| PF04691502 | PI3K/mTOR | MPNSTs | Further study needed | - | [113] |
| Piboserod HCl | Serotonin | pNFs | Further study needed | - | [104] |
| Picropodophyllin | IGF1R | pNFs | Further study needed | - | [103] |
| Ponatinib | FGFR | MPNSTs | Further study needed | - | [119,124,127] |
| PTC596 | BMI1 | HGGs | Further study needed | - | [140] |
| R-1487 | p38α | pNFs | Further study needed | - | [104] |
| Rapamycin/Sirolimus | mTOR | pNFs, cNFs, LGGs, MPNSTs | Phase I Study (NCT01031901- Completed); Phase I Study; (NCT00901849-Completed); Phase II Study (NCT00634270 -Completed); Phase II Study (NCT03433183-Completed) | Monotherapy & Combinatorial | [69,109] |
| Retaspimycin | HSP90AB1 | pNFs | Further study needed | - | [104] |
| Ribociclib | CDK4/6 | MPNSTs | Further study needed | - | [125] |
| Rigosertib | PLK1, PI3K | Melanoma #, MPNSTs | Further study needed | - | [49,116] |
| RMC-7977 | RAS | Gliomas, MPNSTs | Further study needed | - | [87] |
| Romidepsin | HDAC | MPNSTs | Further study needed | - | [119,127] |
| Ruxolitinib | STAT3 | MPNSTs | Further study needed | - | [111] |
| SCH-900776 | CHK1 | pNFs | Further study needed | - | [104] |
| Selumetinib | MEK | pNFs, MPNSTs | FDA-approved for pNFs | Monotherapy | [113] |
| SH-4-54 | STAT3/5 | MPNSTs | Further study needed | - | [113] |
| Sibutramine HCl | Serotonin | pNFs | Further study needed | - | [104] |
| SN-38 | TOP1 | GBMs | Further study needed | - | [136] |
| Sorafenib | RAF | pNFs, MPNSTs | Phase I Study (NCT00727233- Completed) | Monotherapy | [113,119] |
| SU11274 | c-Met | MPNSTs | Further study needed | - | [109] |
| Sunitinib | RTKs | pNFs, MPNSTs | Phase II Study (NCT01402817- Terminated) | Monotherapy | [68,119] |
| TAK-285 | HER2/EGFR | pNFs | Further study needed | - | [104] |
| TAK-632 | RAF/VEGFR | MPNSTs | Further study needed | - | [113] |
| TAK-733 | MEK | pNFs, GBMs | Further study needed | - | [129,136] |
| Teniposide | TOP2A/B | MPNSTs | Further study needed | - | [127] |
| Thapsigargin | SERCA | MPNSTs | Further study needed | - | [124] |
| Tipifarnib | FTI | MPNSTs | Phase II Study (NCT00021541- Completed) | Monotherapy | [113] |
| Tivozanib | VEGFR | pNFs | Further study needed | - | [104] |
| Topotecan | TOP1 | MPNSTs | Further study needed | - | [127,136] |
| Torin-2 | mTORC | MPNSTs | Further study needed | - | [124] |
| Torkinib | mTOR | MPNSTs | Further study needed | - | [113] |
| Trabectedin | Alkylating Agent | MPNSTs | Further study needed | - | [127] |
| Trametinib | MEK | pNFs, MPNSTs | Phase II Study (NCT03741101 -Active, not recruiting) | Monotherapy | [68,104,123,136] |
| Tranilast | NLRP3, TGFB, MAPK | pNFs, MPNSTs | Further study needed | - | [121] |
| Triciribine phosphate | AKT1 | pNFs | Further study needed | - | [104] |
| UC1 | NAB3; Proposed | MPNSTs | Further study needed | - | [54] |
| UNC2250 | MER | MPNSTs | Further study needed | - | [127] |
| Vandetanib | RET, EGFR, VEGFR | MPNSTs | Further study needed | - | [119] |
| Varlitinib tosylate | EGFR | pNFs | Further study needed | - | [104] |
| Verteporfin | YAP | MPNSTs | Further study needed | - | [60] |
| Vincristine | TUBB, TUBA4A | pNFs, LGGs, MPNSTs | Phase II Study (NCT00846430- Completed); Phase III Study (NCT03871257- Active, not recruiting); Phase III Study (NCT04166409-Recruiting) | Combinatorial | [119] |
| Volasertib/BI6727 | PLK1 | Melanoma #, MPNSTs | Further study needed | - | [116,117] |
| Vorinostat | HDAC | MPNSTs | Further study needed | - | [49,119,127,140] |
| Y100/Y100B | Unknown | GBMs | Further study needed | - | [130] |
| Y102 | BORC; Proposed | GBMs, pNFs, MPNSTs | Further study needed | - | [131] |
9. Discussion and Future Directions
9.1. MEK Inhibitor Resistance Screens
9.2. Alternative High-Throughput Screening (HTS) Models for Drug Discovery
9.3. Targeting the Tumor Microenvironment
9.4. The Role of Artificial Intelligence in Drug Discovery
9.5. Addressing “Undruggable” Targets
10. Conclusions
| Name | Source/Model | NF1 Mutation(s) (If Known) | NF1 Status | Used in |
|---|---|---|---|---|
| iPSCs and derived cell lines | ||||
| Patient #2 NF1+/− iPSC | iPSC derived from peripheral blood mononuclear cells from NF1 patient | c.3431_3432dupGT | NF1+/− | [47] |
| Patient #2 NF1−/− iPSC | Patient #2 NF1+/− iPSC with CRISPR KO of remaining wild-type NF1 allele | c.3431_3432dupGT; LOH | NF1−/− | [47] |
| WTC-mEGFP-Safe harbor locus (AAVS1)-cl6 (RRID:CVCL_JM19) | iPSC from healthy individual with eGFP inserted at the safe harbor locus AAVS1 under CAGGS (Coriell Institute (NJ, USA) | n/a | WT | [48] |
| mEGFP-PT | iPSC mEGFP with CRISPR PTEN and TP53 loss | n/a | WT | [48] |
| mEGFP PTCC | iPSC mEGFP iPSC with CRISPR PTEN, TP53, and CDKN2A/B loss | n/a | WT | [48] |
| mEGFP PTN | iPSC mEGFP iPSC with CRISPR PTEN, TP53, and NF1 loss | NF1 CRISPR KO | NF1−/− | [48] |
| C6-a (RUID: 06C53141) | iPSC from healthy individual—RUCDR Infinite Biologics | n/a | WT | [48] |
| C6-a PT | C6-a with CRISPR PTEN and TP53 loss | n/a | WT | [48] |
| C6-a PTCC | C6-a with CRISPR PTEN, TP53 and CDKN2A/B loss | n/a | WT | [48] |
| C6-a PTN | C6-a with CRISPR PTEN, TP53, and NF1 loss | NF1 CRISPR KO | NF1−/− | [48] |
| Organoids derived from cutaneous neurofibroma (cNF) NF1 patient samples | ||||
| NF0002-7 | cNF | NF1 deletion (by whole genome sequencing) | NF1−/− | [61] |
| NF0004 | cNF | No sequencing data available | NF1−/− | [61] |
| NF0004-6_8 | cNF | No sequencing data available | NF1−/− | [61] |
| NF0009 | cNF | NF1 inversion (by whole genome sequencing) | NF1−/− | [61] |
| NF00012 | cNF | NF1 deletion, inversion, and stop gain (by whole genome sequencing) | NF1−/− | [61] |
| Epithelial-like and fibroblast cell lines | ||||
| HSC1 | Epithelial-like primary culture isolated from human spinal nerves | n/a | WT | [116] |
| HSC2 | Primary culture isolated from human spinal nerves | n/a | WT | [116] |
| HFF | Fibroblasts | n/a | WT | [42] |
| Immortalized Schwann cell (SC) lines including those derived from plexiform neurofibromas (pNFs) | ||||
| ipn02.3 2λ | Normal SC from healthy individual | n/a | WT | [41,50,102,103,111,129] |
| ipn02.3 2λ C8 | CRISPR-edited ipn02.3 2λ | Exon 3 (1 bp del) | NF1+/− | [50] |
| ipn02.3 2λ C23 | CRISPR-edited ipn02.3 2λ | Exon 3 (2 bp del), Exon 3 (1 bp del) | NF1−/− | [50] |
| ipn02.8 | Normal SC from healthy individual | n/a | WT | [41,102,103] |
| ipn97.4 | Normal SC from healthy individual | n/a | WT | [41,103] |
| ipNF00.6 | pNF | Gene deletion (>1 Mb); Unknown | NF1−/− | [41] |
| ipNF03.3 | pNF | c.4269 G > A in-frame exon skip; Unknown | NF1−/− | [41] |
| ipNF04.4 | pNF | R2237X; LOH | NF1−/− | [41,102] |
| sipnNF95.12B | pNF | L216P | NF1+/− | [41,102] |
| ipNF05.5 | pNF | c.3456_3457insA; LOH | NF1−/− | [41,50,102,111,129] |
| ipNF05.5-MX (six clone mix) | pNF | c.3456_3457insA; LOH | NF1−/− | [41,102,104] |
| ipNF06.2A | pNF | G848W; Unknown | NF1−/− | [41,102,104] |
| ipNF95.11b C | pNF | c.1756delACTA; LOH | NF1−/− | [41,50,102,103,129,131] |
| ipNF95.11b C/T | pNF | c.1756delACTA; LOH | NF1−/− | [41,103,104] |
| ipNF95.6 | pNF | R816X; R2237X | NF1−/− | [41,102,103,104,111,129] |
| ipnNF09.4 | pNF | c.3456_3457insA | NF1+/− | [41,50] |
| ipnNF95.11C | pNF | c.1756delACTA | NF1+/− | [41,50,102,104,131] |
| HSC1λ | Normal SC—ipn02.3 2λ | n/a | WT | [49,60] |
| HSC1λ N0 (5) | CRISPR-derived from HSC1λ line | NF1 indels in exon 10 on both alleles | NF1−/− | [49,60] |
| HSC1λ N1 (10) | Went through the CRISPR process—unedited | n/a | WT | [49,60] |
| Malignant peripheral nerve sheath tumor (MPNST) cell line models | ||||
| ST88-3/88-3/NF88-3 | MPNST | c.6952T > C; LOH | NF1−/− | [78,113,117,118] |
| NF90-8/90-8/90.8/90-8TL | MPNST | p.Asp1302Tyrfs*5 (c.3904_3910del); LOH | NF1−/− | [69,78,84,111,113,117,118,125] |
| ST88-14/ST8814 ☨ | MPNST | p.Arg304Ter (c.910C > T); LOH | NF1−/− | [50,69,78,84,87,106,109,111,113,116,117,118,125,128] |
| T265 ^ | MPNST | p.Arg304Ter (c.910C > T); LOH | NF1−/− | [54,113,117,128] |
| S1844.1 | MPNST | LOH in both alleles | NF1−/− | [109] |
| S1507.2/S1507-2 | MPNST | Splicing mutation in intron 23-1; deletion in exon 10a | NF1−/− | [109,116] |
| S462 | MPNST | c.6792C > A; LOH | NF1−/− | [60,69,87,109,111,116,124,125,128,129] |
| YST-1 | Sporadic MPNST | n/a | WT | [116] |
| sNF94.3 | MPNST | Microdeletion | NF1+/− | [118,123] |
| SNF10.1 | MPNST | R1276X; Hemizygous deletion | NF1−/− | [123] |
| sNF02.2 | MPNST | c.4868A > T | NF1+/− | [113,118,123] |
| sNF96.2 | MPNST | p.Asn1229Metfs*1; LOH | NF1−/− | [69,113,118,121,123,124,125,131] |
| HS-PSS | Sporadic MPNST | n/a | WT | [116,124] |
| S462.TY/S462 TY | MPNST | p.Tyr2285Ter (c.6855C > A); p.Tyr2264Ter, (c.6792C > A) | NF1−/− | [49,85,113,117,118,128] |
| NCC-MPNST1-C1 | MPNST | Mutation not detected | NF1−/− (Presumed) | [119,127] |
| NCC-MPNST2-C1 | Sporadic MPNST | p.Leu179Tyrfs*11 (c.536_539del) | NF1−/− (Presumed) | [119,127] |
| NCC-MPNST3-C1 | MPNST | p.Arg816Ter (c.2446C > T) | NF1−/− (Presumed) | [119,127] |
| NCC-MPNST3-X2-C1 | From 2nd generation xenograft of MPNST | p.Arg816Ter (c.2446C > T) | NF1−/− (Presumed) | [119] |
| NCC-MPNST4-C1 | Sporadic MPNST | c.5812 + 3delAGTA | NF1−/− (Presumed) | [119,127] |
| NCC-MPNST5-C1 | MPNST | p.Thr586Valfs*18 (c.1756_1759delACTA) | NF1−/− (Presumed) | [119,127] |
| NCC-MPNST6-C1 | Sporadic MPNST | Not Reported | NF1+/+ (Presumed) | [127] |
| NMS-2 | MPNST | c.7062 + 1G > T (c.6999 + 1G > T); LOH | NF1−/− | [125] |
| NF1-08 | MPNST | c.701_730 + 10del; LOH | NF1−/− | [125] |
| NF1-09 | MPNST | c.6792C > A p.(Tyr2264*); c.1186-5_1186-1del | NF1−/− | [125] |
| NF1-18B | MPNST | c.1642-449 > G; LOH | NF1−/− | [125] |
| SP-10 | Sporadic MPNST | g.30922951_31318216del | NF1−/− | [125] |
| Patient-derived MPNST xenograft cell line models | ||||
| JH-2-002 | PDX | Microdeletion; c.6308T > C and c.6309_6310del | NF1−/− | [87] |
| JH-2-031 | PDX | Microdeletion; c.4771dup | NF1−/− | [87] |
| JH-2-079 | PDX | c.5812 + 1G > A; p.C2223X (c.6669C > A) | NF1−/− | [87] |
| NF1-18B | PDOX | c.1642-449 > G; LOH | NF1−/− | [125] |
| SP-10 | PDOX | g.30922951_31318216del | NF1−/− | [125] |
| Melanoma # | ||||
| STS-26T | Melanoma # | n/a | WT | [54,69,86,113,116,117,123,124,128] |
| HS-Sch-2 | Melanoma # | c.3113 + 1G > A; p.Glu91Asnfs*6 (c.270_288del) | NF1−/− | [116,124] |
| High-grade glioma (HGG) cell line models | ||||
| JHH-NF1-GBM1 | NF1-associated Human HGG | Loss of function of NF1 | No detectable protein by western | [142] |
| TM-31 | Human HGG | pLF1247fs*18, homozygous | NF1−/− | [140] |
| LN319 | Human HGG | Total inactivation | NF1−/− | [140] |
| NF1-HGG 17 | HGG from NPcis (Nf1+/− and Tp53+/− in cis) mouse | Complete Nf1 loss | Nf1−/− | [140] |
| NF1-HGG 5653 | HGG from NPcis (Nf1+/− and Tp53+/− in cis) mouse | Complete Nf1 loss | Nf1−/− | [140] |
| NF1-HGG 5746 | HGG from NPcis (Nf1+/− and Tp53+/− in cis) mouse | Complete Nf1 loss | Nf1−/− | [140] |
| Glioblastoma (GBM) cell line models | ||||
| BR 23C | GBM = | I526S | NF1−/− (Presumed) | [136] |
| HSR-GBM1 | GBM = | A1676T | NF1+/− | [136] |
| JHH-68 | Non-NF1 GBM | n/a | WT | [136] |
| JHH-136 | Non-NF1 GBM | n/a | WT | [136] |
| JHH-227 | Non-NF1 GBM | n/a | WT | [136] |
| JHH-505 | Non-NF1 GBM | n/a | WT | [136] |
| JHH-520 | GBM = | Homozygous deletion | NF1−/− | [136] |
| JHU-0879 | Non-NF1 GBM | n/a | WT | [136] |
| JHU-1016B | GBM = | L115T fs*42 | NF1+/− | [136] |
| GBM43 | GBM = | Inactivating mutation; no detectable protein by western | NF1−/− | [137] |
| SB28 | C57BL/6 Mouse GBM | n/a | WT | [137] |
| U251-MG | Non-NF1 GBM | c.2033dupC; no WT allele present | NF1−/− | [130,131,142] |
| U251 ATRX−/− | Non-NF1 GBM; CRISPR modified for ATRX deletion | c.2033dupC; no WT allele present | NF1−/− | [142] |
| U251 ATRX−/2.02 | Non-NF1 GBM; CRISPR modified for ATRX deletion | c.2033dupC; no WT allele present | NF1−/− | [142] |
| U87-MG | Non-NF1 GBM | Proteasome-mediated degradation of NF1 | No detectable protein by western | [130,131] |
| LN229 | GBM = | Total gene deletion | NF1−/− | [87] |
| U373 | Non-NF1 GBM | Unknown | No detectable protein by western | [87] |
| Mouse Embryonic Fibroblast (MEF) cell line models | ||||
| Nf1wt | MEFs derived from Nf1wt E1A-p53 mouse | n/a | WT | [118] |
| Nf1−/− | MEFs derived from Nf1−/− E1A-p53 mouse | Complete Nf1 loss | Nf1−/− | [118] |
| Mouse astrocytoma cell line models | ||||
| Nf1GFAPCKO | Astrocytes from Nf1GFAPCKO mouse | Nf1−/− astrocytes only | Nf1−/− | [106] |
| K5001 | Spontaneous astrocytoma from NPcis (Nf1+/− and Tp53+/− in cis) mouse | Mutant Nf1 allele; loss of WT allele | Nf1−/− | [134] |
| KR158 | Spontaneous astrocytoma from NPcis (Nf1+/− and Tp53+/− in cis) mouse | Mutant Nf1 allele; loss of WT allele | Nf1−/− | [134] |
| K1492 | Spontaneous astrocytoma from NPcis (Nf1+/− and Tp53+/− in cis) mouse | Mutant Nf1 allele; loss of WT allele | Nf1−/− | [134] |
| Mouse models | ||||
| Dhh-Cre; Nf1fl/fl | Mouse | Cre recombinase removes both Nf1 alleles in Schwann cells | Nf1−/− | [110] |
| LSL-Trp53R270H (Cnp-hEGFR background) | Mouse | Conditional mutant alleles of p53 using loxP-STOP-loxP | WT | [128] |
| Saccharomyces cerevisiae (Budding yeast) models | ||||
| MLY41a | Yeast | n/a | WT | [54,130,131] |
| MDW057 | Yeast | n/a | WT | [54,130,131] |
| MDW028 | Yeast | ira2Δ | ira2−/− | [54,130,131] |
| MDW035 | Yeast | erg6Δira2Δ | ira2−/− | [54,130,131] |
| Drosophila melanogaster (Fruit fly) models | ||||
| S2R+ | Drosophila Schneider 2 cells | n/a | WT | [50] |
| S2R+ NF1-KO & | CRISPR-derived Drosophila Schneider 2 cells | del c.148–161, del c.150–160, del c.148–160 | dNf1−/− | [50] |
| Nf1C1 | dNf1-deficient Drosophila model | del c.160–161AT | dNf1−/− | [50] |
| Danio rerio (Zebrafish) models | ||||
| nf1a+/−; nf1b−/−; p53m/m; sox10:mCherry | Zebrafish | Homozygous loss of nf1b and heterozygous for nf1a | nf1a+/−; nf1b−/− | [69] |
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC50 | Half-maximal Activity Concentration |
| AI | Artificial Intelligence |
| CCLE | Cancer Cell Line Encyclopedia |
| CI | Complex I (Mitochondrial) |
| cNFs | Cutaneous Neurofibromas |
| CQ | Chloroquine |
| DSRT | Drug Sensitivity and Resistance Testing |
| ESCs | Embryonic Stem Cells |
| EMT | Epithelial–mesenchymal Transition |
| FDA | Food & Drug Administration |
| FTIs | Farnesyltransferase Inhibitors |
| GAPs | GTPase-activating Proteins |
| GBMs | Glioblastomas |
| GDP | Guanosine Diphosphate |
| GEFs | Guanine Nucleotide Exchange Factors |
| GEMM | Genetically Engineered Mouse Model |
| GIST | Gastrointestinal Stromal Tumor |
| GRD | GAP-related Domain |
| GTP | Guanosine Triphosphate |
| HGGs | High-grade Gliomas |
| HTS | High-throughput screening |
| icNFs | Immortalized Cutaneous Neurofibromas |
| iPSCs | Induced Pluripotent Stem Cells |
| ipNFs | Immortalized Plexiform Neurofibromas |
| KO | Knockout |
| LGGs | Low-Grade Gliomas |
| LOH | Loss-of-Heterozygosity |
| MEFs | Mouse Embryonic Fibroblasts |
| MPNSTs | Malignant Peripheral Nerve Sheath Tumors |
| NCATS | National Center for Advancing Translational Sciences |
| NCI | National Cancer Institute |
| NF1 | Neurofibromatosis type 1 |
| OPGs | Optic Pathway Gliomas |
| PDOX | Patient-derived Orthotopic Xenograft |
| PDX | Patient-derived Xenograft |
| PLK1 | Polo-like Kinase 1 |
| pNFs | Plexiform Neurofibromas |
| RTKs | Receptor Tyrosine Kinases |
| SOS | Son of Sevenless |
| TGF-β2 | Transforming Growth Factor-β2 |
| TNF-α | Tumor Necrosis Factor-α |
| WT | Wild-type |
References
- Lee, T.-S.J.; Chopra, M.; Kim, R.H.; Parkin, P.C.; Barnett-Tapia, C. Incidence and Prevalence of Neurofibromatosis Type 1 and 2: A Systematic Review and Meta-Analysis. Orphanet J. Rare Dis. 2023, 18, 292. [Google Scholar] [CrossRef]
- Gutmann, D.H.; Ferner, R.E.; Listernick, R.H.; Korf, B.R.; Wolters, P.L.; Johnson, K.J. Neurofibromatosis Type 1. Nat. Rev. Dis. Primers 2017, 3, 17004. [Google Scholar] [CrossRef]
- Ruggieri, M.; Praticò, A.D.; Caltabiano, R.; Polizzi, A. Early History of the Different Forms of Neurofibromatosis from Ancient Egypt to the British Empire and beyond: First Descriptions, Medical Curiosities, Misconceptions, Landmarks, and the Persons behind the Syndromes. Am. J. Med. Genet. A 2018, 176, 515–550. [Google Scholar] [CrossRef]
- Zhu, Y.; Ghosh, P.; Charnay, P.; Burns, D.K.; Parada, L.F. Neurofibromas in NF1: Schwann Cell Origin and Role of Tumor Environment. Science 2002, 296, 920–922. [Google Scholar] [CrossRef]
- Yap, Y.-S.; McPherson, J.R.; Ong, C.-K.; Rozen, S.G.; Teh, B.-T.; Lee, A.S.G.; Callen, D.F. The NF1 Gene Revisited—From Bench to Bedside. Oncotarget 2014, 5, 5873–5892. [Google Scholar] [CrossRef]
- Landry, J.P.; Schertz, K.L.; Chiang, Y.-J.; Bhalla, A.D.; Yi, M.; Keung, E.Z.; Scally, C.P.; Feig, B.W.; Hunt, K.K.; Roland, C.L.; et al. Comparison of Cancer Prevalence in Patients with Neurofibromatosis Type 1 at an Academic Cancer Center vs in the General Population from 1985 to 2020. JAMA Netw. Open 2021, 4, e210945. [Google Scholar] [CrossRef] [PubMed]
- Beert, E.; Brems, H.; Daniëls, B.; De Wever, I.; Van Calenbergh, F.; Schoenaers, J.; Debiec-Rychter, M.; Gevaert, O.; De Raedt, T.; Van Den Bruel, A.; et al. Atypical Neurofibromas in Neurofibromatosis Type 1 Are Premalignant Tumors. Genes Chromosomes Cancer 2011, 50, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.M.; Antonescu, C.R.; Fletcher, C.D.M.; Kim, A.; Lazar, A.J.; Quezado, M.M.; Reilly, K.M.; Stemmer-Rachamimov, A.; Stewart, D.R.; Viskochil, D.; et al. Histopathologic Evaluation of Atypical Neurofibromatous Tumors and Their Transformation into Malignant Peripheral Nerve Sheath Tumor in Patients with Neurofibromatosis 1-a Consensus Overview. Hum. Pathol. 2017, 67, 1–10. [Google Scholar] [CrossRef]
- Hirbe, A.C.; Dahiya, S.; Miller, C.A.; Li, T.; Fulton, R.S.; Zhang, X.; McDonald, S.; DeSchryver, K.; Duncavage, E.J.; Walrath, J.; et al. Whole Exome Sequencing Reveals the Order of Genetic Changes during Malignant Transformation and Metastasis in a Single Patient with NF1-Plexiform Neurofibroma. Clin. Cancer Res. 2015, 21, 4201–4211. [Google Scholar] [CrossRef] [PubMed]
- Sohier, P.; Luscan, A.; Lloyd, A.; Ashelford, K.; Laurendeau, I.; Briand-Suleau, A.; Vidaud, D.; Ortonne, N.; Pasmant, E.; Upadhyaya, M. Confirmation of Mutation Landscape of NF1-Associated Malignant Peripheral Nerve Sheath Tumors. Genes Chromosomes Cancer 2017, 56, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Carrió, M.; Gel, B.; Terribas, E.; Zucchiatti, A.C.; Moliné, T.; Rosas, I.; Teulé, Á.; Ramón Y Cajal, S.; López-Gutiérrez, J.C.; Blanco, I.; et al. Analysis of Intratumor Heterogeneity in Neurofibromatosis Type 1 Plexiform Neurofibromas and Neurofibromas with Atypical Features: Correlating Histological and Genomic Findings. Hum. Mutat. 2018, 39, 1112–1125. [Google Scholar] [CrossRef]
- Pemov, A.; Hansen, N.F.; Sindiri, S.; Patidar, R.; Higham, C.S.; Dombi, E.; Miettinen, M.M.; Fetsch, P.; Brems, H.; Chandrasekharappa, S.C.; et al. Low Mutation Burden and Frequent Loss of CDKN2A/B and SMARCA2, but Not PRC2, Define Premalignant Neurofibromatosis Type 1-Associated Atypical Neurofibromas. Neuro-Oncology 2019, 21, 981–992. [Google Scholar] [CrossRef]
- Cortes-Ciriano, I.; Steele, C.D.; Piculell, K.; Al-Ibraheemi, A.; Eulo, V.; Bui, M.M.; Chatzipli, A.; Dickson, B.C.; Borcherding, D.C.; Feber, A.; et al. Genomic Patterns of Malignant Peripheral Nerve Sheath Tumor (MPNST) Evolution Correlate with Clinical Outcome and Are Detectable in Cell-Free DNA. Cancer Discov. 2023, 13, 654–671. [Google Scholar] [CrossRef]
- Lim, Z.; Gu, T.Y.; Tai, B.C.; Puhaindran, M.E. Survival Outcomes of Malignant Peripheral Nerve Sheath Tumors (MPNSTs) with and without Neurofibromatosis Type I (NF1): A Meta-Analysis. World J. Surg. Oncol. 2024, 22, 14. [Google Scholar] [CrossRef]
- Foiadelli, T.; Naso, M.; Licari, A.; Orsini, A.; Magistrali, M.; Trabatti, C.; Luzzi, S.; Mosconi, M.; Savasta, S.; Marseglia, G.L. Advanced Pharmacological Therapies for Neurofibromatosis Type 1-Related Tumors. Acta Biomed. 2020, 91, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Dib, A.; Beaini, S.; Saad, C.; Faraj, S.; El Joueid, Y.; Kotob, Y.; Saoudi, L.; Emmanuel, N. Neurofibromatosis Type 1 System-Based Manifestations and Treatments: A Review. Neurol. Sci. 2023, 44, 1931–1947. [Google Scholar] [CrossRef]
- Romo, C.G.; Piotrowski, A.F.; Campian, J.L.; Diarte, J.; Rodriguez, F.J.; Bale, T.A.; Dahiya, S.; Gutmann, D.H.; Lucas, C.-H.G.; Prichett, L.; et al. Clinical, Histological, and Molecular Features of Gliomas in Adults with Neurofibromatosis Type 1. Neuro Oncol. 2023, 25, 1474–1486. [Google Scholar] [CrossRef] [PubMed]
- Kerashvili, N.; Gutmann, D.H. The Management of Neurofibromatosis Type 1 (NF1) in Children and Adolescents. Expert Rev. Neurother. 2024, 24, 409–420. [Google Scholar] [CrossRef]
- Kotch, C.; de Blank, P.; Gutmann, D.H.; Fisher, M.J. Low-Grade Glioma in Children with Neurofibromatosis Type 1: Surveillance, Treatment Indications, Management, and Future Directions. Child’s Nerv. Syst. 2024, 40, 3241–3250. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, J.; Ge, S.; Jia, R.; Song, X.; Wang, Y.; Fan, X. An Overview of Optic Pathway Glioma with Neurofibromatosis Type 1: Pathogenesis, Risk Factors, and Therapeutic Strategies. Investig. Ophthalmol. Vis. Sci. 2024, 65, 8. [Google Scholar] [CrossRef]
- Amato, A.; Imbimbo, B.P.; Falsini, B. Neurofibromatosis Type 1-Associated Optic Pathway Gliomas: Pathogenesis and Emerging Treatments. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 5636–5653. [Google Scholar] [CrossRef]
- Bellampalli, S.S.; Khanna, R. Towards a Neurobiological Understanding of Pain in Neurofibromatosis Type 1: Mechanisms and Implications for Treatment. Pain 2019, 160, 1007–1018. [Google Scholar] [CrossRef]
- Seizinger, B.R.; Rouleau, G.A.; Lane, A.H.; Farmer, G.; Ozelius, L.J.; Haines, J.L.; Parry, D.M.; Korf, B.R.; Pericak-Vance, M.A.; Faryniarz, A.G. Linkage Analysis in von Recklinghausen Neurofibromatosis (NF1) with DNA Markers for Chromosome 17. Genomics 1987, 1, 346–348. [Google Scholar] [CrossRef]
- Fain, P.R.; Wright, E.; Willard, H.F.; Stephens, K.; Barker, D.F. The Order of Loci in the Pericentric Region of Chromosome 17, Based on Evidence from Physical and Genetic Breakpoints. Am. J. Hum. Genet. 1989, 44, 68–72. [Google Scholar]
- Martin, G.A.; Viskoohil, D.; Bollag, G.; McCabe, P.C.; Crosier, W.J.; Haubruck, H.; Conroy, L.; Clark, R.; O’Connell, P.; Cawthon, R.M.; et al. The GAP-Related Domain of the Neurofibromatosis Type 1 Gene Product Interacts with Ras P21. Cell 1990, 63, 843–849. [Google Scholar] [CrossRef]
- Viskochil, D.; Buchberg, A.M.; Xu, G.; Cawthon, R.M.; Stevens, J.; Wolff, R.K.; Culver, M.; Carey, J.C.; Copeland, N.G.; Jenkins, N.A. Deletions and a Translocation Interrupt a Cloned Gene at the Neurofibromatosis Type 1 Locus. Cell 1990, 62, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.R.; Marchuk, D.A.; Andersen, L.B.; Letcher, R.; Odeh, H.M.; Saulino, A.M.; Fountain, J.W.; Brereton, A.; Nicholson, J.; Mitchell, A.L. Type 1 Neurofibromatosis Gene: Identification of a Large Transcript Disrupted in Three NF1 Patients. Science 1990, 249, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Antônio, J.R.; Goloni-Bertollo, E.M.; Trídico, L.A. Neurofibromatosis: Chronological History and Current Issues. An. Bras. Dermatol. 2013, 88, 329–343. [Google Scholar] [CrossRef]
- Ballester, R.; Marchuk, D.; Boguski, M.; Saulino, A.; Letcher, R.; Wigler, M.; Collins, F. The NF1 Locus Encodes a Protein Functionally Related to Mammalian GAP and Yeast IRA Proteins. Cell 1990, 63, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.; Bollag, G.; Shannon, K. Hyperactive Ras as a Therapeutic Target in Neurofibromatosis Type 1. Am. J. Med. Genet. A 1999, 89, 14–22. [Google Scholar] [CrossRef]
- Gross, A.M.; Wolters, P.L.; Dombi, E.; Baldwin, A.; Whitcomb, P.; Fisher, M.J.; Weiss, B.; Kim, A.; Bornhorst, M.; Shah, A.C.; et al. Selumetinib in Children with Inoperable Plexiform Neurofibromas. N. Engl. J. Med. 2020, 382, 1430–1442. [Google Scholar] [CrossRef]
- Gross, A.M.; Dombi, E.; Wolters, P.L.; Baldwin, A.; Dufek, A.; Herrera, K.; Martin, S.; Derdak, J.; Heisey, K.S.; Whitcomb, P.M.; et al. Long-Term Safety and Efficacy of Selumetinib in Children with Neurofibromatosis Type 1 on a Phase 1/2 Trial for Inoperable Plexiform Neurofibromas. Neuro Oncol. 2023, 25, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Moertel, C.L.; Hirbe, A.C.; Shuhaiber, H.H.; Bielamowicz, K.; Sidhu, A.; Viskochil, D.; Weber, M.D.; Lokku, A.; Smith, L.M.; Foreman, N.K.; et al. ReNeu: A Pivotal, Phase IIb Trial of Mirdametinib in Adults and Children with Symptomatic Neurofibromatosis Type 1-Associated Plexiform Neurofibroma. J. Clin. Oncol. 2025, 43, 716–729. [Google Scholar] [CrossRef]
- Kulkarni, V.S.; Alagarsamy, V.; Solomon, V.R.; Jose, P.A.; Murugesan, S. Drug Repurposing: An Effective Tool in Modern Drug Discovery. Russ. J. Bioorganic Chem. 2023, 49, 157–166. [Google Scholar] [CrossRef]
- Macarron, R.; Banks, M.N.; Bojanic, D.; Burns, D.J.; Cirovic, D.A.; Garyantes, T.; Green, D.V.S.; Hertzberg, R.P.; Janzen, W.P.; Paslay, J.W.; et al. Impact of High-Throughput Screening in Biomedical Research. Nat. Rev. Drug Discov. 2011, 10, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Popova, A.A.; Levkin, P.A. Precision Medicine in Oncology: In Vitro Drug Sensitivity and Resistance Test (DSRT) for Selection of Personalized Anticancer Therapy. Adv. Ther. 2020, 3, 1900100. [Google Scholar] [CrossRef]
- Nijman, S.M.B. Synthetic Lethality: General Principles, Utility and Detection Using Genetic Screens in Human Cells. FEBS Lett. 2011, 585, 1–6. [Google Scholar] [CrossRef]
- Jia, Z.-C.; Yang, X.; Wu, Y.-K.; Li, M.; Das, D.; Chen, M.-X.; Wu, J. The Art of Finding the Right Drug Target: Emerging Methods and Strategies. Pharmacol. Rev. 2024, 76, 896–914. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.A.; Kozakewich, H.P.; Hoffer, F.A.; Lage, J.M.; Weidner, N.; Tepper, R.; Pinkus, G.S.; Morton, C.C.; Corson, J.M. Diagnostic Relevance of Clonal Cytogenetic Aberrations in Malignant Soft-Tissue Tumors. N. Engl. J. Med. 1991, 324, 436–442. [Google Scholar] [CrossRef]
- Frahm, S.; Mautner, V.-F.; Brems, H.; Legius, E.; Debiec-Rychter, M.; Friedrich, R.E.; Knöfel, W.T.; Peiper, M.; Kluwe, L. Genetic and Phenotypic Characterization of Tumor Cells Derived from Malignant Peripheral Nerve Sheath Tumors of Neurofibromatosis Type 1 Patients. Neurobiol. Dis. 2004, 16, 85–91. [Google Scholar] [CrossRef]
- Li, H.; Chang, L.-J.; Neubauer, D.R.; Muir, D.F.; Wallace, M.R. Immortalization of Human Normal and NF1 Neurofibroma Schwann Cells. Lab. Investig. 2016, 96, 1105–1115. [Google Scholar] [CrossRef]
- Ferrer, M.; Gosline, S.J.C.; Stathis, M.; Zhang, X.; Guo, X.; Guha, R.; Ryman, D.A.; Wallace, M.R.; Kasch-Semenza, L.; Hao, H.; et al. Pharmacological and Genomic Profiling of Neurofibromatosis Type 1 Plexiform Neurofibroma-Derived Schwann Cells. Sci. Data 2018, 5, 180106. [Google Scholar] [CrossRef]
- Li, H.; Pemov, A.; Allaway, R.; Muir, D.F.; Chang, L.-J.; Banerjee, J.; Scott, A.J.; Nagy, J.M.W.; Liu, J.; Carrió, M.; et al. Immortalization and Characterization of Schwann Cell Lines Derived from NF1 Associated Cutaneous Neurofibromas. bioRxiv 2025. [Google Scholar] [CrossRef]
- Hatzis, C.; Bedard, P.L.; Birkbak, N.J.; Beck, A.H.; Aerts, H.J.W.L.; Stem, D.F.; Shi, L.; Clarke, R.; Quackenbush, J.; Haibe-Kains, B. Enhancing Reproducibility in Cancer Drug Screening: How Do We Move Forward? Cancer Res. 2014, 74, 4016–4023. [Google Scholar] [CrossRef] [PubMed]
- Niepel, M.; Hafner, M.; Mills, C.E.; Subramanian, K.; Williams, E.H.; Chung, M.; Gaudio, B.; Barrette, A.M.; Stern, A.D.; Hu, B.; et al. A Multi-Center Study on the Reproducibility of Drug-Response Assays in Mammalian Cell Lines. Cell Syst. 2019, 9, 35–48.e5. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Schwenkel, G.J.; Mattingly, R.R.; Sundararaghavan, H.G.; Zhang, Z.G.; Chopp, M. A Fibroblast-Derived Secretome Stimulates the Growth and Invasiveness of 3D Plexiform Neurofibroma Spheroids. Cancers 2024, 16, 2498. [Google Scholar] [CrossRef] [PubMed]
- Lakes, Y.B.; Moye, S.L.; Mo, J.; Tegtmeyer, M.; Nehme, R.; Charlton, M.; Salinas, G.; McKay, R.M.; Eggan, K.; Le, L.Q. Econazole Selectively Induces Cell Death in NF1-Homozygous Mutant Tumor Cells. Cell Rep. Med. 2023, 4, 101309. [Google Scholar] [CrossRef]
- Wang, C.; Sun, M.; Shao, C.; Schlicker, L.; Zhuo, Y.; Harim, Y.; Peng, T.; Tian, W.; Stöffler, N.; Schneider, M.; et al. A Multidimensional Atlas of Human Glioblastoma-like Organoids Reveals Highly Coordinated Molecular Networks and Effective Drugs. NPJ Precis. Oncol. 2024, 8, 19. [Google Scholar] [CrossRef]
- Williams, K.B.; Larsson, A.T.; Keller, B.J.; Chaney, K.E.; Williams, R.L.; Bhunia, M.M.; Draper, G.M.; Jubenville, T.A.; Hudson, W.A.; Georg, G.I.; et al. Pharmacogenomic Synthetic Lethal Screens Reveal Hidden Vulnerabilities and New Therapeutic Approaches for Treatment of NF1-Associated Tumors. Mol. Cancer Ther. 2025, OF1–OF14. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.; Wang, Y.; Bouley, S.J.; Mandigo, T.R.; Sharma, A.; Sengupta, S.; Housden, A.; Perrimon, N.; Walker, J.A.; Housden, B.E. Inhibition of Autophagy as a Novel Treatment for Neurofibromatosis Type 1 Tumors. Mol. Oncol. 2025, 19, 825–851. [Google Scholar] [CrossRef]
- Richter, M.; Piwocka, O.; Musielak, M.; Piotrowski, I.; Suchorska, W.M.; Trzeciak, T. From Donor to the Lab: A Fascinating Journey of Primary Cell Lines. Front. Cell Dev. Biol. 2021, 9, 711381. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, M.; Cortesi, M.; Zamagni, A.; Arienti, C.; Pignatta, S.; Tesei, A. Modeling Neoplastic Disease with Spheroids and Organoids. J. Hematol. Oncol. 2020, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Sakalem, M.E.; De Sibio, M.T.; da Costa, F.A.D.S.; de Oliveira, M. Historical Evolution of Spheroids and Organoids, and Possibilities of Use in Life Sciences and Medicine. Biotechnol. J. 2021, 16, e2000463. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.; Rawe, M.; Johansson, G.; Pang, S.; Soderquist, R.S.; Patel, A.V.; Nelson, S.; Seibel, W.; Ratner, N.; Sanchez, Y. Discovery of a Small Molecule Targeting IRA2 Deletion in Budding Yeast and Neurofibromin Loss in Malignant Peripheral Nerve Sheath Tumor Cells. Mol. Cancer Ther. 2011, 10, 1740–1750. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Williams, J.P.; Rizvi, T.A.; Kordich, J.J.; Witte, D.; Meijer, D.; Stemmer-Rachamimov, A.O.; Cancelas, J.A.; Ratner, N. Plexiform and Dermal Neurofibromas and Pigmentation Are Caused by Nf1 Loss in Desert Hedgehog-Expressing Cells. Cancer Cell 2008, 13, 105–116. [Google Scholar] [CrossRef]
- Freret, M.E.; Gutmann, D.H. Insights into Optic Pathway Glioma Vision Loss from Mouse Models of Neurofibromatosis Type 1. J. Neurosci. Res. 2019, 97, 45–56. [Google Scholar] [CrossRef]
- Chen, Z.; Mo, J.; Brosseau, J.-P.; Shipman, T.; Wang, Y.; Liao, C.-P.; Cooper, J.M.; Allaway, R.J.; Gosline, S.J.C.; Guinney, J.; et al. Spatiotemporal Loss of NF1 in Schwann Cell Lineage Leads to Different Types of Cutaneous Neurofibroma Susceptible to Modification by the Hippo Pathway. Cancer Discov. 2019, 9, 114–129. [Google Scholar] [CrossRef]
- Moy, A.B.; Kamath, A.; Ternes, S.; Kamath, J. The Challenges to Advancing Induced Pluripotent Stem Cell-Dependent Cell Replacement Therapy. Med. Res. Arch. 2023, 11, 4784. [Google Scholar] [CrossRef]
- Venkatesh, A.; Iltis, A.S.; Matthews, K.R.W. Transparency in Controversial Research: A Review of Human Embryo Research Publication Ethical Disclosure Statements. Stem Cell Rep. 2024, 19, 28–36. [Google Scholar] [CrossRef]
- Vélez-Reyes, G.L.; Koes, N.; Ryu, J.H.; Kaufmann, G.; Berner, M.; Weg, M.T.; Wolf, N.K.; Rathe, S.K.; Ratner, N.; Moriarity, B.S.; et al. Transposon Mutagenesis-Guided CRISPR/Cas9 Screening Strongly Implicates Dysregulation of Hippo/YAP Signaling in Malignant Peripheral Nerve Sheath Tumor Development. Cancers 2021, 13, 1584. [Google Scholar] [CrossRef]
- Nguyen, H.T.L.; Kohl, E.; Bade, J.; Eng, S.E.; Tosevska, A.; Al Shihabi, A.; Tebon, P.J.; Hong, J.J.; Dry, S.; Boutros, P.C.; et al. A Platform for Rapid Patient-Derived Cutaneous Neurofibroma Organoid Establishment and Screening. Cell Rep. Methods 2024, 4, 100772. [Google Scholar] [CrossRef]
- Usaj, M.; Tan, Y.; Wang, W.; VanderSluis, B.; Zou, A.; Myers, C.L.; Costanzo, M.; Andrews, B.; Boone, C. TheCellMap.Org: A Web-Accessible Database for Visualizing and Mining the Global Yeast Genetic Interaction Network. G3 2017, 7, 1539–1549. [Google Scholar] [CrossRef]
- Atsoniou, K.; Giannopoulou, E.; Georganta, E.-M.; Skoulakis, E.M.C. Drosophila Contributions towards Understanding Neurofibromatosis 1. Cells 2024, 13, 721. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Janke, L.J.; Gudenas, B.L.; Jin, H.; Fan, Y.; Paré, J.; Clay, M.R.; Northcott, P.A.; Hirbe, A.C.; Cao, X. A Genetic Mouse Model with Postnatal Nf1 and P53 Loss Recapitulates the Histology and Transcriptome of Human Malignant Peripheral Nerve Sheath Tumor. Neuro-Oncol. Adv. 2021, 3, vdab129. [Google Scholar] [CrossRef]
- Dehner, C.; Moon, C.I.; Zhang, X.; Zhou, Z.; Miller, C.; Xu, H.; Wan, X.; Yang, K.; Mashl, J.; Gosline, S.J.C.; et al. Chromosome 8 Gain Is Associated with High-Grade Transformation in MPNST. JCI Insight 2021, 6, e146351. [Google Scholar] [CrossRef]
- Larsson, A.T.; Bhatia, H.; Calizo, A.; Pollard, K.; Zhang, X.; Conniff, E.; Tibbitts, J.F.; Rono, E.; Cummins, K.; Osum, S.H.; et al. Ex Vivo to In Vivo Model of Malignant Peripheral Nerve Sheath Tumors for Precision Oncology. Neuro Oncol. 2023, 25, 2044–2057. [Google Scholar] [CrossRef]
- Shin, J.; Padmanabhan, A.; de Groh, E.D.; Lee, J.-S.; Haidar, S.; Dahlberg, S.; Guo, F.; He, S.; Wolman, M.A.; Granato, M.; et al. Zebrafish Neurofibromatosis Type 1 Genes Have Redundant Functions in Tumorigenesis and Embryonic Development. Dis. Models Mech. 2012, 5, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Ki, D.H.; He, S.; Rodig, S.; Look, A.T. Overexpression of PDGFRA Cooperates with Loss of NF1 and P53 to Accelerate the Molecular Pathogenesis of Malignant Peripheral Nerve Sheath Tumors. Oncogene 2017, 36, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Ki, D.H.; Oppel, F.; Durbin, A.D.; Look, A.T. Mechanisms Underlying Synergy between DNA Topoisomerase I-Targeted Drugs and mTOR Kinase Inhibitors in NF1-Associated Malignant Peripheral Nerve Sheath Tumors. Oncogene 2019, 38, 6585–6598. [Google Scholar] [CrossRef] [PubMed]
- Isakson, S.H.; Rizzardi, A.E.; Coutts, A.W.; Carlson, D.F.; Kirstein, M.N.; Fisher, J.; Vitte, J.; Williams, K.B.; Pluhar, G.E.; Dahiya, S.; et al. Genetically Engineered Minipigs Model the Major Clinical Features of Human Neurofibromatosis Type 1. Commun. Biol. 2018, 1, 158. [Google Scholar] [CrossRef] [PubMed]
- Uthoff, J.; Larson, J.; Sato, T.S.; Hammond, E.; Schroeder, K.E.; Rohret, F.; Rogers, C.S.; Quelle, D.E.; Darbro, B.W.; Khanna, R.; et al. Longitudinal Phenotype Development in a Minipig Model of Neurofibromatosis Type 1. Sci. Rep. 2020, 10, 5046. [Google Scholar] [CrossRef]
- Simanshu, D.K.; Nissley, D.V.; McCormick, F. RAS Proteins and Their Regulators in Human Disease. Cell 2017, 170, 17–33. [Google Scholar] [CrossRef]
- Scheffzek, K.; Shivalingaiah, G. Ras-Specific GTPase-Activating Proteins-Structures, Mechanisms, and Interactions. Cold Spring Harb. Perspect. Med. 2019, 9, a031500. [Google Scholar] [CrossRef]
- Grech, V.S.; Lotsaris, K.; Touma, T.E.; Kefala, V.; Rallis, E. The Role of Artificial Intelligence in Identifying NF1 Gene Variants and Improving Diagnosis. Genes 2025, 16, 560. [Google Scholar] [CrossRef]
- Hiatt, K.K.; Ingram, D.A.; Zhang, Y.; Bollag, G.; Clapp, D.W. Neurofibromin GTPase-Activating Protein-Related Domains Restore Normal Growth in Nf1-/- Cells. J. Biol. Chem. 2001, 276, 7240–7245. [Google Scholar] [CrossRef] [PubMed]
- Lake, D.; Corrêa, S.A.L.; Müller, J. Negative Feedback Regulation of the ERK1/2 MAPK Pathway. Cell. Mol. Life Sci. 2016, 73, 4397–4413. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Moye, S.L.; McKay, R.M.; Le, L.Q. Neurofibromin and Suppression of Tumorigenesis: Beyond the GAP. Oncogene 2022, 41, 1235–1251. [Google Scholar] [CrossRef]
- Yan, N.; Ricca, C.; Fletcher, J.; Glover, T.; Seizinger, B.R.; Manne, V. Farnesyltransferase Inhibitors Block the Neurofibromatosis Type I (NF1) Malignant Phenotype. Cancer Res. 1995, 55, 3569–3575. [Google Scholar]
- Park, J.-I.; Powers, J.F.; Tischler, A.S.; Strock, C.J.; Ball, D.W.; Nelkin, B.D. GDNF-Induced Leukemia Inhibitory Factor Can Mediate Differentiation via the MEK/ERK Pathway in Pheochromocytoma Cells Derived from Nf1-Heterozygous Knockout Mice. Exp. Cell Res. 2005, 303, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Mattingly, R.R.; Kraniak, J.M.; Dilworth, J.T.; Mathieu, P.; Bealmear, B.; Nowak, J.E.; Benjamins, J.A.; Tainsky, M.A.; Reiners, J.J. The Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase Kinase Inhibitor PD184352 (CI-1040) Selectively Induces Apoptosis in Malignant Schwannoma Cell Lines. J. Pharmacol. Exp. Ther. 2006, 316, 456–465. [Google Scholar] [CrossRef]
- Li, H.; Velasco-Miguel, S.; Vass, W.C.; Parada, L.F.; DeClue, J.E. Epidermal Growth Factor Receptor Signaling Pathways Are Associated with Tumorigenesis in the Nf1:P53 Mouse Tumor Model. Cancer Res. 2002, 62, 4507–4513. [Google Scholar]
- Boumelha, J.; Molina-Arcas, M.; Downward, J. Facts and Hopes on RAS Inhibitors and Cancer Immunotherapy. Clin. Cancer Res. 2023, 29, 5012–5020. [Google Scholar] [CrossRef]
- Widemann, B.C.; Dombi, E.; Gillespie, A.; Wolters, P.L.; Belasco, J.; Goldman, S.; Korf, B.R.; Solomon, J.; Martin, S.; Salzer, W.; et al. Phase 2 Randomized, Flexible Crossover, Double-Blinded, Placebo-Controlled Trial of the Farnesyltransferase Inhibitor Tipifarnib in Children and Young Adults with Neurofibromatosis Type 1 and Progressive Plexiform Neurofibromas. Neuro Oncol. 2014, 16, 707–718. [Google Scholar] [CrossRef]
- Wojtkowiak, J.W.; Fouad, F.; LaLonde, D.T.; Kleinman, M.D.; Gibbs, R.A.; Reiners, J.J.; Borch, R.F.; Mattingly, R.R. Induction of Apoptosis in Neurofibromatosis Type 1 Malignant Peripheral Nerve Sheath Tumor Cell Lines by a Combination of Novel Farnesyl Transferase Inhibitors and Lovastatin. J. Pharmacol. Exp. Ther. 2008, 326, 1–11. [Google Scholar] [CrossRef]
- Reiners, J.J.; Mathieu, P.A.; Gargano, M.; George, I.; Shen, Y.; Callaghan, J.F.; Borch, R.F.; Mattingly, R.R. Synergistic Suppression of NF1 Malignant Peripheral Nerve Sheath Tumor Cell Growth in Culture and Orthotopic Xenografts by Combinational Treatment with Statin and Prodrug Farnesyltransferase Inhibitor PAMAM G4 Dendrimers. Cancers 2023, 16, 89. [Google Scholar] [CrossRef]
- Sane, K.M.; Mynderse, M.; Lalonde, D.T.; Dean, I.S.; Wojtkowiak, J.W.; Fouad, F.; Borch, R.F.; Reiners, J.J.; Gibbs, R.A.; Mattingly, R.R. A Novel Geranylgeranyl Transferase Inhibitor in Combination with Lovastatin Inhibits Proliferation and Induces Autophagy in STS-26T MPNST Cells. J. Pharmacol. Exp. Ther. 2010, 333, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.-Y.; Xu, M.; Lu, Y.; Liu, J.; Staedtke, V. Pan-RAS Inhibitor RMC-7977 Is Efficacious in Treating NF1-Related Tumors. Neurooncol. Adv. 2025, 7, vdaf065. [Google Scholar] [CrossRef] [PubMed]
- Alessi, D.R.; Cuenda, A.; Cohen, P.; Dudley, D.T.; Saltiel, A.R. PD 098059 Is a Specific Inhibitor of the Activation of Mitogen-Activated Protein Kinase Kinase In Vitro and In Vivo. J. Biol. Chem. 1995, 270, 27489–27494. [Google Scholar] [CrossRef]
- Akinleye, A.; Furqan, M.; Mukhi, N.; Ravella, P.; Liu, D. MEK and the Inhibitors: From Bench to Bedside. J. Hematol. Oncol. 2013, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.J.M.; McCormack, P.L. Trametinib: First Global Approval. Drugs 2013, 73, 1245–1254. [Google Scholar] [CrossRef]
- Tran, B.; Cohen, M.S. The Discovery and Development of Binimetinib for the Treatment of Melanoma. Expert Opin. Drug Discov. 2020, 15, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Jessen, W.J.; Miller, S.J.; Jousma, E.; Wu, J.; Rizvi, T.A.; Brundage, M.E.; Eaves, D.; Widemann, B.; Kim, M.-O.; Dombi, E.; et al. MEK Inhibition Exhibits Efficacy in Human and Mouse Neurofibromatosis Tumors. J. Clin. Investig. 2013, 123, 340–347. [Google Scholar] [CrossRef]
- Gross, A.M.; O’Sullivan Coyne, G.; Dombi, E.; Tibery, C.; Herrick, W.G.; Martin, S.; Angus, S.P.; Shern, J.F.; Rhodes, S.D.; Foster, J.C.; et al. Selumetinib in Adults with NF1 and Inoperable Plexiform Neurofibroma: A Phase 2 Trial. Nat. Med. 2025, 31, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Sarin, K.Y.; Kincaid, J.; Sell, B.; Shahryari, J.; Duncton, M.A.J.; Morefield, E.; Sun, W.; Prieto, K.; Chavez-Chiang, O.; de Moran Segura, C.; et al. Development of a MEK Inhibitor, NFX-179, as a Chemoprevention Agent for Squamous Cell Carcinoma. Sci. Transl. Med. 2023, 15, eade1844. [Google Scholar] [CrossRef]
- Sarin, K.Y.; Bradshaw, M.; O’Mara, C.; Shahryari, J.; Kincaid, J.; Kempers, S.; Tu, J.H.; Dhawan, S.; DuBois, J.; Wilson, D.; et al. Effect of NFX-179 MEK Inhibitor on Cutaneous Neurofibromas in Persons with Neurofibromatosis Type 1. Sci. Adv. 2024, 10, eadk4946. [Google Scholar] [CrossRef]
- Dasgupta, B.; Yi, Y.; Chen, D.Y.; Weber, J.D.; Gutmann, D.H. Proteomic Analysis Reveals Hyperactivation of the Mammalian Target of Rapamycin Pathway in Neurofibromatosis 1-Associated Human and Mouse Brain Tumors. Cancer Res. 2005, 65, 2755–2760. [Google Scholar] [CrossRef]
- Johannessen, C.M.; Reczek, E.E.; James, M.F.; Brems, H.; Legius, E.; Cichowski, K. The NF1 Tumor Suppressor Critically Regulates TSC2 and mTOR. Proc. Natl. Acad. Sci. USA 2005, 102, 8573–8578. [Google Scholar] [CrossRef]
- Kaul, A.; Toonen, J.A.; Cimino, P.J.; Gianino, S.M.; Gutmann, D.H. Akt- or MEK-Mediated mTOR Inhibition Suppresses Nf1 Optic Glioma Growth. Neuro-Oncology 2015, 17, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.; Widemann, B.C.; Wolters, P.; Dombi, E.; Vinks, A.A.; Cantor, A.; Korf, B.; Perentesis, J.; Gutmann, D.H.; Schorry, E.; et al. Sirolimus for Non-Progressive NF1-Associated Plexiform Neurofibromas: An NF Clinical Trials Consortium Phase II Study. Pediatr. Blood Cancer 2014, 61, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, N.J.; Prabhu, S.P.; Reddy, A.T.; Fisher, M.J.; Packer, R.; Goldman, S.; Robison, N.J.; Gutmann, D.H.; Viskochil, D.H.; Allen, J.C.; et al. A Phase II Study of Continuous Oral mTOR Inhibitor Everolimus for Recurrent, Radiographic-Progressive Neurofibromatosis Type 1-Associated Pediatric Low-Grade Glioma: A Neurofibromatosis Clinical Trials Consortium Study. Neuro-Oncology 2020, 22, 1527–1535. [Google Scholar] [CrossRef]
- Schreck, K.C.; Allen, A.N.; Wang, J.; Pratilas, C.A. Combination MEK and mTOR Inhibitor Therapy Is Active in Models of Glioblastoma. Neuro-Oncol. Adv. 2020, 2, vdaa138. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.M.; Farouk Sait, S.; Dunn, G.; Sullivan, A.; Bruckert, B.; Sun, D. Integrated Drug Mining Reveals Actionable Strategies Inhibiting Plexiform Neurofibromas. Brain Sci. 2022, 12, 720. [Google Scholar] [CrossRef] [PubMed]
- Kraniak, J.M.; Chalasani, A.; Wallace, M.R.; Mattingly, R.R. Development of 3D Culture Models of Plexiform Neurofibroma and Initial Application for Phenotypic Characterization and Drug Screening. Exp. Neurol. 2018, 299, 289–298. [Google Scholar] [CrossRef]
- Zamora, P.O.; Altay, G.; Santamaria, U.; Dwarshuis, N.; Donthi, H.; Moon, C.I.; Bakalar, D.; Zamora, M. Drug Responses in Plexiform Neurofibroma Type I (PNF1) Cell Lines Using High-Throughput Data and Combined Effectiveness and Potency. Cancers 2023, 15, 5811. [Google Scholar] [CrossRef]
- Lippincott, M.J. High-Content Microscopy for Characterizing and Predicting Drug Response in NF1-/- Schwann Cell Cultures and NF1 Patient-Derived Tumor Organoids. In Proceedings of the NF Conference, Washington, DC, USA, 21–24 June 2025. [Google Scholar]
- Banerjee, S.; Byrd, J.N.; Gianino, S.M.; Harpstrite, S.E.; Rodriguez, F.J.; Tuskan, R.G.; Reilly, K.M.; Piwnica-Worms, D.R.; Gutmann, D.H. The Neurofibromatosis Type 1 Tumor Suppressor Controls Cell Growth by Regulating Signal Transducer and Activator of Transcription-3 Activity In Vitro and In Vivo. Cancer Res. 2010, 70, 1356–1366. [Google Scholar] [CrossRef]
- Monga, M.; Sausville, E. Developmental Therapeutics Program at the NCI: Molecular Target and Drug Discovery Process. Leukemia 2002, 16, 520–526. [Google Scholar] [CrossRef]
- Fletcher, J.S.; Pundavela, J.; Ratner, N. After Nf1 Loss in Schwann Cells, Inflammation Drives Neurofibroma Formation. Neuro-Oncol. Adv. 2020, 2, i23–i32. [Google Scholar] [CrossRef]
- Rad, E.; Dodd, K.; Thomas, L.; Upadhyaya, M.; Tee, A. STAT3 and HIF1α Signaling Drives Oncogenic Cellular Phenotypes in Malignant Peripheral Nerve Sheath Tumors. Mol. Cancer Res. 2015, 13, 1149–1160. [Google Scholar] [CrossRef]
- Fletcher, J.S.; Springer, M.G.; Choi, K.; Jousma, E.; Rizvi, T.A.; Dombi, E.; Kim, M.-O.; Wu, J.; Ratner, N. STAT3 Inhibition Reduces Macrophage Number and Tumor Growth in Neurofibroma. Oncogene 2019, 38, 2876–2884. [Google Scholar] [CrossRef]
- Gampala, S.; Shah, F.; Zhang, C.; Rhodes, S.D.; Babb, O.; Grimard, M.; Wireman, R.S.; Rad, E.; Calver, B.; Bai, R.-Y.; et al. Exploring Transcriptional Regulators Ref-1 and STAT3 as Therapeutic Targets in Malignant Peripheral Nerve Sheath Tumours. Br. J. Cancer 2021, 124, 1566–1580. [Google Scholar] [CrossRef] [PubMed]
- Ling, A.; Gruener, R.F.; Fessler, J.; Huang, R.S. More than Fishing for a Cure: The Promises and Pitfalls of High Throughput Cancer Cell Line Screens. Pharmacol. Ther. 2018, 191, 178–189. [Google Scholar] [CrossRef]
- Guo, J.; Grovola, M.R.; Xie, H.; Coggins, G.E.; Duggan, P.; Hasan, R.; Huang, J.; Lin, D.W.; Song, C.; Witek, G.M.; et al. Comprehensive Pharmacological Profiling of Neurofibromatosis Cell Lines. Am. J. Cancer Res. 2017, 7, 923–934. [Google Scholar]
- Nagabushan, S.; Lau, L.M.S.; Barahona, P.; Wong, M.; Sherstyuk, A.; Marshall, G.M.; Tyrrell, V.; Wegner, E.A.; Ekert, P.G.; Cowley, M.J.; et al. Efficacy of MEK Inhibition in a Recurrent Malignant Peripheral Nerve Sheath Tumor. NPJ Precis. Oncol. 2021, 5, 9. [Google Scholar] [CrossRef]
- De Blank, P.M.K.; Gross, A.M.; Akshintala, S.; Blakeley, J.O.; Bollag, G.; Cannon, A.; Dombi, E.; Fangusaro, J.; Gelb, B.D.; Hargrave, D.; et al. MEK Inhibitors for Neurofibromatosis Type 1 Manifestations: Clinical Evidence and Consensus. Neuro-Oncology 2022, 24, 1845–1856. [Google Scholar] [CrossRef]
- Kolberg, M.; Bruun, J.; Murumägi, A.; Mpindi, J.P.; Bergsland, C.H.; Høland, M.; Eilertsen, I.A.; Danielsen, S.A.; Kallioniemi, O.; Lothe, R.A. Drug Sensitivity and Resistance Testing Identifies PLK1 Inhibitors and Gemcitabine as Potent Drugs for Malignant Peripheral Nerve Sheath Tumors. Mol. Oncol. 2017, 11, 1156–1171. [Google Scholar] [CrossRef]
- Guo, J.; Chaney, K.E.; Choi, K.; Witek, G.; Patel, A.V.; Xie, H.; Lin, D.; Whig, K.; Xiong, Y.; Schultz, D.C.; et al. Polo-like Kinase 1 as a Therapeutic Target for Malignant Peripheral Nerve Sheath Tumors (MPNST) and Schwannomas. Am. J. Cancer Res. 2020, 10, 856–869. [Google Scholar]
- Semenova, G.; Stepanova, D.S.; Deyev, S.M.; Chernoff, J. Medium Throughput Biochemical Compound Screening Identifies Novel Agents for Pharmacotherapy of Neurofibromatosis Type 1. Biochimie 2017, 135, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Oyama, R.; Kito, F.; Takahashi, M.; Hattori, E.; Noguchi, R.; Takai, Y.; Sakumoto, M.; Qiao, Z.; Toki, S.; Sugawara, M.; et al. Establishment and Characterization of Patient-Derived Cancer Models of Malignant Peripheral Nerve Sheath Tumors. Cancer Cell Int. 2020, 20, 58. [Google Scholar] [CrossRef]
- Arima, Y.; Hayashi, H.; Kamata, K.; Goto, T.M.; Sasaki, M.; Kuramochi, A.; Saya, H. Decreased Expression of Neurofibromin Contributes to Epithelial–Mesenchymal Transition in Neurofibromatosis Type 1. Exp. Dermatol. 2010, 19, e136–e141. [Google Scholar] [CrossRef] [PubMed]
- Harigai, R.; Sakai, S.; Nobusue, H.; Hirose, C.; Sampetrean, O.; Minami, N.; Hata, Y.; Kasama, T.; Hirose, T.; Takenouchi, T.; et al. Tranilast Inhibits the Expression of Genes Related to Epithelial-Mesenchymal Transition and Angiogenesis in Neurofibromin-Deficient Cells. Sci. Rep. 2018, 8, 6069. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Nagano, O.; Ishimoto, T.; Yae, T.; Suzuki, Y.; Shinoda, T.; Nakamura, S.; Niwa, S.; Ikeda, S.; Koga, H.; et al. Tumor Necrosis Factor-Alpha Regulates Transforming Growth Factor-Beta-Dependent Epithelial-Mesenchymal Transition by Promoting Hyaluronan-CD44-Moesin Interaction. J. Biol. Chem. 2010, 285, 4060–4073. [Google Scholar] [CrossRef]
- Kahen, E.J.; Brohl, A.; Yu, D.; Welch, D.; Cubitt, C.L.; Lee, J.K.; Chen, Y.; Yoder, S.J.; Teer, J.K.; Zhang, Y.O.; et al. Neurofibromin Level Directs RAS Pathway Signaling and Mediates Sensitivity to Targeted Agents in Malignant Peripheral Nerve Sheath Tumors. Oncotarget 2018, 9, 22571–22585. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, J.; Creus-Bachiller, E.; Zhang, X.; Martínez-Iniesta, M.; Ortega-Bertran, S.; Guha, R.; Thomas, C.J.; Wallace, M.R.; Romagosa, C.; Salazar-Huayna, L.; et al. A High-Throughput Screening Platform Identifies Novel Combination Treatments for Malignant Peripheral Nerve Sheath Tumors. Mol. Cancer Ther. 2022, 21, 1246–1258. [Google Scholar] [CrossRef]
- Ortega-Bertran, S.; Fernández-Rodríguez, J.; Magallón-Lorenz, M.; Zhang, X.; Creus-Bachiller, E.; Diazgranados, A.P.; Uriarte-Arrazola, I.; Mazuelas, H.; Blanco, I.; Valverde, C.; et al. Triple Combination of MEK, BET, and CDK Inhibitors Significantly Reduces Human Malignant Peripheral Nerve Sheath Tumors in Mouse Models. Clin. Cancer Res. 2025, 31, 907–920. [Google Scholar] [CrossRef] [PubMed]
- De Raedt, T.; Beert, E.; Pasmant, E.; Luscan, A.; Brems, H.; Ortonne, N.; Helin, K.; Hornick, J.L.; Mautner, V.; Kehrer-Sawatzki, H.; et al. PRC2 Loss Amplifies Ras-Driven Transcription and Confers Sensitivity to BRD4-Based Therapies. Nature 2014, 514, 247–251. [Google Scholar] [CrossRef]
- Tsuchiya, R.; Yoshimatsu, Y.; Noguchi, R.; Sin, Y.; Ono, T.; Akiyama, T.; Kosako, H.; Yoshida, A.; Ohtori, S.; Kawai, A.; et al. Integrating Analysis of Proteome Profile and Drug Screening Identifies Therapeutic Potential of MET Pathway for the Treatment of Malignant Peripheral Nerve Sheath Tumor. Expert Rev. Proteom. 2023, 20, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Rahrmann, E.P.; Watson, A.L.; Keng, V.W.; Choi, K.; Moriarity, B.S.; Beckmann, D.A.; Wolf, N.K.; Sarver, A.; Collins, M.H.; Moertel, C.L.; et al. Forward Genetic Screen for Malignant Peripheral Nerve Sheath Tumor Formation Identifies New Genes and Pathways Driving Tumorigenesis. Nat. Genet. 2013, 45, 756–766. [Google Scholar] [CrossRef]
- Wang, W.; Cui, X.-W.; Gu, Y.-H.; Wei, C.-J.; Li, Y.-H.; Ren, J.-Y.; Chung, M.-H.; Aimaier, R.; Zhang, H.-B.; Li, Q.-F.; et al. Combined Cyclin-Dependent Kinase Inhibition Overcomes MAPK/Extracellular Signal–Regulated Kinase Kinase Inhibitor Resistance in Plexiform Neurofibroma of Neurofibromatosis Type I. J. Investig. Dermatol. 2022, 142, 613–623.e7. [Google Scholar] [CrossRef]
- Allaway, R.J.; Wood, M.D.; Downey, S.L.; Bouley, S.J.; Traphagen, N.A.; Wells, J.D.; Batra, J.; Melancon, S.N.; Ringelberg, C.; Seibel, W.; et al. Exploiting Mitochondrial and Metabolic Homeostasis as a Vulnerability in NF1 Deficient Cells. Oncotarget 2018, 9, 15860–15875. [Google Scholar] [CrossRef]
- Bouley, S.J.; Grassetti, A.V.; Allaway, R.J.; Wood, M.D.; Hou, H.W.; Burdon Dasbach, I.R.; Seibel, W.; Wu, J.; Gerber, S.A.; Dragnev, K.H.; et al. Chemical Genetic Screens Reveal Defective Lysosomal Trafficking as Synthetic Lethal with NF1 Loss. J. Cell Sci. 2024, 137, jcs262343. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, D.H.; McLellan, M.D.; Hussain, I.; Wallis, J.W.; Fulton, L.L.; Fulton, R.S.; Magrini, V.; Demeter, R.; Wylie, T.; Kandoth, C.; et al. Somatic Neurofibromatosis Type 1 (NF1) Inactivation Characterizes NF1-Associated Pilocytic Astrocytoma. Genome Res. 2013, 23, 431–439. [Google Scholar] [CrossRef]
- Ayasa, L.A.; Rahhal, S.; Najjar, A.K.; Suboh, B.N.; Aliwaiai, M.; Daqour, A.M.; Bakri, I. Glioblastoma Multiforme in a Patient with Neurofibromatosis Type 1: A Case Report and Review of Literature. J. Surg. Case Rep. 2024, 2024, rjae517. [Google Scholar] [CrossRef] [PubMed]
- Henrich, C.J.; Cartner, L.K.; Wilson, J.A.; Fuller, R.W.; Rizzo, A.E.; Reilly, K.M.; McMahon, J.B.; Gustafson, K.R. Deguelins, Natural Product Modulators of NF1-Defective Astrocytoma Cell Growth Identified by High-Throughput Screening of Partially Purified Natural Product Extracts. J. Nat. Prod. 2015, 78, 2776–2781. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, W.; Zheng, W. Deguelin, a Novel Anti-Tumorigenic Agent Targeting Apoptosis, Cell Cycle Arrest and Anti-Angiogenesis for Cancer Chemoprevention. Mol. Clin. Oncol. 2013, 1, 215–219. [Google Scholar] [CrossRef]
- Wilson, K.M.; Mathews-Griner, L.A.; Williamson, T.; Guha, R.; Chen, L.; Shinn, P.; McKnight, C.; Michael, S.; Klumpp-Thomas, C.; Binder, Z.A.; et al. Mutation Profiles in Glioblastoma 3D Oncospheres Modulate Drug Efficacy. SLAS Technol. 2019, 24, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Mirchia, K.; Payne, E.; Liu, S.J.; Al-Adli, N.; Peeran, Z.; Shukla, P.; Young, J.S.; Gupta, R.; Wu, J.; et al. Multiplatform Molecular Profiling and Functional Genomic Screens Identify Prognostic Signatures and Mechanisms Underlying MEK Inhibitor Response in Somatic NF1 Mutant Glioblastoma. bioRxiv 2024. [Google Scholar] [CrossRef]
- Olsen, J.J. Identification of Treatment Concentrations of Defactinib or VS-6766 for the Treatment of Patients with Glioblastoma; Emory University: Atlanta, GA, USA, 2024. [Google Scholar]
- Robinson, G.W.; Orr, B.A.; Dhanda, S.K.; Lin, T.; Sabin, N.D.; Han, K.; Kostecka, A.; Onar-Thomas, A.; Roussel, M.F.; Stewart, C.; et al. TRLS-16. Results from SJDAWN: A St Jude Children’s Research Hospital Phase 1 Study Evaluating Molecularly Driven Doublet Therapies for All Children with Refractory or Recurrent Central Nervous System (CNS) Malignant Neoplasms and Young Adults with SHH Medulloblastoma. Neuro-Oncology 2024, 26, 0. [Google Scholar] [CrossRef]
- Dougherty, J.; Harvey, K.; Liou, A.; Labella, K.; Moran, D.; Brosius, S.; De Raedt, T. Identification of Therapeutic Sensitivities in a Spheroid Drug Combination Screen of Neurofibromatosis Type I Associated High Grade Gliomas. PLoS ONE 2023, 18, e0277305. [Google Scholar] [CrossRef]
- Cichowski, K.; Shih, T.S.; Schmitt, E.; Santiago, S.; Reilly, K.; McLaughlin, M.E.; Bronson, R.T.; Jacks, T. Mouse Models of Tumor Development in Neurofibromatosis Type 1. Science 1999, 286, 2172–2176. [Google Scholar] [CrossRef]
- Dubey, S.; Rai, S.; Guillen, F.; Iyer, A.; Aung, S.; Yuan, M.; Eberhart, C.G.; Rodriguez, F.J. High-Throughput Targeted Drug Screening for NF1-Associated High-Grade Gliomas with ATRX Deficiency. bioRxiv 2025. [Google Scholar] [CrossRef]
- Johansson, G.; Mahller, Y.Y.; Collins, M.H.; Kim, M.-O.; Nobukuni, T.; Perentesis, J.; Cripe, T.P.; Lane, H.A.; Kozma, S.C.; Thomas, G.; et al. Effective in Vivo Targeting of the Mammalian Target of Rapamycin Pathway in Malignant Peripheral Nerve Sheath Tumors. Mol. Cancer Ther. 2008, 7, 1237–1245. [Google Scholar] [CrossRef]
- Johannessen, C.M.; Johnson, B.W.; Williams, S.M.G.; Chan, A.W.; Reczek, E.E.; Lynch, R.C.; Rioth, M.J.; McClatchey, A.; Ryeom, S.; Cichowski, K. TORC1 Is Essential for NF1-Associated Malignancies. Curr. Biol. 2008, 18, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Bhola, P.; Banerjee, S.; Mukherjee, J.; Balasubramanium, A.; Arun, V.; Karim, Z.; Burrell, K.; Croul, S.; Gutmann, D.H.; Guha, A. Preclinical In Vivo Evaluation of Rapamycin in Human Malignant Peripheral Nerve Sheath Explant Xenograft. Int. J. Cancer 2010, 126, 563–571. [Google Scholar] [CrossRef]
- Emoto, C.; Fukuda, T.; Mizuno, T.; Cox, S.; Schniedewind, B.; Christians, U.; Widemann, B.C.; Fisher, M.J.; Weiss, B.; Perentesis, J.; et al. Age-Dependent Changes in Sirolimus Metabolite Formation in Patients with Neurofibromatosis Type 1. Ther. Drug Monit. 2015, 37, 395–399. [Google Scholar] [CrossRef]
- Weiss, B.; Widemann, B.C.; Wolters, P.; Dombi, E.; Vinks, A.; Cantor, A.; Perentesis, J.; Schorry, E.; Ullrich, N.; Gutmann, D.H.; et al. Sirolimus for Progressive Neurofibromatosis Type 1-Associated Plexiform Neurofibromas: A Neurofibromatosis Clinical Trials Consortium Phase II Study. Neuro-Oncology 2015, 17, 596–603. [Google Scholar] [CrossRef]
- Slopis, J.M.; Arevalo, O.; Bell, C.S.; Hebert, A.A.; Northrup, H.; Riascos, R.F.; Samuels, J.A.; Smith, K.C.; Tate, P.; Koenig, M.K. Treatment of Disfiguring Cutaneous Lesions in Neurofibromatosis-1 with Everolimus: A Phase II, Open-Label, Single-Arm Trial. Drugs R D 2018, 18, 295–302. [Google Scholar] [CrossRef]
- Watson, A.L.; Anderson, L.K.; Greeley, A.D.; Keng, V.W.; Rahrmann, E.P.; Halfond, A.L.; Powell, N.M.; Collins, M.H.; Rizvi, T.; Moertel, C.L.; et al. Co-Targeting the MAPK and PI3K/AKT/mTOR Pathways in Two Genetically Engineered Mouse Models of Schwann Cell Tumors Reduces Tumor Grade and Multiplicity. Oncotarget 2014, 5, 1502–1514. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.J.; Shih, C.-S.; Rhodes, S.D.; Armstrong, A.E.; Wolters, P.L.; Dombi, E.; Zhang, C.; Angus, S.P.; Johnson, G.L.; Packer, R.J.; et al. Cabozantinib for Neurofibromatosis Type 1-Related Plexiform Neurofibromas: A Phase 2 Trial. Nat. Med. 2021, 27, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Widemann, B.C.; Meyer, C.F.; Cote, G.M.; Chugh, R.; Milhem, M.M.; Van Tine, B.A.; Kim, A.; Turpin, B.; Dombi, E.; Jayaprakash, N.; et al. SARC016: Phase II Study of Everolimus in Combination with Bevacizumab in Sporadic and Neurofibromatosis Type 1 (NF1) Related Refractory Malignant Peripheral Nerve Sheath Tumors (MPNST). J. Clin. Oncol. 2016, 34, 11053. [Google Scholar] [CrossRef]
- Kim, A.; Lu, Y.; Okuno, S.H.; Reinke, D.; Maertens, O.; Perentesis, J.; Basu, M.; Wolters, P.L.; De Raedt, T.; Chawla, S.; et al. Targeting Refractory Sarcomas and Malignant Peripheral Nerve Sheath Tumors in a Phase I/II Study of Sirolimus in Combination with Ganetespib (SARC023). Sarcoma 2020, 2020, 5784876. [Google Scholar] [CrossRef]
- Akshintala, S.; Mallory, N.C.; Lu, Y.; Ballman, K.V.; Schuetze, S.M.; Chugh, R.; Maki, R.G.; Reinke, D.K.; Widemann, B.C.; Kim, A. Outcome of Patients with Malignant Peripheral Nerve Sheath Tumors Enrolled on Sarcoma Alliance for Research Through Collaboration (SARC) Phase II Trials. Oncologist 2023, 28, 453–459. [Google Scholar] [CrossRef]
- Manji, G.A.; Stanton, L.J.; Hirbe, A.C.; Ge, L.; Sta Ana, S.; Titus, S.; Labadie, B.W.; May, M.S.; Lyu, Y.; Chrisinger, J.S.A.; et al. Phase II Study of Pexidartinib Plus Sirolimus in Unresectable Malignant Peripheral Nerve Sheath Tumors Identifies M2 Macrophage Activation. JCO Oncol. Adv. 2025, 2, e2400083. [Google Scholar] [CrossRef]
- McCowage, G.B.; Mueller, S.; Pratilas, C.A.; Hargrave, D.R.; Moertel, C.L.; Whitlock, J.; Fox, E.; Hingorani, P.; Russo, M.W.; Dasgupta, K.; et al. Trametinib in Pediatric Patients with Neurofibromatosis Type 1 (NF-1)–Associated Plexiform Neurofibroma: A Phase I/IIa Study. J. Clin. Oncol. 2018, 36, 10504. [Google Scholar] [CrossRef]
- Mueller, S.; Reddy, A.T.; Dombi, E.; Allen, J.; Packer, R.; Clapp, W.; Goldman, S.; Schorry, E.; Tonsgard, J.; Blakeley, J.; et al. NFB-17. MEK Inhibitor Binimetinib Shows Clinical Activity in Children with Neurofibromatosis Type 1-Associated Plexiform Neurofibromas: A Report from PNOC and the NF Clinical Trials Consortium. Neuro-Oncology 2020, 22, iii420–iii421. [Google Scholar] [CrossRef]
- Wang, D.; Ge, L.; Guo, Z.; Li, Y.; Zhu, B.; Wang, W.; Wei, C.; Li, Q.; Wang, Z. Efficacy and Safety of Trametinib in Neurofibromatosis Type 1-Associated Plexiform Neurofibroma and Low-Grade Glioma: A Systematic Review and Meta-Analysis. Pharmaceuticals 2022, 15, 956. [Google Scholar] [CrossRef]
- Fukuda, M.; Mukohara, T.; Kuwata, T.; Sunami, K.; Naito, Y. Efficacy of Trametinib in Neurofibromatosis Type 1-Associated Gastrointestinal Stromal Tumors: A Case Report. JCO Precis. Oncol. 2024, 8, e2300649. [Google Scholar] [CrossRef] [PubMed]
- Hargrave, D.R.; Terashima, K.; Hara, J.; Kordes, U.R.; Upadhyaya, S.A.; Sahm, F.; Bouffet, E.; Packer, R.J.; Witt, O.; Sandalic, L.; et al. Phase II Trial of Dabrafenib Plus Trametinib in Relapsed/Refractory BRAF V600–Mutant Pediatric High-Grade Glioma. J. Clin. Oncol. 2023, 41, 5174–5183. [Google Scholar] [CrossRef]
- Higham, C.S.; Steinberg, S.M.; Dombi, E.; Perry, A.; Helman, L.J.; Schuetze, S.M.; Ludwig, J.A.; Staddon, A.; Milhem, M.M.; Rushing, D.; et al. SARC006: Phase II Trial of Chemotherapy in Sporadic and Neurofibromatosis Type 1 Associated Chemotherapy-Naive Malignant Peripheral Nerve Sheath Tumors. Sarcoma 2017, 2017, 8685638. [Google Scholar] [CrossRef]
- Nishida, Y.; Urakawa, H.; Nakayama, R.; Kobayashi, E.; Ozaki, T.; Ae, K.; Matsumoto, Y.; Tsuchiya, H.; Goto, T.; Hiraga, H.; et al. Phase II Clinical Trial of Pazopanib for Patients with Unresectable or Metastatic Malignant Peripheral Nerve Sheath Tumors. Int. J. Cancer 2021, 148, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pollard, K.; Calizo, A.; Pratilas, C.A. Activation of Receptor Tyrosine Kinases Mediates Acquired Resistance to MEK Inhibition in Malignant Peripheral Nerve Sheath Tumors. Cancer Res. 2021, 81, 747–762. [Google Scholar] [CrossRef]
- Rauner, G.; Gupta, P.B.; Kuperwasser, C. From 2D to 3D and beyond: The Evolution and Impact of In Vitro Tumor Models in Cancer Research. Nat. Methods 2025, 22, 1776–1787. [Google Scholar] [CrossRef] [PubMed]
- Tomkinson, J.; Mattson, C.; Mattson-Hoss, M.; Guzman, G.; Sarnoff, H.; Bouley, S.J.; Walker, J.A.; Way, G.P. High-Content Microscopy and Machine Learning Characterize a Cell Morphology Signature of NF1 Genotype in Schwann Cells. Glial Health Res. 2025, 2, 100009. [Google Scholar] [CrossRef]
- Jiang, C.; McKay, R.M.; Le, L.Q. Tumorigenesis in Neurofibromatosis Type 1: Role of the Microenvironment. Oncogene 2021, 40, 5781–5787. [Google Scholar] [CrossRef] [PubMed]
- White, E.E.; Rhodes, S.D. The NF1+/- Immune Microenvironment: Dueling Roles in Neurofibroma Development and Malignant Transformation. Cancers 2024, 16, 994. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, H.; Sharma, P.; Sahi, S. Advances in Artificial Intelligence (AI)-Assisted Approaches in Drug Screening. Artif. Intell. Chem. 2024, 2, 100039. [Google Scholar] [CrossRef]
- Engleman, K. Healx Announces First Patient Dosed in Phase 2 Trial Evaluating HLX-1502 for the Treatment of Neurofibromatosis Type 1. 2025. Available online: https://healx.ai/healx-announces-first-patient-dosed-in-phase-2-trial-evaluating-hlx-1502-for-the-treatment-of-neurofibromatosis-type-1/ (accessed on 6 August 2025).
- Gates, L. IU Researcher, AI-Powered Biotech Firm Test Tumor-Shrinking Drug for Rare Disease. 2025. Available online: https://news.iu.edu/live/news/45736-iu-researcher-ai-powered-biotech-firm-test (accessed on 6 August 2025).
- Cox, A.D.; Der, C.J. “Undruggable KRAS”: Druggable After All. Genes Dev. 2025, 39, 132–162. [Google Scholar] [CrossRef]
- Desai, D.; Kantliwala, S.V.; Vybhavi, J.; Ravi, R.; Patel, H.; Patel, J. Review of AlphaFold 3: Transformative Advances in Drug Design and Therapeutics. Cureus 2024, 16, e63646. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouley, S.J.; Housden, B.E.; Walker, J.A. Neurofibromatosis Type 1 and the Search for Effective Tumor Therapies Using High-Throughput Drug Screening. Curr. Oncol. 2025, 32, 649. https://doi.org/10.3390/curroncol32110649
Bouley SJ, Housden BE, Walker JA. Neurofibromatosis Type 1 and the Search for Effective Tumor Therapies Using High-Throughput Drug Screening. Current Oncology. 2025; 32(11):649. https://doi.org/10.3390/curroncol32110649
Chicago/Turabian StyleBouley, Stephanie J., Benjamin E. Housden, and James A. Walker. 2025. "Neurofibromatosis Type 1 and the Search for Effective Tumor Therapies Using High-Throughput Drug Screening" Current Oncology 32, no. 11: 649. https://doi.org/10.3390/curroncol32110649
APA StyleBouley, S. J., Housden, B. E., & Walker, J. A. (2025). Neurofibromatosis Type 1 and the Search for Effective Tumor Therapies Using High-Throughput Drug Screening. Current Oncology, 32(11), 649. https://doi.org/10.3390/curroncol32110649






