Predicting the Consistency of Vestibular Schwannoma and Its Implication in the Retrosigmoid Approach: A Single-Center Analysis
Simple Summary
Abstract
1. Introduction
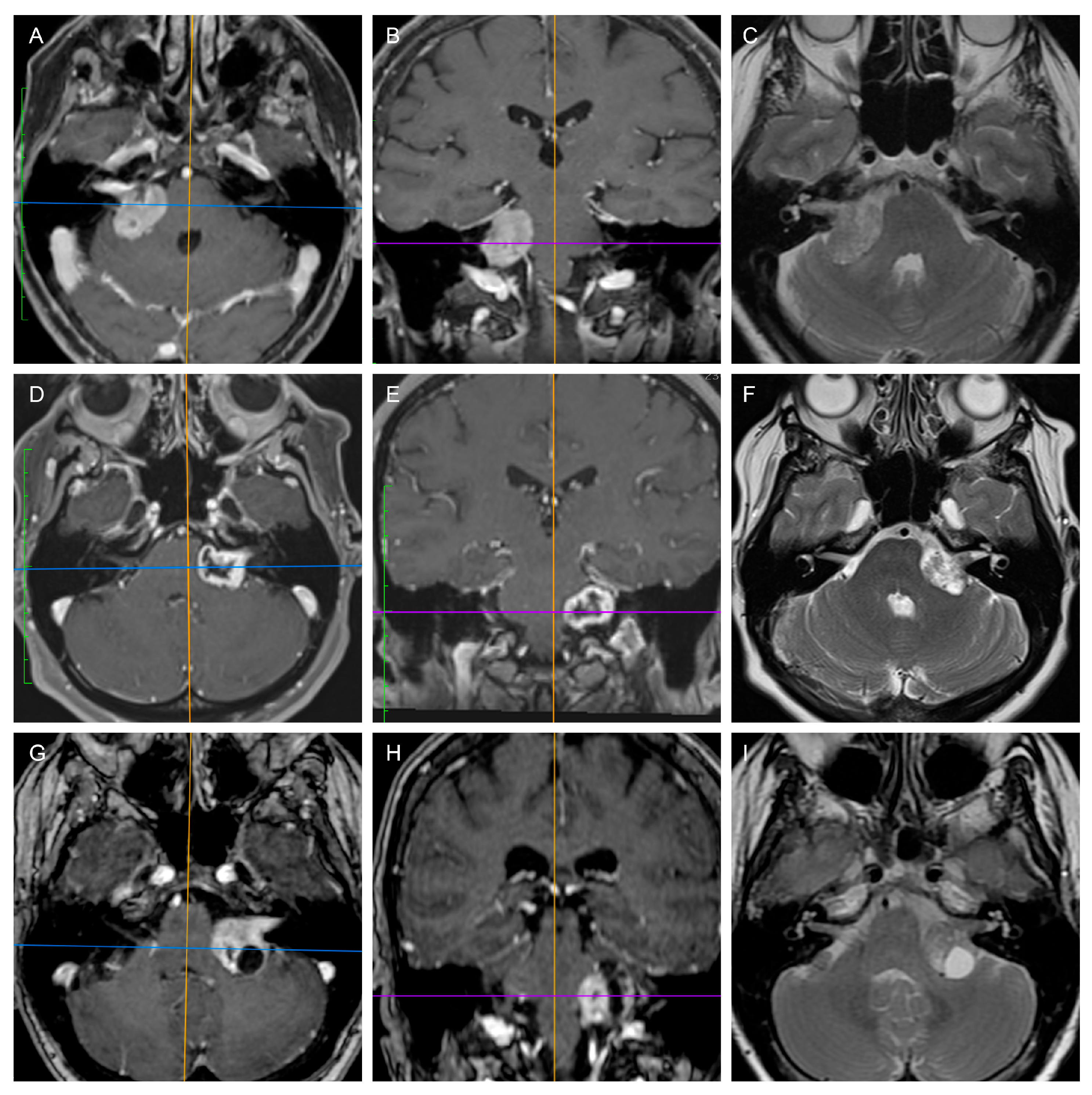
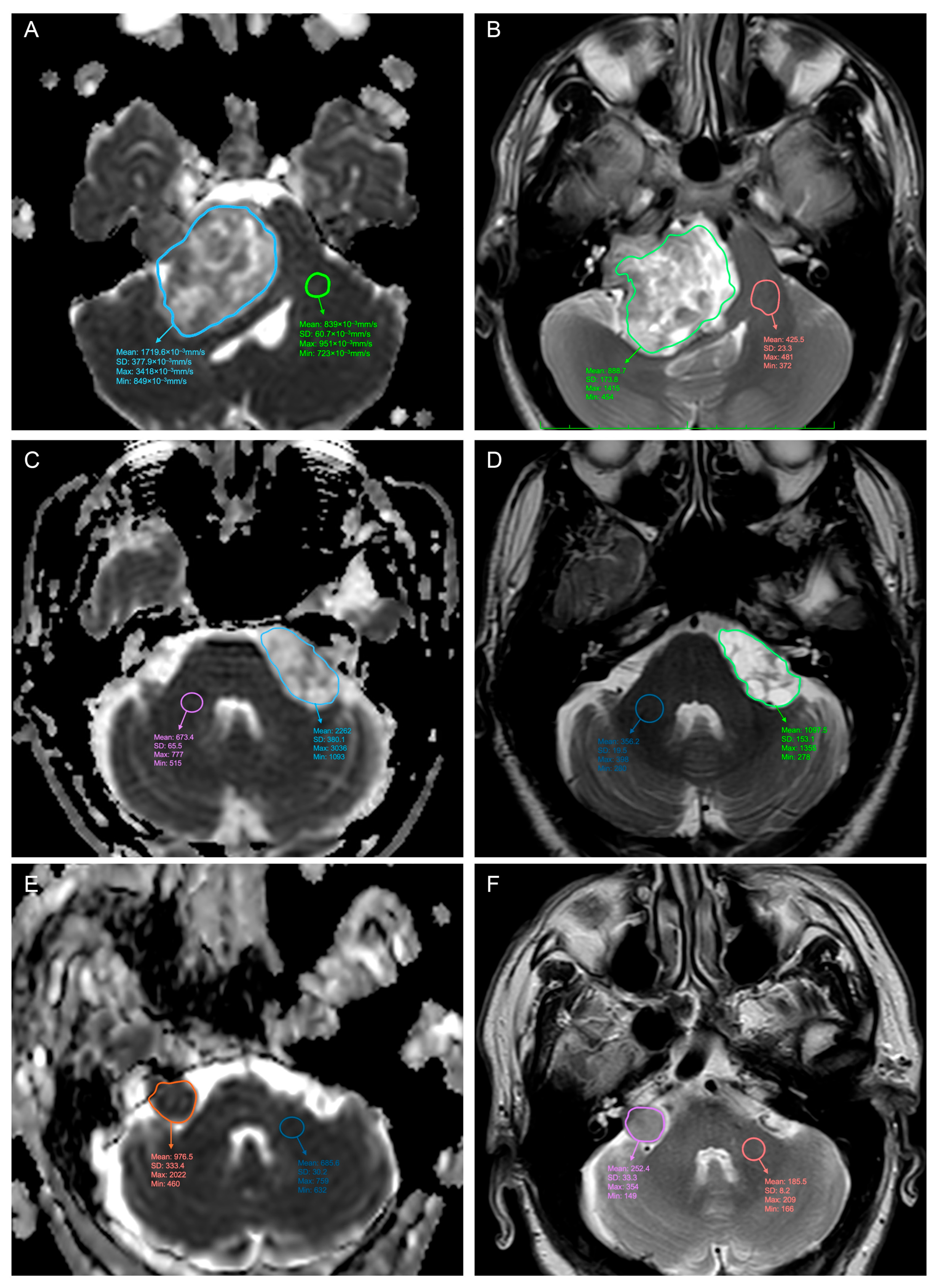
2. Materials and Methods
3. Results
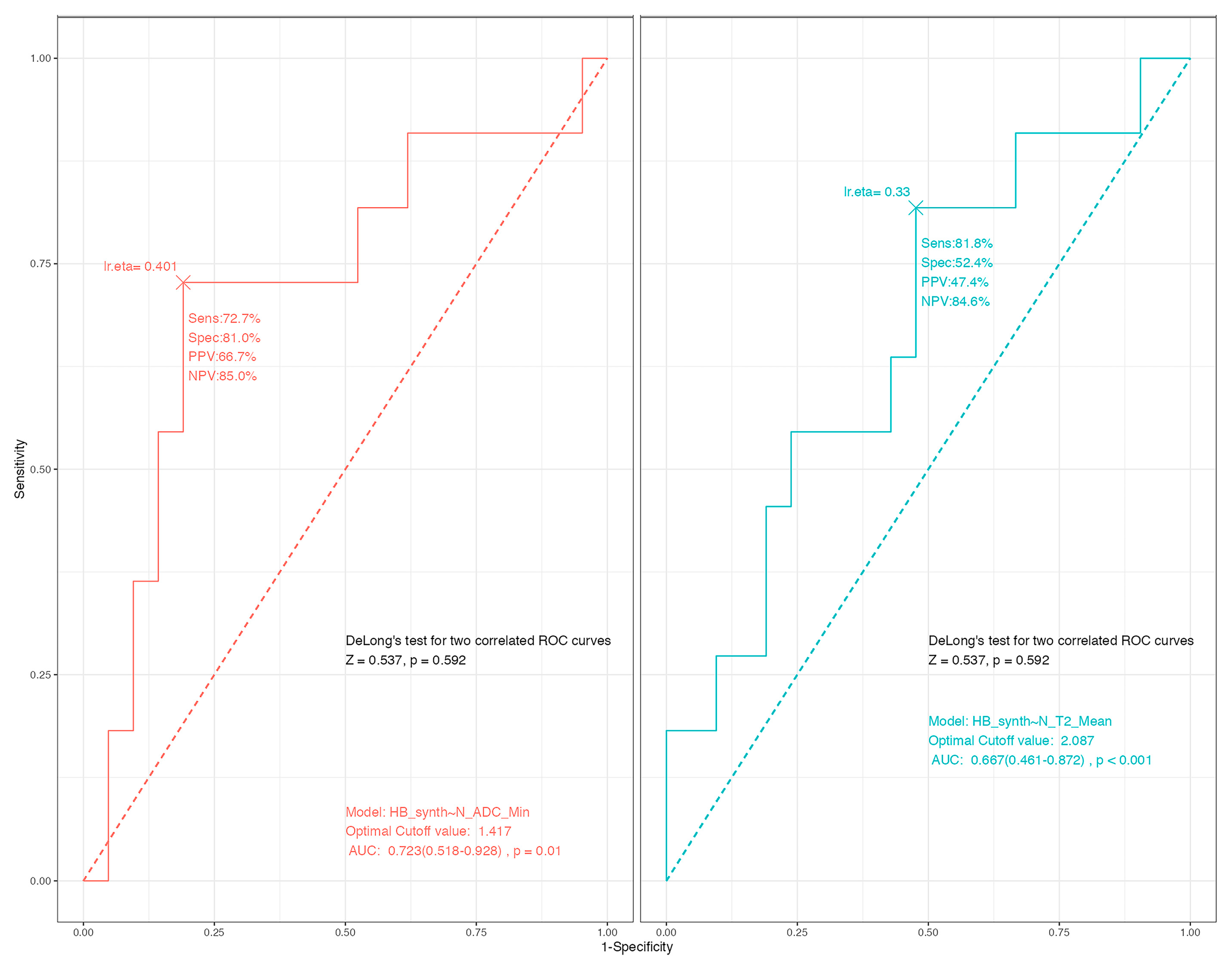
3.1. Predicting the Consistency
3.2. Association with the Outcome
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| VS | Vestibular Schwannoma |
| SVS | Solid Vestibular Schwannoma |
| CVS | Cystic Vestibular Schwannoma |
| MRI | Magnetic Resonance Imaging |
| ADC | Apparent Diffusion Coefficient |
| DWI | Diffusion Weighted Imaging |
| UA | Ultrasonic Aspirator |
| RS | Retrosigmoid Approach |
| FN | Facial Nerve |
| HB | House–Brackmann |
| AAO-HNS | American Academy of Otolaryngology-Head and Neck Surgery |
| CPA | CerebelloPontine Angle |
| IAC | Internal Auditory Canal |
| EOR | Extent Of Resetion |
| GTR | Gross-Total Resection |
| NTR | Near-Total Resection |
| STR | Sub-Total Resection |
| ANOVA | Analysis of Variance |
| LOS | Length of Stay |
| ROC | Receiving Operating Characteristics |
| AUC | Area Under the Curve |
| VIF | Variance Inflation Factor |
Appendix A
| Radiological Characteristics | Specification | Total (N = 33) |
|---|---|---|
| Max Diameter (mm) | Mean (SD) | 23.8 (8.7) |
| Range | 12.0–48.6 | |
| Tumor Volume (cm3) | Mean (SD) | 6.7 (8.2) |
| Range | 0.7–38.3 | |
| Koos Grade | 2 | 10 (30.3%) |
| 3 | 12 (36.4%) | |
| 4 | 11 (33.3%) | |
| Samii Classification | T3a | 10 (30.3%) |
| T3b | 10 (30.3%) | |
| T4a | 9 (27.3%) | |
| T4b | 4 (14.1%) | |
| CVS vs. SVS | Cystic * | 15 (45.5%) |
| Solid | 18 (54.5%) | |
| Brainstem Edema | Absence | 27 (81.8%) |
| Presence | 6 (18.2%) | |
| N-T2mean (N = 32) | Mean (SD) | 2.1 (0.4) |
| Range | 1.3–3.0 | |
| N-T2max (N = 32) | Mean (SD) | 2.5 (0.6) |
| Range | 1.5–3.5 | |
| N-ADCmean (10−3 mm/s) | Mean (SD) | 2.0 (0.4) |
| Range | 1.1–3.3 | |
| N-ADCmin (10−3 mm/s) | Mean (SD) | 1.6 (0.4) |
| Range | 0.6–2.4 |
| Perioperative Characteristics | Specification | Total (N = 33) |
|---|---|---|
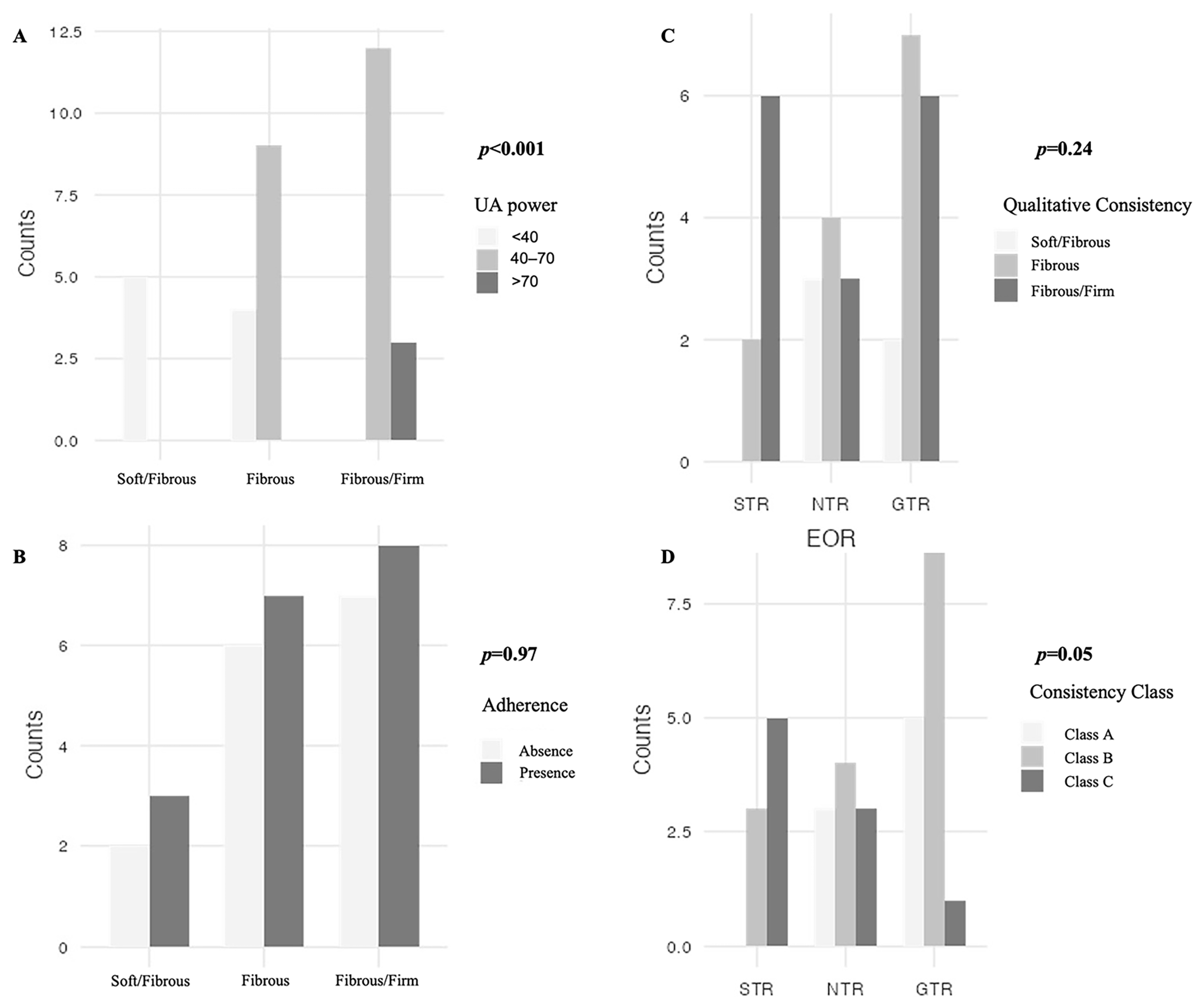
| Qualitative Consistency | Soft with Fibrous Areas | 5 (15.2%) |
| Fibrous | 13 (39.4%) | |
| Fibrous/Firm | 15 (45.5%) | |
| UA power range | <40 | 9 (27.3%) |
| 40–70 | 21 (63.6%) | |
| >70 | 3 (9.1%) | |
| Adherences | Absence | 15 (45.5%) |
| Presence | 18 (54.5%) | |
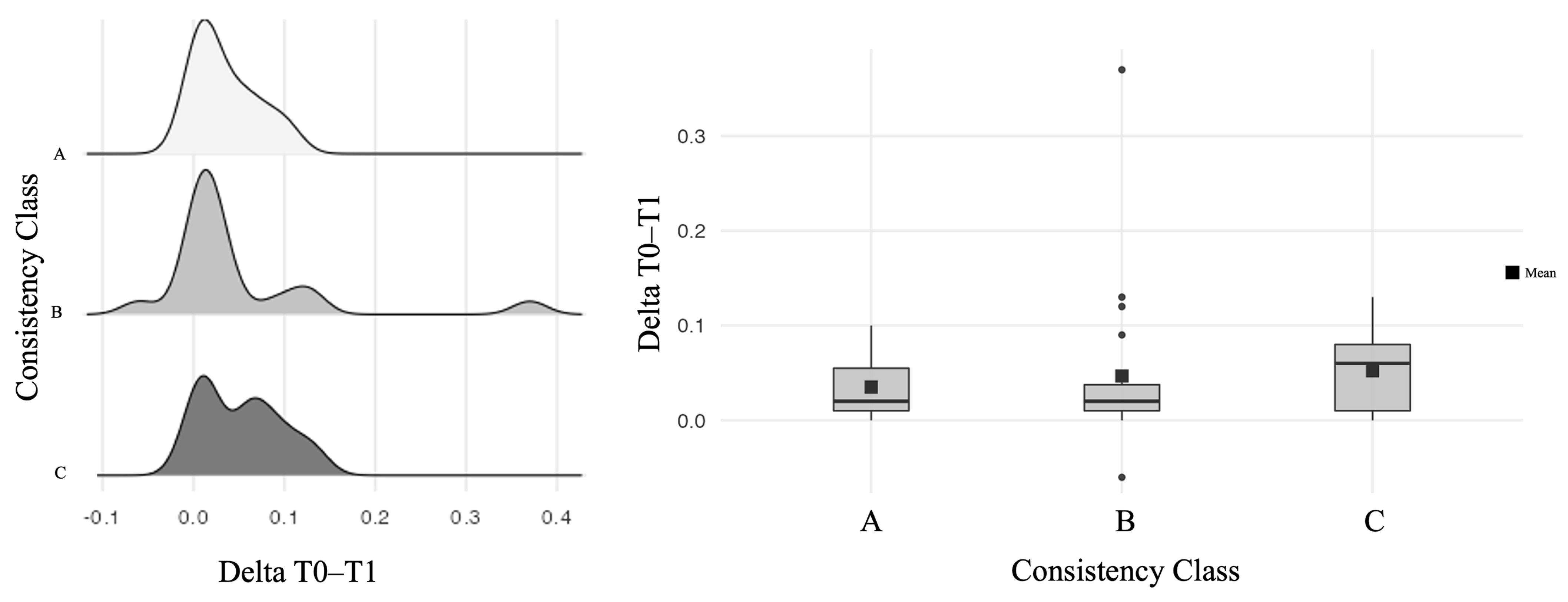
| Delta Threshold T0–T1 | Mean (SD) | 0.03 (0.04) |
| Operative Time (min) | Mean (SD) | 394.2 (105.5) |
| Range | 210–630 | |
| Extent Of Resection | STR | 8 (24.2%) |
| NTR | 10 (30.3%) | |
| GTR | 15 (45.5%) | |
| Length of stay (LOS) | Mean (SD) | 8.0 (5.2) |
| Range | 3.0–30.0 | |
| Preoperative HB Grade | 1 | 27 (81.8%) |
| 2 | 4 (12.1%) | |
| 3 | 1 (3.0%) | |
| 5 | 1 (3.0%) | |
| Immediate Postoperative HB Grade | 1 | 10 (30.3%) |
| 2 | 12 (36.4%) | |
| 3 | 5 (15.2%) | |
| 4 | 4 (12.1%) | |
| 5 | 2 (6.1%) | |
| HB Grade at Follow-up (12 months) | 1 | 23 (69.7%) |
| 2 | 4 (12.1%) | |
| 3 | 2 (6.1%) | |
| 4 | 4 (12.1%) |
| Variable | Group (Number) | Mean | Statistic Test | Statistic | df | p |
|---|---|---|---|---|---|---|
| N-T2mean | SVS (17) | 1.87 | Student’s t | −3.65 | 30.0 | <0.001 |
| CVS (15) | 2.36 | Mann–Whitney U | 41.0 | <0.001 | ||
| N-T2max | SVS (17) | 2.35 | Student’s t | −1.89 | 30.0 | 0.069 |
| CVS (15) | 2.71 | Mann–Whitney U | 84.0 | 0.099 | ||
| N-ADCmean | SVS (18) | 1.72 | Student’s t | −4.28 | 31.0 | <0.001 |
| CVS (15) | 2.27 | Mann–Whitney U | 35.0 | <0.001 | ||
| N-ADCmin | SVS (18) | 1.47 | Student’s t | −2.25 | 31.0 | 0.032 |
| CVS (15) | 1.79 | Mann–Whitney U | 77.0 | 0.036 |
| Radiological Characteristics | Immediate Postoperative HB | |||
|---|---|---|---|---|
| HB < 3 (N = 22) | HB ≥ 3 (N = 11) | Total (N = 33) | p Value | |
| Max Diameter | 0.26 1 | |||
| Mean (SD) | 22.6 (7.0) | 26.3 (11.2) | 23.8 (8.7) | |
| Range | 12.0–33.0 | 15.0–48.6 | 12.0–48.6 | |
| Tumor Volume | 0.05 1 | |||
| Mean (SD) | 4.7 (3.5) | 10.6 (12.8) | 6.7 (8.2) | |
| Range | 0.7–12.2 | 1.4–38.3 | 0.7–38.3 | |
| Koos Grade | 0.18 2 | |||
| 2 | 8.0 (36.4%) | 2.0 (18.2%) | 10.0 (30.3%) | |
| 3 | 9.0 (40.9%) | 3.0 (27.3%) | 12.0 (36.4%) | |
| 4 | 5.0 (22.7%) | 6.0 (54.5%) | 11.0 (33.3%) | |
| Samii Classification | 0.26 2 | |||
| T3a | 8.0 (36.4%) | 2.0 (18.2%) | 10.0 (30.3%) | |
| T3b | 7.0 (31.8%) | 3.0 (27.3%) | 10.0 (30.3%) | |
| T4a | 6.0 (27.3%) | 3.0 (27.3%) | 9.0 (27.3%) | |
| T4b | 1.0 (4.5%) | 3.0 (27.3%) | 4.0 (12.1%) | |
| Brainstem Edema | 0.06 2 | |||
| Absence | 20.0 (90.9%) | 7.0 (63.6%) | 27.0 (81.8%) | |
| Presence | 2.0 (9.1%) | 4.0 (36.4%) | 6.0 (18.2%) | |
| SV type | 0.46 2 | |||
| SVS | 11.0 (50.0%) | 7.0 (63.6%) | 18.0 (54.5%) | |
| CVS | 11.0 (50.0%) | 4.0 (36.4%) | 15.0 (45.5%) | |
| N-T2mean | 0.11 1; 0.13 3 | |||
| N-Miss | 1.0 | 0.0 | 1.0 | |
| Mean (SD) | 2.2 (0.4) | 1.9 (0.4) | 2.1 (0.4) | |
| Range | 1.5–3.0 | 1.3–2.8 | 1.3–3.0 | |
| N-T2max | 0.22 1; 0.28 3 | |||
| N-Miss | 1.0 | 0.0 | 1.0 | |
| Mean (SD) | 2.6 (0.5) | 2.4 (0.6) | 2.5 (0.5) | |
| Range | 1.6–3.5 | 1.5–3.0 | 1.5–3.5 | |
| N-ADCmean | 0.26 1; 0.27 3 | |||
| Mean (SD) | 2.0 (0.5) | 1.8 (0.4) | 2.0 (0.4) | |
| Range | 1.5–3.3 | 1.1–2.4 | 1.1–3.3 | |
| N-ADCmin | 0.11 1; 0.04 3 | |||
| Mean (SD) | 1.7 (0.4) | 1.5 (0.4) | 1.6 (0.4) | |
| Range | 0.6–2.4 | 1.0–2.3 | 0.6–2.4 | |
| Intraoperative Characteristics | Immediate Postoperative HB | |||
|---|---|---|---|---|
| HB < 3 (N = 22) | HB ≥ 3 (N = 11) | Total (N = 33) | p Value | |
| Consistency Classes | 0.17 2 | |||
| A | 7.0 (31.8%) | 1.0 (9.1%) | 8.0 (24.2%) | |
| B | 11.0 (50.0%) | 5.0 (45.5%) | 16.0 (48.5%) | |
| C | 4.0 (18.2%) | 5.0 (45.5%) | 9.0 (27.3%) | |
| Adherence | 0.03 2 | |||
| Absence | 13.0 (59.1%) | 2.0 (18.2%) | 15.0 (45.5%) | |
| Presence | 9.0 (40.9%) | 9.0 (81.8%) | 18.0 (54.5%) | |
| Preoperative HB | 0.04 2 | |||
| <3 | 22.0 (100.0%) | 9.0 (81.8%) | 31.0 (93.9%) | |
| ≥3 | 0.0 (0.0%) | 2.0 (18.2%) | 2.0 (6.1%) | |
| Delta T0–T1 | 0.03 1 | |||
| Mean (SD) | 0.0 (0.0) | 0.1 (0.1) | 0.0 (0.0) | |
| Range | 0.0–0.1 | −0.1–0.1 | −0.1–0.1 | |
| Operative Time | 0.17 1 | |||
| Mean (SD) | 376.4 (86.7) | 429.9 (133.1) | 394.2 (105.5) | |
| Range | 210.0–520.0 | 250.0–630.0 | 210.0–630.0 | |
| EOR | <0.01 2 | |||
| STR | 1.0 (4.5%) | 7.0 (63.6%) | 8.0 (24.2%) | |
| NTR | 8.0 (36.4%) | 2.0 (18.2%) | 10.0 (30.3%) | |
| GTR | 13.0 (59.1%) | 2.0 (18.2%) | 15.0 (45.5%) | |
| Radiological Characteristics | HB 12-Month FU | |||
|---|---|---|---|---|
| HB < 3 (N = 28) | HB ≥ 3 (N = 5) | Total (N = 33) | p Value | |
| Max Diameter | 0.003 1 | |||
| Mean (SD) | 22.0 (6.5) | 33.9 (13.0) | 23.8 (8.7) | |
| Range | 12.0–33.0 | 17.0–48.6 | 12.0–48.6 | |
| Tumor Volume | <0.001 1 | |||
| Mean (SD) | 4.4 (3.2) | 19.2 (15.4) | 6.7 (8.2) | |
| Range | 0.7–12.2 | 2.2–38.3 | 0.7–38.3 | |
| Koos Grade | 0.05 2 | |||
| 2 | 10.0 (35.7%) | 0.0 (0.0%) | 10.0 (30.3%) | |
| 3 | 11.0 (39.3%) | 1.0 (20.0%) | 12.0 (36.4%) | |
| 4 | 7.0 (25.0%) | 4.0 (80.0%) | 11.0 (33.3%) | |
| Samii Classification | <0.01 2 | |||
| T3a | 10.0 (35.7%) | 0.0 (0.0%) | 10.0 (30.3%) | |
| T3b | 9.0 (32.1%) | 1.0 (20.0%) | 10.0 (30.3%) | |
| T4a | 8.0 (28.6%) | 1.0 (20.0%) | 9.0 (27.3%) | |
| T4b | 1.0 (3.6%) | 3.0 (60.0%) | 4.0 (12.1%) | |
| Brainstem Edema | 0.008 2 | |||
| Absence | 25.0 (89.3%) | 2.0 (40.0%) | 27.0 (81.8%) | |
| Presence | 3.0 (10.7%) | 3.0 (60.0%) | 6.0 (18.2%) | |
| SV type | 0.22 2 | |||
| SVS | 14.0 (50.0%) | 4.0 (80.0%) | 18.0 (54.5%) | |
| CVS | 14.0 (50.0%) | 1.0 (20.0%) | 15.0 (45.5%) | |
| N-T2mean | 0.08 1; 0.09 3 | |||
| N-Miss | 1.0 | 0.0 | 1.0 | |
| Mean (SD) | 2.2 (0.4) | 1.8 (0.3) | 2.1 (0.4) | |
| Range | 1.4–3.0 | 1.3–2.1 | 1.3–3.0 | |
| N-T2max | 0.68 1; 0.86 3 | |||
| N-Miss | 1.0 | 0.0 | 1.0 | |
| Mean (SD) | 2.5 (0.5) | 2.4 (0.7) | 2.5 (0.5) | |
| Range | 1.5–3.5 | 1.5–3.0 | 1.5–3.5 | |
| N-ADCmean | 0.41 1; 0.32 3 | |||
| Mean (SD) | 2.0 (0.5) | 1.8 (0.4) | 2.0 (0.4) | |
| Range | 1.1–3.3 | 1.4–2.2 | 1.1–3.3 | |
| N-ADCmin | 0.58 1; 0.46 3 | |||
| Mean (SD) | 1.6 (0.4) | 1.5 (0.3) | 1.6 (0.4) | |
| Range | 0.6–2.4 | 1.2–1.9 | 0.6–2.4 | |
| Intraoperative Characteristics | HB 12-Month FU | |||
|---|---|---|---|---|
| HB < 3 (N = 28) | HB ≥ 3 (N = 5) | Total (N = 33) | p Value | |
| Consistency Classes | 0.19 2 | |||
| A | 7.0 (25.0%) | 1.0 (20.0%) | 8.0 (24.2%) | |
| B | 15.0 (53.6%) | 1.0 (20.0%) | 16.0 (48.5%) | |
| C | 6.0 (21.4%) | 3.0 (60.0%) | 9.0 (27.3%) | |
| Adherence | 0.22 2 | |||
| Absence | 14.0 (50.0%) | 1.0 (20.0%) | 15.0 (45.5%) | |
| Presence | 14.0 (50.0%) | 4.0 (80.0%) | 18.0 (54.5% | |
| Preoperative HB | 0.16 2 | |||
| <3 | 27.0 (96.4%) | 4.0 (80.0%) | 31.0 (93.9%) | |
| ≥3 | 1.0 (3.6%) | 1.0 (20.0%) | 2.0 (6.1%) | |
| Delta T0–T1 | 0.006 1 | |||
| Mean (SD) | 0.0 (0.0) | 0.1 (0.1) | 0.0 (0.0) | |
| Range | −0.1–0.1 | 0.0–0.1 | −0.1–0.1 | |
| Operative Time | 0.001 1 | |||
| Mean (SD) | 370.7 (83.8) | 526.0 (126.4) | 394.2 (105.5) | |
| Range | 210.0–520.0 | 345.0–630.0 | 210.0–630.0 | |
| EOR | 0.01 2 | |||
| STR | 4.0 (14.3%) | 4.0 (80.0%) | 8.0 (24.2%) | |
| NTR | 9.0 (32.1%) | 1.0 (20.0%) | 10.0 (30.3%) | |
| GTR | 15.0 (53.6%) | 0.0 (0.0%) | 15.0 (45.5%) | |
References
- Thakur, J.D.; Khan, I.S.; Shorter, C.D.; Sonic, A.; Gardner, G.L.; Guthikonda, B.; Nanda, A. Do Cystic Vestibular Schwannomas Have Worse Surgical Outcomes? Systematic Analysis of the Literature. Neurosurg. Focus 2012, 33, E12. [Google Scholar] [CrossRef]
- Wu, X.; Song, G.; Wang, X.; Li, M.; Chen, G.; Guo, H.; Bao, Y.; Liang, J. Comparison of Surgical Outcomes in Cystic and Solid Vestibular Schwannomas: A Systematic Review and Meta-Analysis. Neurosurg. Rev. 2021, 44, 1889–1902. [Google Scholar] [CrossRef]
- Tang, I.P.; Freeman, S.R.; Rutherford, S.A.; King, A.T.; Ramsden, R.T.; Lloyd, S.K.W. Surgical Outcomes in Cystic Vestibular Schwannoma versus Solid Vestibular Schwannoma. Otol. Neurotol. 2014, 35, 1266–1270. [Google Scholar] [CrossRef]
- Han, J.H.; Baek, K.H.; Lee, Y.W.; Hur, Y.K.; Kim, H.J.; Moon, I.S. Comparison of Clinical Characteristics and Surgical Outcomes of Cystic and Solid Vestibular Schwannomas. Otol. Neurotol. 2018, 39, e381–e386. [Google Scholar] [CrossRef]
- Almefty, R.O.; Xu, D.S.; Mooney, M.A.; Montoure, A.; Naeem, K.; Coons, S.W.; Spetzler, R.F.; Porter, R.W. Comparison of Surgical Outcomes and Recurrence Rates of Cystic and Solid Vestibular Schwannomas. J. Neurol. Surg. B Skull Base 2021, 82, 333–337. [Google Scholar] [CrossRef]
- Zhang, L.; Ostrander, B.T.; Duhon, B.; Moshitaghi, O.; Lee, J.; Harris, M.; Hardesty, D.A.; Prevedello, D.M.; Schwartz, M.S.; Dodson, E.E.; et al. Comparison of Postoperative Outcomes in Cystic Versus Solid Vestibular Schwannoma in a Multi-Institutional Cohort. Otol. Neurotol. 2023, 45, 92–99. [Google Scholar] [CrossRef]
- Lee, T.K.Y.; Lund, W.S.; Adams, C.B.T. Factors Influencing the Preservation of the Facial Nerve during Acoustic Surgery. Br. J. Neurosurg. 1990, 4, 5–8. [Google Scholar] [CrossRef]
- Giordano, M.; Samii, A.; Samii, M.; Nabavi, A. Magnetic Resonance Imaging-Apparent Diffusion Coefficient Assessment of Vestibular Schwannomas: Systematic Approach, Methodology, and Pitfalls. World Neurosurg. 2019, 125, e820–e823. [Google Scholar] [CrossRef] [PubMed]
- Kunigelis, K.E.; Hosokawa, P.; Arnone, G.; Raban, D.; Starr, A.; Gurau, A.; Sunshine, A.; Bunn, J.; Thaker, A.A.; Youssef, A.S. The Predictive Value of Preoperative Apparent Diffusion Coefficient (ADC) for Facial Nerve Outcomes after Vestibular Schwannoma Resection: Clinical Study. Acta Neurochir. 2020, 162, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.M.; Ung, T.H.; Thompson, J.A.; Ovard, O.; Olson, M.; Hirt, L.; Hosokawa, P.; Thaker, A.; Youssef, A.S. Refining the Predictive Value of Preoperative Apparent Diffusion Coefficient (ADC) by Whole-Tumor Analysis for Facial Nerve Outcomes in Vestibular Schwannomas. Acta Neurochir. 2024, 166, 168. [Google Scholar] [CrossRef] [PubMed]
- Di Perna, G.; De Marco, R.; Baldassarre, B.M.; Lo Bue, E.; Cofano, F.; Zeppa, P.; Ceroni, L.; Penner, F.; Melcarne, A.; Garbossa, D.; et al. Facial Nerve Outcome Score: A New Score to Predict Long-Term Facial Nerve Function after Vestibular Schwannoma Surgery. Front. Oncol. 2023, 13, 1153662. [Google Scholar] [CrossRef]
- De Marco, R.; Lo Bue, E.; Di Perna, G.; Penner, F.; Vercelli, A.; Baldassarre, B.M.; Albera, R.; Garbossa, D.; Zenga, F. Introducing Endoscopic Assistance on Routinary Basis for Vestibular Schwannomas Resection: A Single Centre Acceptance Analysis. Neurochirurgie 2024, 70, 101524. [Google Scholar] [CrossRef] [PubMed]
- Goldbrunner, R.; Weller, M.; Regis, J.; Lund-Johansen, M.; Stavrinou, P.; Reuss, D.; Evans, D.G.; Lefranc, F.; Sallabanda, K.; Falini, A.; et al. EANO Guideline on the Diagnosis and Treatment of Vestibular Schwannoma. Neuro Oncol. 2020, 22, 31–45. [Google Scholar] [CrossRef]
- House, J.W.; Brackmann, D.E. Facial Nerve Grading System. Otolaryngol. Head Neck Surg. 1985, 93, 146–147. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, E.; Wiet, M.R.; Flanagan, S.; Dispenza, F.; Giannuzzi, A.; Mancini, F.; Sanna, M. Cystic Vestibular Schwannoma: Classification, Management, and Facial Nerve Outcomes. Otol. Neurotol. 2009, 30, 826–834. [Google Scholar] [CrossRef]
- Koos, W.T.; Day, J.D.; Matula, C.; Levy, D.I. Neurotopographic Considerations in the Microsurgical Treatment of Small Acoustic Neurinomas. J. Neurosurg. 1998, 88, 506–512. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, L.; Han, D.; Mao, Y.; Yang, J.; Wang, Z.; Jia, W.; Zhong, P.; Jia, H. Summary and Consensus in 7th International Conference on Acoustic Neuroma: An Update for the Management of Sporadic Acoustic Neuromas. World J. Otorhinolaryngol. Head Neck Surg. 2016, 2, 234. [Google Scholar] [CrossRef]
- Smith, K.A.; Leever, J.D.; Chamoun, R.B. Predicting Consistency of Meningioma by Magnetic Resonance Imaging. J. Neurol. Surg. B Skull Base 2015, 76, 225. [Google Scholar] [CrossRef]
- MacIelak, R.J.; Harris, M.S.; Mattingly, J.K.; Shah, V.S.; Prevedello, L.M.; Adunka, O.F. Can an Imaging Marker of Consistency Predict Intraoperative Experience and Clinical Outcomes for Vestibular Schwannomas? A Retrospective Review. J. Neurol. Surg. B Skull Base 2021, 82, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Zada, G.; Yashar, P.; Robison, A.; Winer, J.; Khalessi, A.; Mack, W.J.; Giannotta, S.L. A Proposed Grading System for Standardizing Tumor Consistency of Intracranial Meningiomas. Neurosurg. Focus 2013, 35, E1. [Google Scholar] [CrossRef]
- Charabi, S.; Klinken, L.; Tos, M.; Thomsen, J. Histopathology and Growth Pattern of Cystic Acoustic Neuromas. Laryngoscope 1994, 104, 1348–1352. [Google Scholar] [CrossRef] [PubMed]
- Park, C.K.; Kim, D.C.; Park, S.H.; Jeong, E.K.; Sun, H.P.; Dong, G.K.; Jung, H.W. Microhemorrhage, a Possible Mechanism for Cyst Formation in Vestibular Schwannomas. J. Neurosurg. 2006, 105, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, N.; Behari, S.; Pal, L.; Banerji, D.; Sahu, R.N.; Jain, V.K. Giant Vestibular Schwannomas: Focusing on the Differences between the Solid and the Cystic Variants. Br. J. Neurosurg. 2008, 22, 550–556. [Google Scholar] [CrossRef]
- Wanibuchi, M.; Fukushima, T.; Friedman, A.H.; Watanabe, K.; Akiyama, Y.; Mikami, T.; Iihoshi, S.; Murakami, T.; Sugino, T.; Mikuni, N. Hearing Preservation Surgery for Vestibular Schwannomas via the Retrosigmoid Transmeatal Approach: Surgical Tips. Neurosurg. Rev. 2014, 37, 431–444. [Google Scholar] [CrossRef]
- Rizk, A.R.; Adam, A.; Gugel, I.; Schittenhelm, J.; Tatagiba, M.; Ebner, F.H. Implications of Vestibular Schwannoma Consistency: Analysis of 140 Cases Regarding Radiologic and Clinical Features. World Neurosurg. 2017, 99, 159–163. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Z.; Hu, X.; Yang, D.; Zheng, Z. Comparison of Clinical Features and Surgical Outcomes of Cystic and Solid Vestibular Schwannoma. World Neurosurg. 2025, 203, 124470. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, J.; Tos, M.; Sanna, M.; Moffat, D.A. New and Modified Reporting Systems from the Consensus Meeting on Systems for Reporting Results in Vestibular Schwannoma. Otol. Neurotol. 2003, 24, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Soyama, N.; Kuratsu, J.I.; Ushio, Y. Correlation between Magnetic Resonance Images and Histology in Meningiomas: T2-Weighted Images Indicate Collagen Contents in Tissues. Neurol. Med. Chir. 1995, 35, 438–441. [Google Scholar] [CrossRef]
- Kashimura, H.; Inoue, T.; Ogasawara, K.; Arai, H.; Otawara, Y.; Kanbara, Y.; Ogawa, A. Prediction of Meningioma Consistency Using Fractional Anisotropy Value Measured by Magnetic Resonance Imaging. J. Neurosurg. 2007, 107, 784–787. [Google Scholar] [CrossRef]
- Chernov, M.F.; Kasuya, H.; Nakaya, K.; Kato, K.; Ono, Y.; Yoshida, S.; Muragaki, Y.; Suzuki, T.; Iseki, H.; Kubo, O.; et al. 1H-MRS of Intracranial Meningiomas: What It Can Add to Known Clinical and MRI Predictors of the Histopathological and Biological Characteristics of the Tumor? Clin. Neurol. Neurosurg. 2011, 113, 202–212. [Google Scholar] [CrossRef]
- Murphy, M.C.; Huston, J.; Glaser, K.J.; Manduca, A.; Meyer, F.B.; Lanzino, G.; Morris, J.M.; Felmlee, J.P.; Ehman, R.L. Preoperative Assessment of Meningioma Stiffness Using Magnetic Resonance Elastography. J. Neurosurg. 2013, 118, 643–648. [Google Scholar] [CrossRef]
- Bunevicius, A.; Schregel, K.; Sinkus, R.; Golby, A.; Patz, S. MR Elastography of Brain Tumors. NeuroImage Clin. 2020, 25, 102109. [Google Scholar] [CrossRef] [PubMed]
- Duhon, B.H.; Thompson, K.; Fisher, M.; Kaul, V.F.; Nguyen, H.T.N.; Harris, M.S.; Varadarajan, V.; Adunka, O.F.; Prevedello, D.M.; Kolipaka, A.; et al. Tumor Biomechanical Stiffness by Magnetic Resonance Elastography Predicts Surgical Outcomes and Identifies Biomarkers in Vestibular Schwannoma and Meningioma. Sci. Rep. 2024, 14, 14561. [Google Scholar] [CrossRef] [PubMed]
- Shiroishi, M.S.; Cen, S.Y.; Tamrazi, B.; D’Amore, F.; Lerner, A.; King, K.S.; Kim, P.E.; Law, M.; Hwang, D.H.; Boyko, O.B.; et al. Predicting Meningioma Consistency on Preoperative Neuroimaging Studies. Neurosurg. Clin. N. Am. 2016, 27, 145–154. [Google Scholar] [CrossRef]
- Surov, A.; Meyer, H.J.; Wienke, A. Correlation between Apparent Diffusion Coefficient (ADC) and Cellularity Is Different in Several Tumors: A Meta-Analysis. Oncotarget 2017, 8, 59492. [Google Scholar] [CrossRef] [PubMed]
- Darbar, A.; Waqas, M.; Enam, S.F.; Mahmood, S.D.; Darbar, A.; Waqas, M.; Enam, S.F.; Mahmood, S.D. Use of Preoperative Apparent Diffusion Coefficients to Predict Brain Tumor Grade. Cureus 2018, 10, e2284. [Google Scholar] [CrossRef]
- Bano, S.; Waraich, M.M.; Khan, M.A.; Buzdar, S.A.; Manzur, S. Diagnostic Value of Apparent Diffusion Coefficient for the Accurate Assessment and Differentiation of Intracranial Meningiomas. Acta Radiol. Short Rep. 2013, 2, 204798161351248. [Google Scholar] [CrossRef]
- Baskan, O.; Silav, G.; Bolukbasi, F.H.; Canoz, O.; Geyik, S.; Elmaci, I. Relation of Apparent Diffusion Coefficient with Ki-67 Proliferation Index in Meningiomas. Br. J. Radiol. 2016, 89, 20140842. [Google Scholar] [CrossRef]
- Chuang, C.C.; Chang, C.S.; Tyan, Y.S.; Chuang, K.S.; Tu, H.T.; Huang, C.F. Use of Apparent Diffusion Coefficients in Evaluating the Response of Vestibular Schwannomas to Gamma Knife Surgery: Clinical Article. J. Neurosurg. 2012, 117, 63–68. [Google Scholar] [CrossRef]
- Copeland, W.R.; Hoover, J.M.; Morris, J.M.; Driscoll, C.L.W.; Link, M.J. Use of Preoperative MRI to Predict Vestibular Schwannoma Intraoperative Consistency and Facial Nerve Outcome. J. Neurol. Surg. B Skull Base 2013, 74, 347–350. [Google Scholar] [CrossRef]
- Yiping, L.; Ji, X.; Daoying, G.; Bo, Y. Prediction of the Consistency of Pituitary Adenoma: A Comparative Study on Diffusion-Weighted Imaging and Pathological Results. J. Neuroradiol. 2016, 43, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Della Pepa, G.M.; Menna, G.; Stifano, V.; Pezzullo, A.M.; Auricchio, A.M.; Rapisarda, A.; Caccavella, V.M.; La Rocca, G.; Sabatino, G.; Marchese, E.; et al. Predicting Meningioma Consistency and Brain-Meningioma Interface with Intraoperative Strain Ultrasound Elastography: A Novel Application to Guide Surgical Strategy. Neurosurg. Focus 2021, 50, E15. [Google Scholar] [CrossRef] [PubMed]
| Intraoperative Consistency (Qualitative) | Ultrasonic Aspirator (UA) Power Range | Adherence |
|---|---|---|
| Soft with fibrous areas (1) | <40 (1) | Yes (1) |
| Fibrous (2) | 40–70 (2) | No (0) |
| Fibrous/Firm (3) | >70 (3) | |
| Class A ≤ 3 | Class B = 4–5 | Class C = 6–7 |
| Radiological and Intraoperative Characteristics | Consistency Class | ||||
|---|---|---|---|---|---|
| Class A (N = 8) | Class B (N = 16) | Class C (N = 9) | Total (N = 33) | p Value | |
| Max Diameter | 0.027 2 | ||||
| Mean (SD) | 20.8 (6.3) | 21.7 (8.0) | 30.2 (9.1) | 23.8 (8.7) | |
| Range | 14.0–33.0 | 12.0–40.0 | 22.0–48.6 | 12.0–48.6 | |
| Tumor Volume | 0.015 2 | ||||
| Mean (SD) | 3.9 (3.0) | 4.4 (4.7) | 13.2 (12.4) | 6.7 (8.2) | |
| Range | 0.8–8.7 | 0.7–18.5 | 3.8–38.3 | 0.7–38.3 | |
| Koos Grade | 0.017 1 | ||||
| 2 | 3.0 (37.5%) | 7.0 (43.8%) | 0.0 (0.0%) | 10.0 (30.3%) | |
| 3 | 5.0 (62.5%) | 4.0 (25.0%) | 3.0 (33.3%) | 12.0 (36.4%) | |
| 4 | 0.0 (0.0%) | 5.0 (31.2%) | 6.0 (66.7%) | 11.0 (33.3%) | |
| Samii Classification | 0.002 1 | ||||
| T3a | 3.0 (37.5%) | 7.0 (43.8%) | 0.0 (0.0%) | 10.0 (30.3%) | |
| T3b | 5.0 (62.5%) | 4.0 (25.0%) | 1.0 (11.1%) | 10.0 (30.3%) | |
| T4a | 0.0 (0.0%) | 4.0 (25.0%) | 5.0 (55.6%) | 9.0 (27.3%) | |
| T4b | 0.0 (0.0%) | 1.0 (6.2%) | 3.0 (33.3%) | 4.0 (12.1%) | |
| N-T2mean | 0.068 2 | ||||
| N-Miss | 1.0 | 0.0 | 0.0 | 1.0 | |
| Mean (SD) | 2.4 (0.4) | 2.1 (0.5) | 1.9 (0.3) | 2.1 (0.4) | |
| Range | 1.9–3.0 | 1.4–2.9 | 1.3–2.3 | 1.3–3.0 | |
| N-ADCmin | 0.027 2 | ||||
| Mean (SD) | 1.9 (0.4) | 1.7 (0.4) | 1.3 (0.4) | 1.6 (0.4) | |
| Range | 1.4–2.4 | 1.0–2.3 | 0.6–1.9 | 0.6–2.4 | |
| Operative Time (min) | 0.066 2 | ||||
| Mean (SD) | 356.2 (66.6) | 374.9 (111.2) | 462.2 (100.3) | 394.2 (105.5) | |
| Range | 250–445 | 210–630 | 300–630 | 210–630 | |
| EOR | 0.015 1 | ||||
| STR | 0.0 (0.0%) | 3.0 (18.8%) | 5.0 (55.6%) | 8.0 (24.2%) | |
| NTR | 3.0 (37.5%) | 4.0 (25.0%) | 3.0 (33.3%) | 10.0 (30.3%) | |
| GTR | 5.0 (62.5%) | 9.0 (56.2%) | 1.0 (11.1%) | 15.0 (45.5%) | |
| Linear Regression Dependent Variable N-T2wmean | 95% CI | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictor | Estimate | SE | t | p | Stand. Estimate | Lower | Upper | |
| Intercept a | 1.90600 | 0.35108 | 5.429 | <0.001 | ||||
| VS Type | CVS–SVS | 0.51126 | 0.13049 | 3.918 | <0.001 | 1.1395 | 0.5405 | 1.739 |
| Tumor Volume | 0.00792 | 0.00666 | 1.189 | 0.246 | 0.1771 | −0.1296 | 0.484 | |
| Consistency Class | B–A | −0.42070 | 0.13845 | −3.039 | 0.006 | −0.9377 | −1.5733 | −0.302 |
| C–A | −0.55195 | 0.17175 | −3.214 | 0.004 | −1.2302 | −2.0186 | −0.442 | |
| Operative time | 1.47 × 10−4 | 6.59 × 10−4 | 0.223 | 0.825 | 0.0356 | −0.2927 | 0.364 | |
| EOR | NTR–STR | 0.33901 | 0.16108 | 2.105 | 0.046 | 0.7556 | 0.0162 | 1.495 |
| GTR–STR | 0.29739 | 0.17700 | 1.680 | 0.105 | 0.6629 | −0.1496 | 1.475 | |
| Delta T0–T1 | −0.82178 | 0.82024 | −1.002 | 0.326 | −0.1314 | −0.4014 | 0.139 | |
| Linear regression for N-ADCmin | 95% CI | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictor | Estimate | SE | t | p | Stand. Estimate | Lower | Upper | |
| Intercept a | 0.64636 | 0.3601 | 1.795 | 0.085 | ||||
| VS Type | CVS–SVS | 0.63426 | 0.1565 | 4.052 | <0.001 | 1.484 | 0.7284 | 2.2406 |
| Tumor Volume | −0.00639 | 0.0101 | −0.631 | 0.534 | −0.123 | −0.5236 | 0.2783 | |
| Consistency Class | B–A | −0.26317 | 0.1373 | −1.916 | 0.067 | −0.616 | −1.2794 | 0.0475 |
| C–A | −0.66921 | 0.1733 | −3.861 | <0.001 | −1.566 | −2.4037 | −0.7289 | |
| Operative time | 0.00159 | 7.36 × 10−4 | 2.162 | 0.041 | 0.393 | 0.0178 | 0.7679 | |
| EOR | NTR–STR | 0.22648 | 0.1707 | 1.327 | 0.197 | 0.530 | −0.2945 | 1.3547 |
| GTR–STR | 0.43400 | 0.1936 | 2.242 | 0.034 | 1.016 | 0.0806 | 1.9510 | |
| Delta T0–T1 | 4.19394 | 1.6463 | 2.547 | 0.018 | 0.426 | 0.0810 | 0.7720 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Marco, R.; Morana, G.; Sgambetterra, S.; Penner, F.; Melcarne, A.; Garbossa, D.; Lanotte, M.; Albera, R.; Zenga, F. Predicting the Consistency of Vestibular Schwannoma and Its Implication in the Retrosigmoid Approach: A Single-Center Analysis. Curr. Oncol. 2025, 32, 647. https://doi.org/10.3390/curroncol32110647
De Marco R, Morana G, Sgambetterra S, Penner F, Melcarne A, Garbossa D, Lanotte M, Albera R, Zenga F. Predicting the Consistency of Vestibular Schwannoma and Its Implication in the Retrosigmoid Approach: A Single-Center Analysis. Current Oncology. 2025; 32(11):647. https://doi.org/10.3390/curroncol32110647
Chicago/Turabian StyleDe Marco, Raffaele, Giovanni Morana, Silvia Sgambetterra, Federica Penner, Antonio Melcarne, Diego Garbossa, Michele Lanotte, Roberto Albera, and Francesco Zenga. 2025. "Predicting the Consistency of Vestibular Schwannoma and Its Implication in the Retrosigmoid Approach: A Single-Center Analysis" Current Oncology 32, no. 11: 647. https://doi.org/10.3390/curroncol32110647
APA StyleDe Marco, R., Morana, G., Sgambetterra, S., Penner, F., Melcarne, A., Garbossa, D., Lanotte, M., Albera, R., & Zenga, F. (2025). Predicting the Consistency of Vestibular Schwannoma and Its Implication in the Retrosigmoid Approach: A Single-Center Analysis. Current Oncology, 32(11), 647. https://doi.org/10.3390/curroncol32110647






