Defining the Prognostic Significance of BRAF V600E in Early-Stage Colon Cancer: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
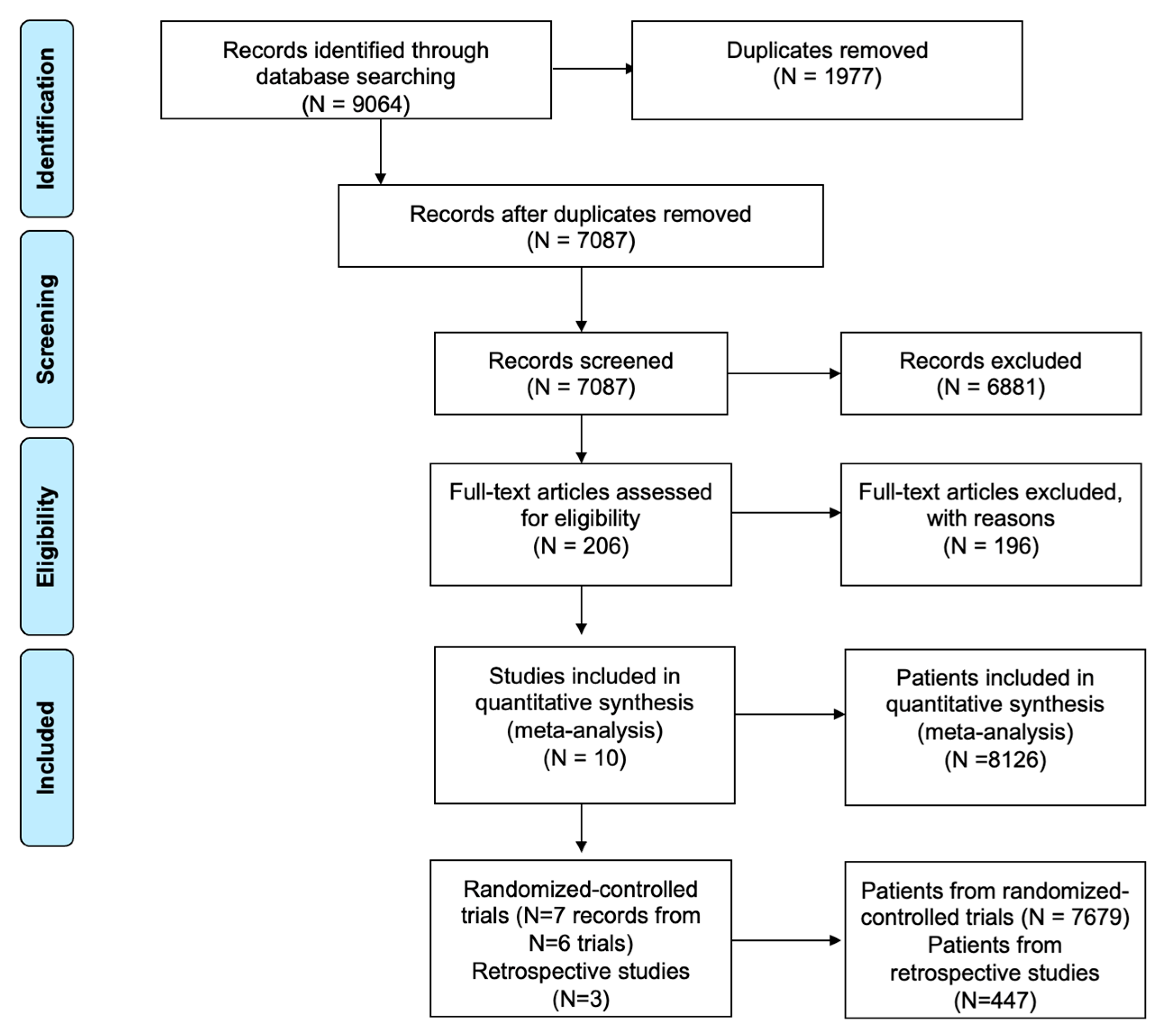
2.2. Deduplication of Search Results
2.3. Primary Outcomes
2.4. Inclusion and Exclusion Criteria
2.5. Meta-Analysis
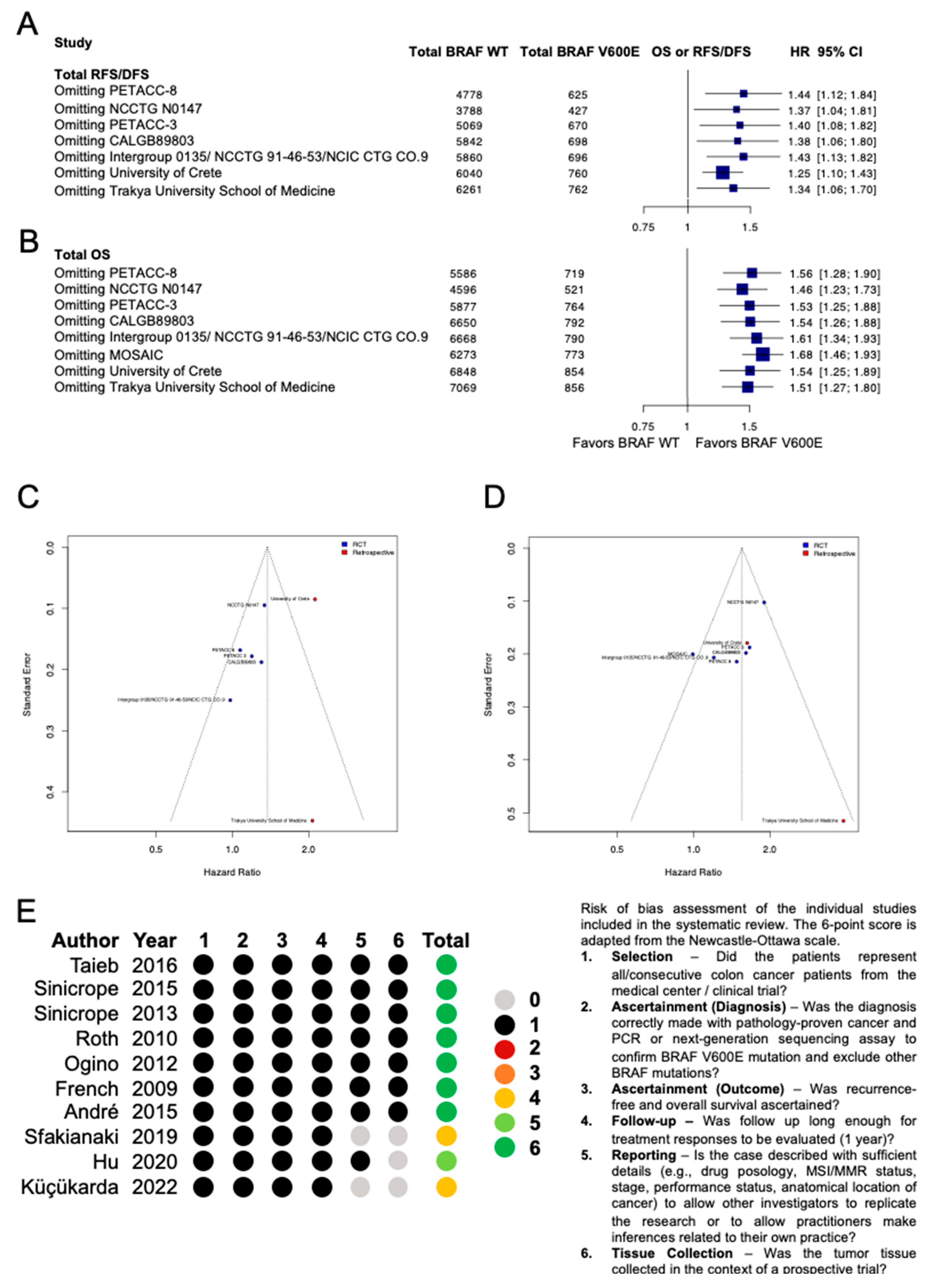
2.6. Quality Assessment
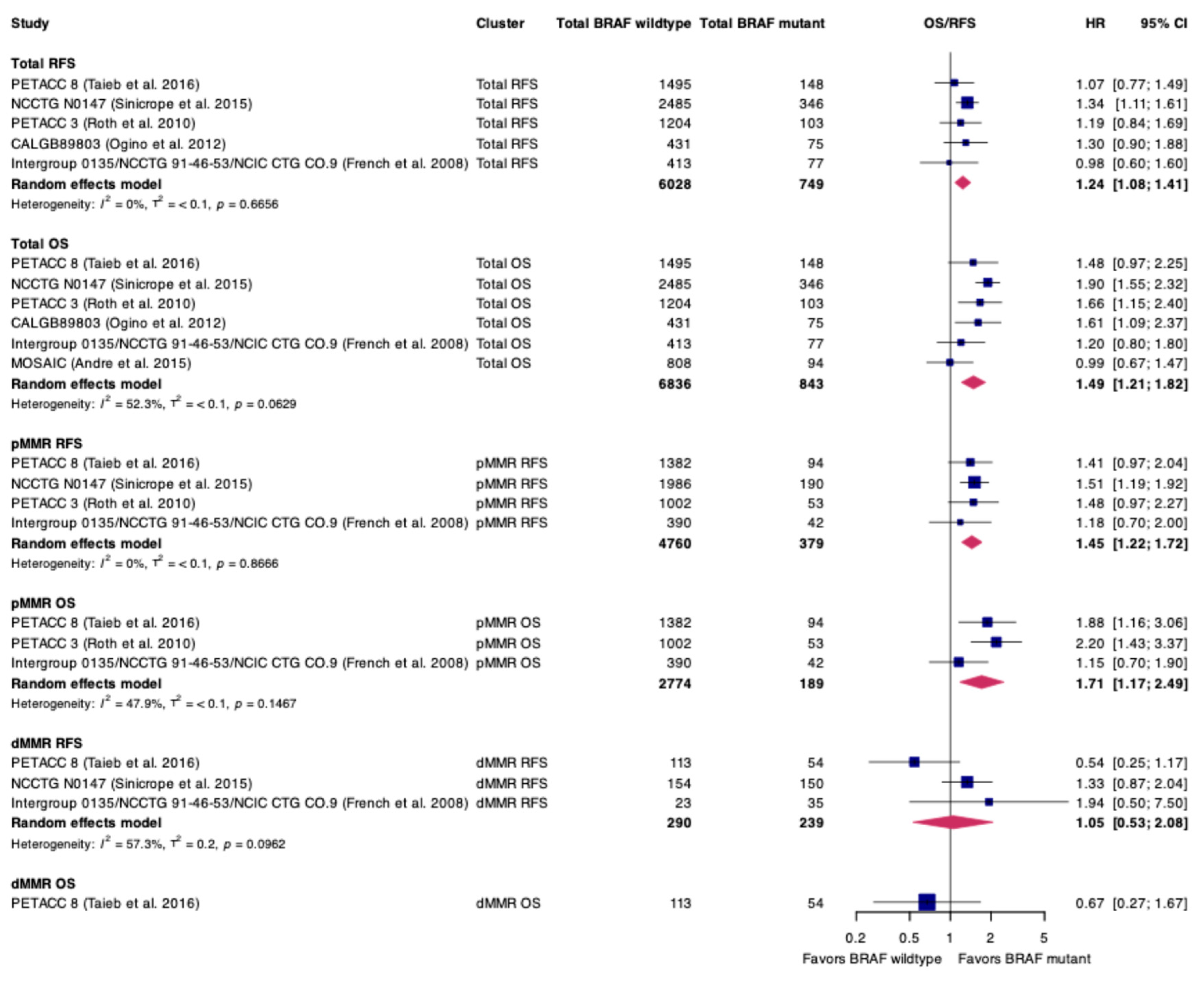
3. Results
3.1. Characteristics of Included Studies and Patients
| Study | Name of Study | Type of Study | Stage | N Total | N BRAF V600E | N BRAF Wild-Type | MSI/MMR Status Included | Adjuvant Treatment Regimen | Definition of OS Endpoint | Definition of RFS/DFS Endpoint |
|---|---|---|---|---|---|---|---|---|---|---|
| Taieb et al., 2016 [27] | PETACC-8 | RCT | 3 | 1643 | 148 | 1495 | Yes | 4 months of Oxaliplatin, Leucovorin, Fluorouracil +/− Cetuximab | Time from randomization until death from any cause. | DFS: time between randomization and local or metastatic recurrence, diagnosis of a second colon or rectal cancer, or death, whichever occurred first. |
| Sinicrope et al., 2015 [36] | NCCTG N0147 | RCT | 3 | 2831 | 346 | 2485 | No | 6 months of Oxaliplatin, Leucovorin, Fluorouracil +/− Cetuximab | Time from randomization until date of death; surviving patients were censored for OS at the date of last contact/follow-up. All patients were censored at 8 years post-randomization. | DFS: time from randomization until disease recurrence, or were censored at the date of last disease assessment if no recurrence occurred. For DFS, patients having died without recurrence were considered an event using date of death. |
| Sinicrope et al., 2013 [37] | NCCTG N0147 | RCT | 3 | 2480 | 340 | 2140 | Yes | 6 months of Oxaliplatin, Leucovorin, Fluorouracil +/− Cetuximab | Time from randomization until date of death; surviving patients were censored for OS at the date of last contact/follow-up. All patients were censored at 8 years post-randomization. | DFS: time from randomization until disease recurrence, or were censored at the date of last disease assessment if no recurrence occurred. For DFS, patients having died without recurrence were considered an event using date of death. |
| Roth et al., 2010 [28] | PETACC-3 | RCT | 2–3 | 1307 | 103 | 1204 | Yes | 6 months of Fluorouracil or Leucovorin +/− Irinotexan | Time from random assignment until death from any cause | RFS: time from the date of random assignment to the first date of local, regional, or distant relapse, the occurrence of a second primary colon cancer, or death. |
| Ogino et al., 2012 [38] | CALGB89803 | RCT | 3 | 506 | 75 | 431 | No | 8 months of Leucovorin and Fluorouracil +/− Irinotecan | Time from the study enrollment to death from any cause. | DFS: time from the study enrollment to cancer recurrence, occurrence of a new primary colon cancer, or death from any cause. |
| French et al., 2008 [39] | Intergroup 0135/NCCTG 91-46-53/NCIC CTG CO.9 | RCT | 2–3 | 490 | 77 | 413 | Yes | Fluoruoracil and Leucovorin + high- vs. standard-dose Levamisole | Time from random assignment to the date of death or last contact. Censored at 8 years. | DFS: time from random assignment to the date of disease recurrence or death. |
| André et al., 2015 [29] | MOSAIC | RCT | 2–3 | 902 | 94 | 808 | No | 6 months of Flurouracil or Leucovorin +/− Oxaliplatin | Time from random assignment to the date of death as a result of any cause. | DFS: time from random assignment to relapse or death, whichever occurred first. The occurrence of a second colorectal cancer was considered a relapse, whereas non-colorectal tumors were disregarded. |
| Sfakianaki et al., 2019 [40] | University of Crete | Retrospective | 2–3 | 246 | 13 | 233 | No | Fluorouracil or Capecitabine + Leucovorin and Oxaliplatin | Time from the date of surgery to date of death. | DFS: time between the date of colectomy to the first documented disease progression, second primary colon cancer, or death. |
| Küçükarda et al., 2022 [41] | Trakya University School of Medicine | Retrospective | 1–3 | 23 | 11 | 12 | No | Not described | Time between diagnosis and death for any cause. | DFS: time from the diagnosis to disease recurrence or development of distant metastasis. |
| Hu et al., 2020 [42] | Guangzhou Medical University | Retrospective | 2–3 | 178 | 17 | 161 | Yes | 3 vs. 6 months of Oxaliplatin, Leucovorin and Fluorouracil | OS not reported, thus not defined. | DFS: time from surgery to the first event of local or metastatic recurrence, second primary cancer, or death from any cause. |
3.2. Quality Assessment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CC | Colon cancer |
| OS | Overall survival |
| DFS | Disease-free survival |
| HR | Hazard ratio |
| WT | Wild-type |
| ORR | Overall response rate |
References
- Dankner, M.; Rose, A.A.N.; Rajkumar, S.; Siegel, P.M.; Watson, I.R. Classifying BRAF alterations in cancer: New rational therapeutic strategies for actionable mutations. Oncogene 2018, 37, 3183–3199. [Google Scholar] [CrossRef]
- Kazandjian, S.; Rousselle, E.; Dankner, M.; Cescon, D.W.; Spreafico, A.; Ma, K.; Kavan, P.; Batist, G.; Rose, A.A.N. The Clinical, Genomic, and Transcriptomic Landscape of BRAF Mutant Cancers. Cancers 2024, 16, 445. [Google Scholar] [CrossRef]
- Dankner, M. Targeted Therapy for Colorectal Cancers With Non-V600 BRAF Mutations: Perspectives for Precision Oncology. JCO Precis. Oncol. 2018, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dankner, M.; Wang, Y.; Fazelzad, R.; Johnson, B.; Nebhan, C.A.; Dagogo-Jack, I.; Myall, N.J.; Richtig, G.; Bracht, J.W.P.; Gerlinger, M.; et al. Clinical Activity of Mitogen-Activated Protein Kinase-Targeted Therapies in Patients With Non-V600 BRAF-Mutant Tumors. JCO Precis. Oncol. 2022, 6, e2200107. [Google Scholar] [CrossRef]
- Jones, J.C.; Renfro, L.A.; Al-Shamsi, H.O.; Schrock, A.B.; Rankin, A.; Zhang, B.Y.; Kasi, P.M.; Voss, J.S.; Leal, A.D.; Sun, J.; et al. (Non-V600) BRAF Mutations Define a Clinically Distinct Molecular Subtype of Metastatic Colorectal Cancer. J. Clin. Oncol. 2017, 35, 2624–2630. [Google Scholar] [CrossRef]
- Hauschild, A.; Grob, J.J.; Demidov, L.V.; Jouary, T.; Gutzmer, R.; Millward, M.; Rutkowski, P.; Blank, C.U.; Miller, W.H., Jr.; Kaempgen, E.; et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012, 380, 358–365. [Google Scholar] [CrossRef]
- Sosman, J.A.; Kim, K.B.; Schuchter, L.; Gonzalez, R.; Pavlick, A.C.; Weber, J.S.; McArthur, G.A.; Hutson, T.E.; Moschos, S.J.; Flaherty, K.T.; et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N. Engl. J. Med. 2012, 366, 707–714. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.T.; Infante, J.R.; Daud, A.; Gonzalez, R.; Kefford, R.F.; Sosman, J.; Hamid, O.; Schuchter, L.; Cebon, J.; Ibrahim, N.; et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Engl. J. Med. 2012, 367, 1694–1703. [Google Scholar] [CrossRef]
- Larkin, J.; Ascierto, P.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Desai, J.; Chan, E.; Hecht, J.R.; O’Dwyer, P.J.; Maru, D.; Morris, V.; Janku, F.; Dasari, A.; Chung, W.; et al. Phase II Pilot Study of Vemurafenib in Patients With Metastatic BRAF-Mutated Colorectal Cancer. J. Clin. Oncol. 2015, 33, 4032–4038. [Google Scholar] [CrossRef]
- Hyman, D.M.; Puzanov, I.; Subbiah, V.; Faris, J.E.; Chau, I.; Blay, J.Y.; Wolf, J.; Raje, N.S.; Diamond, E.L.; Hollebecque, A.; et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N. Engl. J. Med. 2015, 373, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, R.B.; Ebi, H.; Turke, A.B.; Coffee, E.M.; Nishino, M.; Cogdill, A.P.; Brown, R.D.; Della Pelle, P.; Dias-Santagata, D.; Hung, K.E.; et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012, 2, 227–235. [Google Scholar] [CrossRef]
- Prahallad, A.; Sun, C.; Huang, S.; Di Nicolantonio, F.; Salazar, R.; Zecchin, D.; Beijersbergen, R.L.; Bardelli, A.; Bernards, R. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 2012, 483, 100–103. [Google Scholar] [CrossRef]
- Yaeger, R.; Cercek, A.; O’Reilly, E.M.; Reidy, D.L.; Kemeny, N.; Wolinsky, T.; Capanu, M.; Gollub, M.J.; Rosen, N.; Berger, M.F.; et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin. Cancer Res. 2015, 21, 1313–1320. [Google Scholar] [CrossRef]
- Kopetz, S.; Guthrie, K.A.; Morris, V.K.; Lenz, H.J.; Magliocco, A.M.; Maru, D.; Yan, Y.; Lanman, R.; Manyam, G.; Hong, D.S.; et al. Randomized Trial of Irinotecan and Cetuximab With or Without Vemurafenib in BRAF-Mutant Metastatic Colorectal Cancer (SWOG S1406). J. Clin. Oncol. 2021, 39, 285–294. [Google Scholar] [CrossRef]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Grothey, A.; Van Cutsem, E.; Yaeger, R.; Wasan, H.; Yoshino, T.; Desai, J.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J. Clin. Oncol. 2021, 39, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Taieb, J.; Yaeger, R.; Yoshino, T.; Grothey, A.; Maiello, E.; Elez, E.; Dekervel, J.; Ross, P.; Ruiz-Casado, A.; et al. ANCHOR CRC: Results From a Single-Arm, Phase II Study of Encorafenib Plus Binimetinib and Cetuximab in Previously Untreated BRAF(V600E)-Mutant Metastatic Colorectal Cancer. J. Clin. Oncol. 2023, 41, 2628–2637. [Google Scholar] [CrossRef]
- Kopetz, S.; Yoshino, T.; Van Cutsem, E.; Eng, C.; Kim, T.W.; Wasan, H.S.; Desai, J.; Ciardiello, F.; Yaeger, R.; Maughan, T.S.; et al. Encorafenib, cetuximab and chemotherapy in BRAF-mutant colorectal cancer: A randomized phase 3 trial. Nat. Med. 2025, 31, 901–908. [Google Scholar] [CrossRef]
- Boku, S.; Satake, H.; Ohta, T.; Mitani, S.; Kawakami, K.; Suzuki, Y.; Matsumoto, T.; Terazawa, T.; Yamazaki, E.; Hasegawa, H.; et al. TRESBIEN (OGSG 2101): Encorafenib, binimetinib and cetuximab for early recurrent stage II/III BRAF V600E-mutated colorectal cancer. Future Oncol. 2022, 18, 4153–4160. [Google Scholar] [CrossRef]
- Yaeger, R.; Shi, Q.; Dueck, A.C.; Dib, E.G.; Kazmi, S.M.A.; Alese, O.B.; Krishnamurthi, S.S.; Nixon, A.B.; Shergill, A.; O’Reilly, E.M.; et al. A randomized trial of consolidation-targeted adjuvant therapy with encorafenib and cetuximab versus usual care for patients with stage II/III BRAF V600E colon cancer: Alliance for Clinical Trials in Oncology A022004. J. Clin. Oncol. 2023, 41, TPS3641. [Google Scholar] [CrossRef]
- Ciappina, G.; Toscano, E.; Ottaiano, A.; Capuozzo, M.; Consolo, P.; Maiorana, E.; Carroccio, P.; Franchina, T.; Ieni, A.; Di Mauro, A.; et al. Negative Hyperselection in Metastatic Colorectal Cancer for First-Line Anti-EGFR Therapy: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 2216. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, R.; Cercek, A.; Chou, J.F.; Sylvester, B.E.; Kemeny, N.E.; Hechtman, J.F.; Ladanyi, M.; Rosen, N.; Weiser, M.R.; Capanu, M.; et al. BRAF mutation predicts for poor outcomes after metastasectomy in patients with metastatic colorectal cancer. Cancer 2014, 120, 2316–2324. [Google Scholar] [CrossRef]
- Richman, S.D.; Seymour, M.T.; Chambers, P.; Elliott, F.; Daly, C.L.; Meade, A.M.; Taylor, G.; Barrett, J.H.; Quirke, P. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: Results from the MRC FOCUS trial. J. Clin. Oncol. 2009, 27, 5931–5937. [Google Scholar] [CrossRef]
- Clarke, C.N.; Kopetz, E.S. BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: Clinical characteristics, clinical behavior, and response to targeted therapies. J. Gastrointest. Oncol. 2015, 6, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Taieb, J.; Zaanan, A.; Le Malicot, K.; Julié, C.; Blons, H.; Mineur, L.; Bennouna, J.; Tabernero, J.; Mini, E.; Folprecht, G.; et al. Prognostic Effect of BRAF and KRAS Mutations in Patients With Stage III Colon Cancer Treated With Leucovorin, Fluorouracil, and Oxaliplatin With or Without Cetuximab: A Post Hoc Analysis of the PETACC-8 Trial. JAMA Oncol. 2016, 2, 643–653. [Google Scholar] [CrossRef]
- Roth, A.D.; Tejpar, S.; Delorenzi, M.; Yan, P.; Fiocca, R.; Klingbiel, D.; Dietrich, D.; Biesmans, B.; Bodoky, G.; Barone, C.; et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: Results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J. Clin. Oncol. 2010, 28, 466–474. [Google Scholar] [CrossRef] [PubMed]
- André, T.; de Gramont, A.; Vernerey, D.; Chibaudel, B.; Bonnetain, F.; Tijeras-Raballand, A.; Scriva, A.; Hickish, T.; Tabernero, J.; Van Laethem, J.L.; et al. Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. J. Clin. Oncol. 2015, 33, 4176–4187. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Babineau, J. Product Review: Covidence (Systematic Review Software). J. Can. Health Libr. Assoc./J. L’association Bibliothèques Santé Can. 2014, 35, 68–71. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Lazaratos, A.M.; Maritan, S.M.; Quaiattini, A.; Darlix, A.; Ratosa, I.; Ferraro, E.; Griguolo, G.; Guarneri, V.; Pellerino, A.; Hofer, S.; et al. Intrathecal trastuzumab versus alternate routes of delivery for HER2-targeted therapies in patients with HER2+ breast cancer leptomeningeal metastases. Breast 2023, 69, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Sinicrope, F.A.; Mahoney, M.R.; Yoon, H.H.; Smyrk, T.C.; Thibodeau, S.N.; Goldberg, R.M.; Nelson, G.D.; Sargent, D.J.; Alberts, S.R. Analysis of Molecular Markers by Anatomic Tumor Site in Stage III Colon Carcinomas from Adjuvant Chemotherapy Trial NCCTG N0147 (Alliance). Clin. Cancer Res. 2015, 21, 5294–5304. [Google Scholar] [CrossRef]
- Sinicrope, F.A.; Mahoney, M.R.; Smyrk, T.C.; Thibodeau, S.N.; Warren, R.S.; Bertagnolli, M.M.; Nelson, G.D.; Goldberg, R.M.; Sargent, D.J.; Alberts, S.R. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J. Clin. Oncol. 2013, 31, 3664–3672. [Google Scholar] [CrossRef]
- Ogino, S.; Shima, K.; Meyerhardt, J.A.; McCleary, N.J.; Ng, K.; Hollis, D.; Saltz, L.B.; Mayer, R.J.; Schaefer, P.; Whittom, R.; et al. Predictive and prognostic roles of BRAF mutation in stage III colon cancer: Results from intergroup trial CALGB 89803. Clin. Cancer Res. 2012, 18, 890–900. [Google Scholar] [CrossRef]
- French, A.J.; Sargent, D.J.; Burgart, L.J.; Foster, N.R.; Kabat, B.F.; Goldberg, R.; Shepherd, L.; Windschitl, H.E.; Thibodeau, S.N. Prognostic significance of defective mismatch repair and BRAF V600E in patients with colon cancer. Clin. Cancer Res. 2008, 14, 3408–3415. [Google Scholar] [CrossRef]
- Sfakianaki, M.; Papadaki, C.; Tzardi, M.; Trypaki, M.; Alam, S.; Lagoudaki, E.D.; Messaritakis, I.; Zoras, O.; Mavroudis, D.; Georgoulias, V.; et al. Loss of LKB1 Protein Expression Correlates with Increased Risk of Recurrence and Death in Patients with Resected, Stage II or III Colon Cancer. Cancer Res. Treat. 2019, 51, 1518–1526. [Google Scholar] [CrossRef]
- Küçükarda, A.; Gökyer, A.; Sayın, S.; Gökmen, İ.; Özcan, E.; Köstek, O.; Hacıoğlu, M.B.; Uzunoğlu, S.; Çiçin, İ.; Erdoğan, B. Prognostic Factors for Survival in Transverse Colon Cancers. J. Gastrointest. Cancer 2022, 53, 31–40. [Google Scholar] [CrossRef]
- Hu, H.; Wu, Z.; Wang, C.; Huang, Y.; Zhang, J.; Cai, Y.; Xie, X.; Li, J.; Shen, C.; Li, W.; et al. Duration of FOLFOX Adjuvant Chemotherapy in High-Risk Stage II and Stage III Colon Cancer With Deficient Mismatch Repair. Front. Oncol. 2020, 10, 579478. [Google Scholar] [CrossRef]
- Gavin, P.G.; Colangelo, L.H.; Fumagalli, D.; Tanaka, N.; Remillard, M.Y.; Yothers, G.; Kim, C.; Taniyama, Y.; Kim, S.I.; Choi, H.J.; et al. Mutation profiling and microsatellite instability in stage II and III colon cancer: An assessment of their prognostic and oxaliplatin predictive value. Clin. Cancer Res. 2012, 18, 6531–6541. [Google Scholar] [CrossRef]
- Hutchins, G.; Southward, K.; Handley, K.; Magill, L.; Beaumont, C.; Stahlschmidt, J.; Richman, S.; Chambers, P.; Seymour, M.; Kerr, D.; et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J. Clin. Oncol. 2011, 29, 1261–1270. [Google Scholar] [CrossRef]
- Domingo, E.; Camps, C.; Kaisaki, P.J.; Parsons, M.J.; Mouradov, D.; Pentony, M.M.; Makino, S.; Palmieri, M.; Ward, R.L.; Hawkins, N.J.; et al. Mutation burden and other molecular markers of prognosis in colorectal cancer treated with curative intent: Results from the QUASAR 2 clinical trial and an Australian community-based series. Lancet Gastroenterol. Hepatol. 2018, 3, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Formica, V.; Sera, F.; Cremolini, C.; Riondino, S.; Morelli, C.; Arkenau, H.T.; Roselli, M. KRAS and BRAF Mutations in Stage II and III Colon Cancer: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2022, 114, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Dong, C.; Cao, Y.; Fang, X.; Zhong, C.; Li, D.; Yuan, Y. Prognostic Role of BRAF Mutation in Stage II/III Colorectal Cancer Receiving Curative Resection and Adjuvant Chemotherapy: A Meta-Analysis Based on Randomized Clinical Trials. PLoS ONE 2016, 11, e0154795. [Google Scholar] [CrossRef] [PubMed]
- Pentheroudakis, G.; Raptou, G.; Kotoula, V.; Wirtz, R.M.; Vrettou, E.; Karavasilis, V.; Gourgioti, G.; Gakou, C.; Syrigos, K.N.; Bournakis, E.; et al. Immune response gene expression in colorectal cancer carries distinct prognostic implications according to tissue, stage and site: A prospective retrospective translational study in the context of a hellenic cooperative oncology group randomised trial. PLoS ONE 2015, 10, e0124612. [Google Scholar] [CrossRef]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef]
- Venderbosch, S.; Nagtegaal, I.D.; Maughan, T.S.; Smith, C.G.; Cheadle, J.P.; Fisher, D.; Kaplan, R.; Quirke, P.; Seymour, M.T.; Richman, S.D.; et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: A pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin. Cancer Res. 2014, 20, 5322–5330. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Elez, E.; Kopetz, S.; Tabernero, J.; Bekaii-Saab, T.; Taieb, J.; Yoshino, T.; Manji, G.; Fernandez, K.; Abbattista, A.; Zhang, X.; et al. SEAMARK: Phase II study of first-line encorafenib and cetuximab plus pembrolizumab for MSI-H/dMMR BRAFV600E-mutant mCRC. Future Oncol. 2023, 20, 653–663. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dankner, M.; Dubé, L.-R.; Sorin, M.; Stein, A.J.B.; Nowakowski, A.; Park, C.L.; Magrill, J.; Lazaratos, A.-M.; Romero, J.M.; Batist, G.; et al. Defining the Prognostic Significance of BRAF V600E in Early-Stage Colon Cancer: A Systematic Review and Meta-Analysis. Curr. Oncol. 2025, 32, 624. https://doi.org/10.3390/curroncol32110624
Dankner M, Dubé L-R, Sorin M, Stein AJB, Nowakowski A, Park CL, Magrill J, Lazaratos A-M, Romero JM, Batist G, et al. Defining the Prognostic Significance of BRAF V600E in Early-Stage Colon Cancer: A Systematic Review and Meta-Analysis. Current Oncology. 2025; 32(11):624. https://doi.org/10.3390/curroncol32110624
Chicago/Turabian StyleDankner, Matthew, Laurie-Rose Dubé, Mark Sorin, Andrew J. B. Stein, Alexander Nowakowski, Changsu Lawrence Park, Jamie Magrill, Anna-Maria Lazaratos, Joan Miguel Romero, Gerald Batist, and et al. 2025. "Defining the Prognostic Significance of BRAF V600E in Early-Stage Colon Cancer: A Systematic Review and Meta-Analysis" Current Oncology 32, no. 11: 624. https://doi.org/10.3390/curroncol32110624
APA StyleDankner, M., Dubé, L.-R., Sorin, M., Stein, A. J. B., Nowakowski, A., Park, C. L., Magrill, J., Lazaratos, A.-M., Romero, J. M., Batist, G., Kavan, P., Rose, A. A. N., & Ma, K. (2025). Defining the Prognostic Significance of BRAF V600E in Early-Stage Colon Cancer: A Systematic Review and Meta-Analysis. Current Oncology, 32(11), 624. https://doi.org/10.3390/curroncol32110624






