A Novel Frailty Index Can Predict the Short-Term Outcomes of Esophagectomy in Older Patients with Esophageal Cancer
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. The Cohort
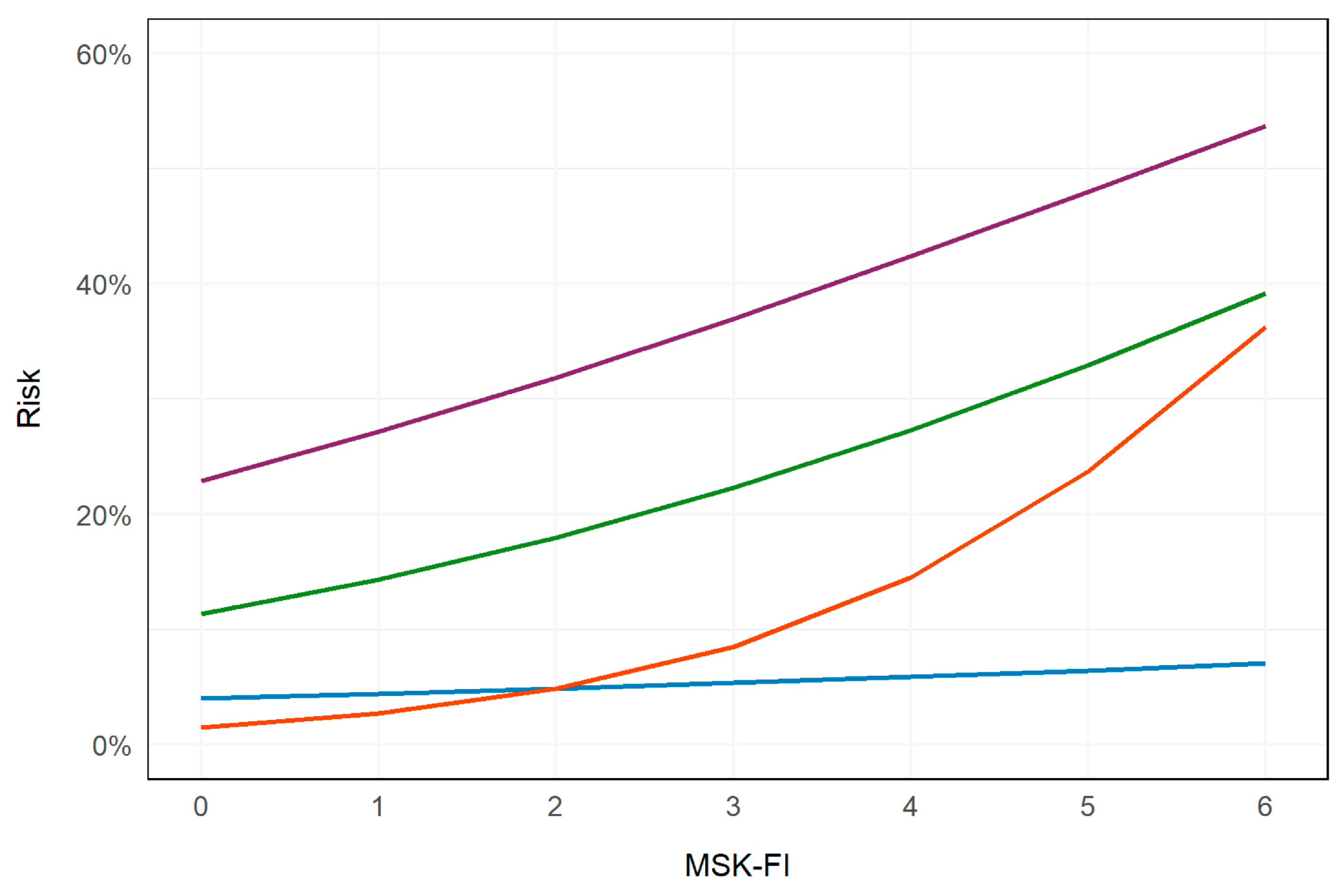
3.2. Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trumper, M.; Ross, P.J.; Cunningham, D.; Norman, A.R.; Hawkins, R.; Seymour, M.; Harper, P.; Iveson, T.; Nicolson, M.; Hickish, T. Efficacy and tolerability of chemotherapy in elderly patients with advanced oesophago-gastric cancer: A pooled analysis of three clinical trials. Eur. J. Cancer 2006, 42, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Wildiers, H.; Heeren, P.; Puts, M.; Topinkova, E.; Janssen-Heijnen, M.L.; Extermann, M.; Falandry, C.; Artz, A.; Brain, E.; Colloca, G.; et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J. Clin. Oncol. 2014, 32, 2595–2603. [Google Scholar] [CrossRef] [PubMed]
- Hurria, A.; Togawa, K.; Mohile, S.G.; Owusu, C.; Klepin, H.D.; Gross, C.P.; Lichtman, S.M.; Gajra, A.; Bhatia, S.; Katheria, V.; et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J. Clin. Oncol. 2011, 29, 3457–3465. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.M.; Shahrokni, A. Moving beyond Karnofsky and ECOG Performance Status Assessments with New Technologies. J. Oncol. 2016, 2016, 6186543. [Google Scholar] [CrossRef] [PubMed]
- Kirkhus, L.; Šaltytė Benth, J.; Rostoft, S.; Grønberg, B.H.; Hjermstad, M.J.; Selbæk, G.; Wyller, T.B.; Harneshaug, M.; Jordhøy, M.S. Geriatric assessment is superior to oncologists’ clinical judgement in identifying frailty. Br. J. Cancer 2017, 117, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Shahrokni, A.; Tin, A.; Alexander, K.; Sarraf, S.; Afonso, A.; Filippova, O.; Harris, J.; Downey, R.J.; Vickers, A.J.; Korc-Grodzicki, B. Development and Evaluation of a New Frailty Index for Older Surgical Patients With Cancer. JAMA Netw. Open 2019, 2, e193545. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.S.; Flynn, J.R.; Panageas, K.S.; Shahrokni, A.; Tin, A.L.; Bello, D.M.; Ariyan, C.E.; Brady, M.S.; Coit, D.G.; Bartlett, E.K. Assessment of Frailty Can Guide Decision Making for Utilization of Sentinel Lymph Node Biopsy in Patients with Thick Melanoma. Ann. Surg. Oncol. 2021, 28, 9031–9038. [Google Scholar] [CrossRef] [PubMed]
- Filippova, O.T.; Tin, A.L.; Alonso, J.; Vickers, A.J.; Tew, W.P.; Gardner, G.J.; Sonoda, Y.; Roche, K.L.; Zivanovic, O.; Chi, D.S.; et al. Frailty based on the memorial Sloan Kettering Frailty Index is associated with surgical decision making, clinical trial participation, and overall survival among older women with ovarian cancer. Gynecol. Oncol. 2021, 161, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Dobaria, V.; Hadaya, J.; Sanaiha, Y.; Aguayo, E.; Sareh, S.; Benharash, P. The Pragmatic Impact of Frailty on Outcomes of Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2021, 112, 108–115. [Google Scholar] [CrossRef]
- Karunungan, K.L.; Hadaya, J.; Tran, Z.; Sanaiha, Y.; Mandelbaum, A.; Sha’Shonda, L.R.; Benharash, P. Frailty is independently associated with worse outcomes after elective anatomic lung resection. Ann. Thorac. Surg. 2021, 112, 1639–1646. [Google Scholar] [CrossRef]
- Hadaya, J.; Sanaiha, Y.; Juillard, C.; Benharash, P. Impact of frailty on clinical outcomes and resource use following emergency general surgery in the United States. PLoS ONE 2021, 16, e0255122. [Google Scholar] [CrossRef] [PubMed]
- Morito, A.; Harada, K.; Iwatsuki, M.; Maeda, Y.; Mitsuura, C.; Toihata, T.; Kosumi, K.; Eto, K.; Iwagami, S.; Baba, Y.; et al. Frailty Assessed by the Clinical Frailty Scale is Associated with Prognosis After Esophagectomy. Ann. Surg. Oncol. 2023, 30, 3725–3732. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Hastie, D.J.; Fan, G.H.; Addonizio, E.A.; Han, J.; Karagozian, R. Clinical frailty is a risk factor of adverse outcomes in patients with esophageal cancer undergoing esophagectomy: Analysis of 2011–2017 US hospitals. Dis. Esophagus 2022, 35, doac002. [Google Scholar] [CrossRef] [PubMed]
- Swisher, S.G.; Deford, L.; Merriman, K.W.; Walsh, G.L.; Smythe, R.; Vaporicyan, A.; Ajani, J.A.; Brown, T.; Komaki, R.; Roth, J.A.; et al. Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J. Thorac. Cardiovasc. Surg. 2000, 119, 1126–1132. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Birkmeyer, J.D.; Siewers, A.E.; Finlayson, E.V.; Stukel, T.A.; Lucas, F.L.; Batista, I.; Welch, H.G.; Wennberg, D.E. Hospital volume and surgical mortality in the United States. N. Engl. J. Med. 2002, 346, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.H.; Bull, D.A.; Harpole, D.H.; Rentz, J.J.; Neumayer, L.A.; Pappas, T.N.; Daley, J.; Henderson, W.G.; Krasnicka, B.; Khuri, S.F. Outcomes after esophagectomy: A ten-year prospective cohort. Ann. Thorac. Surg. 2003, 75, 217–222; discussion 222. [Google Scholar] [CrossRef] [PubMed]
- Ra, J.; Paulson, E.C.; Kucharczuk, J.; Armstrong, K.; Wirtalla, C.; Rapaport-Kelz, R.; Kaiser, L.R.; Spitz, F.R. Postoperative Mortality After Esophagectomy for Cancer: Development of a Preoperative Risk Prediction Model. Ann. Surg. Oncol. 2008, 15, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Raymond, D.P.; Seder, C.W.; Wright, C.D.; Magee, M.J.; Kosinski, A.S.; Cassivi, S.D.; Grogan, E.L.; Blackmon, S.H.; Allen, M.S.; Park, B.J.; et al. Predictors of Major Morbidity or Mortality After Resection for Esophageal Cancer: A Society of Thoracic Surgeons General Thoracic Surgery Database Risk Adjustment Model. Ann. Thorac. Surg. 2016, 102, 207–214. [Google Scholar] [CrossRef]
- Markar, S.R.; Low, D.E. Physiology, Not Chronology, Dictates Outcomes after Esophagectomy for Esophageal Cancer: Outcomes in Patients 80 Years and Older. Ann. Surg. Oncol. 2013, 20, 1020–1026. [Google Scholar] [CrossRef]
- Moy, R.H.; Sabwa, S.; Maron, S.B.; Shcherba, M.; Apollo, A.; Janjigian, Y.Y.; Ku, G.Y.; Tew, W.P.; Wu, A.J.; Jones, D.R.; et al. A nutritional management algorithm in older patients with locally advanced esophageal cancer. J. Geriatr. Oncol. 2022, 13, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Pisarska, M.; Małczak, P.; Major, P.; Wysocki, M.; Budzyński, A.; Pędziwiatr, M. Enhanced recovery after surgery protocol in oesophageal cancer surgery: Systematic review and meta-analysis. PLoS ONE 2017, 12, e0174382. [Google Scholar] [CrossRef] [PubMed]
- Mariette, C.; Markar, S.R.; Dabakuyo-Yonli, T.S.; Meunier, B.; Pezet, D.; Collet, D.; D’Journo, X.B.; Brigand, C.; Perniceni, T.; Carrère, N.; et al. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N. Engl. J. Med. 2019, 380, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.D.; Nobel, T.B.; Hsu, M.; Tan, K.S.; Chudgar, N.; Yan, S.; Rusch, V.W.; Jones, D.R.; Rocco, G.; Molena, D.; et al. Improved Preoperative Risk-Assessment Tools Are Needed to Guide Informed Decision-Making Before Esophagectomy. Ann. Surg. 2020. Publish Ahead of Print. [Google Scholar] [CrossRef] [PubMed]
- Hodari, A.; Hammoud, Z.T.; Borgi, J.F.; Tsiouris, A.; Rubinfeld, I.S. Assessment of morbidity and mortality after esophagectomy using a modified frailty index. Ann. Thorac. Surg. 2013, 96, 1240–1245. [Google Scholar] [CrossRef]
- Ghaferi, A.A.; Birkmeyer, J.D.; Dimick, J.B. Variation in hospital mortality associated with inpatient surgery. N. Engl. J. Med. 2009, 361, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Attwood, K.; Arya, S.; Hall, D.E.; Johanning, J.M.; Gabriel, E.; Visioni, A.; Nurkin, S.; Kukar, M.; Hochwald, S.; et al. Association of Frailty With Failure to Rescue After Low-Risk and High-Risk Inpatient Surgery. JAMA Surg. 2018, 153, e180214. [Google Scholar] [CrossRef] [PubMed]
- Silber, J.H.; Williams, S.V.; Krakauer, H.; Schwartz, J.S. Hospital and patient characteristics associated with death after surgery: A study of adverse occurrence and failure to rescue. Med. Care 1992, 30, 615–629. [Google Scholar] [CrossRef]
- Liou, D.Z.; Serna-Gallegos, D.; Mirocha, J.; Bairamian, V.; Alban, R.F.; Soukiasian, H.J. Predictors of Failure to Rescue After Esophagectomy. Ann. Thorac. Surg. 2018, 105, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, E.; Fan, Z.; Birkmeyer, J.D. Outcomes in Octogenarians Undergoing High-Risk Cancer Operation: A National Study. J. Am. Coll. Surg. 2007, 205, 729–734. [Google Scholar] [CrossRef]
- Stahl, C.C.; Hanseman, D.J.; Wima, K.; Sutton, J.M.; Wilson, G.C.; Hohmann, S.F.; Shah, S.A.; Abbott, D.E. Increasing Age Is a Predictor of Short-Term Outcomes in Esophagectomy: A Propensity Score Adjusted Analysis. J. Gastrointest. Surg. 2014, 18, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Scotte, F.; Bossi, P.; Carola, E.; Cudennec, T.; Dielenseger, P.; Gomes, F.; Knox, S.; Strasser, F. Addressing the quality of life needs of older patients with cancer: A SIOG consensus paper and practical guide. Ann. Oncol. 2018, 29, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Marano, L.; Carbone, L.; Poto, G.E.; Gambelli, M.; Nguefack Noudem, L.L.; Grassi, G.; Manasci, F.; Curreri, G.; Giuliani, A.; Piagnerelli, R.; et al. Handgrip strength predicts length of hospital stay in an abdominal surgical setting: The role of frailty beyond age. Aging Clin. Exp. Res. 2022, 34, 811–817. [Google Scholar] [CrossRef]
- Rubenstein, L.Z.; Stuck, A.E.; Siu, A.L.; Wieland, D. Impacts of geriatric evaluation and management programs on defined outcomes: Overview of the evidence. J. Am. Geriatr. Soc. 1991, 39, 8S–16S; discussion 17S–18S. [Google Scholar] [CrossRef]
- Vlacich, G.; Samson, P.P.; Perkins, S.M.; Roach, M.C.; Parikh, P.J.; Bradley, J.D.; Lockhart, A.C.; Puri, V.; Meyers, B.F.; Kozower, B.; et al. Treatment utilization and outcomes in elderly patients with locally advanced esophageal carcinoma: A review of the National Cancer Database. Cancer Med. 2017, 6, 2886–2896. [Google Scholar] [CrossRef] [PubMed]
- Faiz, Z.; Lemmens, V.E.P.P.; Siersema, P.D.; Nieuwenhuijzen, G.A.P.; Wouters, M.W.J.M.; Rozema, T.; Coebergh, J.W.W.; Wijnhoven, B.P.L. Increased Resection Rates and Survival among Patients Aged 75 Years and Older with Esophageal Cancer: A Dutch Nationwide Population-Based Study. World J. Surg. 2012, 36, 2872–2878. [Google Scholar] [CrossRef] [PubMed]
- Tougeron, D.; Hamidou, H.; Scotté, M.; Di Fiore, F.; Antonietti, M.; Paillot, B.; Michel, P. Esophageal cancer in the elderly: An analysis of the factors associated with treatment decisions and outcomes. BMC Cancer 2010, 10, 510. [Google Scholar] [CrossRef]
- Sabel, M.S.; Smith, J.L.; Nava, H.R.; Mollen, K.; Douglass, H.O.; Gibbs, J.F. Esophageal resection for carcinoma in patients older than 70 years. Ann. Surg. Oncol. 2002, 9, 210–214. [Google Scholar] [CrossRef] [PubMed]
- van Hagen, P.; Hulshof, M.; Van Lanschot, J.; Steyerberg, E.; Henegouwen, M.V.B.; Wijnhoven, B.; Richel, D.; Nieuwenhuijzen, G.; Hospers, G.; Bonenkamp, J. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
| Characteristic | Total |
|---|---|
| Age at surgery, years | 71 (68–75) |
| Male sex | 361 (81) |
| Race | |
| White | 395 (88) |
| White Hispanic | 4 (0.9) |
| Black | 6 (1.3) |
| Asian | 25 (5.6) |
| Unknown | 17 (3.8) |
| Body mass index | 27.7 (24.9–31.0) |
| American Society of Anesthesiologists Physical Status | |
| 2 | 25 (5.6) |
| 3 | 375 (84) |
| 4 | 47 (11) |
| Memorial Sloan Kettering Frailty Index score | |
| 0 | 73 (16) |
| 1 | 125 (28) |
| 2 | 118 (26) |
| 3 | 57 (13) |
| 4 | 38 (8.5) |
| 5 | 19 (4.3) |
| ≥6 | 17 (3.8) |
| Forced expiratory volume, L | 2.9 (2.3–3.4) |
| Unknown forced expiratory volume | 121 |
| ECOG performance status | |
| 0 | 218 (49) |
| 1 | 210 (47) |
| 2 | 19 (4.3) |
| Pulmonary comorbidities | 52 (12) |
| Cardiac comorbidities | 316 (71) |
| Diabetes comorbidities | 104 (23) |
| Renal comorbidities | 11 (2.5) |
| History of smoking cigarettes | |
| Current | 27 (6.0) |
| Former | 285 (64) |
| Never | 135 (30) |
| Tumor type | |
| Adenocarcinoma | 393 (88) |
| Squamous cell carcinoma | 48 (11) |
| Other | 6 (1.3) |
| Siewert classification | |
| 1 | 191 (43%) |
| 2 | 153 (34) |
| 3 | 29 (6) |
| NA | 74 (17) |
| Clinical stage | |
| 1 | 57 (13) |
| 2 | 57 (13) |
| 3 | 276 (62) |
| 4 | 55 (12) |
| NA | 2 (0.1) |
| Neoadjuvant treatment | 360 (81) |
| Chemoradiation | 349 (78) |
| Type of operation | |
| Ivor Lewis esophagectomy | 384 (86) |
| Three-hole esophagectomy | 32 (7) |
| Transhiatal esophagectomy | 16 (4) |
| Partial or complete gastrectomy and esophagectomy | 5 (1) |
| Other | 10 (2) |
| Minimally invasive surgical approach | 248 (55) |
| Resection margin | |
| R0 | 423 (95%) |
| R+ | 24 (5%) |
| Outcome | No. | Event No. | OR | 95% CI | p |
|---|---|---|---|---|---|
| Death within 90 days of surgery | 446 | 22 | 1.14 | 0.87–1.47 | 0.3 |
| Major complication within 30 days of surgery | 447 | 144 | 1.24 | 1.09–1.41 | 0.001 |
| Readmission within 30 days of discharge | 432 | 81 | 1.31 | 1.13–1.52 | <0.001 |
| Discharge to a facility | 432 | 31 | 1.86 | 1.49–2.37 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boerner, T.; Sewell, M.; Tin, A.L.; Vickers, A.J.; Harrington-Baksh, C.; Bains, M.S.; Bott, M.J.; Park, B.J.; Sihag, S.; Jones, D.R.; et al. A Novel Frailty Index Can Predict the Short-Term Outcomes of Esophagectomy in Older Patients with Esophageal Cancer. Curr. Oncol. 2024, 31, 4685-4694. https://doi.org/10.3390/curroncol31080349
Boerner T, Sewell M, Tin AL, Vickers AJ, Harrington-Baksh C, Bains MS, Bott MJ, Park BJ, Sihag S, Jones DR, et al. A Novel Frailty Index Can Predict the Short-Term Outcomes of Esophagectomy in Older Patients with Esophageal Cancer. Current Oncology. 2024; 31(8):4685-4694. https://doi.org/10.3390/curroncol31080349
Chicago/Turabian StyleBoerner, Thomas, Marisa Sewell, Amy L. Tin, Andrew J. Vickers, Caitlin Harrington-Baksh, Manjit S. Bains, Matthew J. Bott, Bernard J. Park, Smita Sihag, David R. Jones, and et al. 2024. "A Novel Frailty Index Can Predict the Short-Term Outcomes of Esophagectomy in Older Patients with Esophageal Cancer" Current Oncology 31, no. 8: 4685-4694. https://doi.org/10.3390/curroncol31080349
APA StyleBoerner, T., Sewell, M., Tin, A. L., Vickers, A. J., Harrington-Baksh, C., Bains, M. S., Bott, M. J., Park, B. J., Sihag, S., Jones, D. R., Downey, R. J., Shahrokni, A., & Molena, D. (2024). A Novel Frailty Index Can Predict the Short-Term Outcomes of Esophagectomy in Older Patients with Esophageal Cancer. Current Oncology, 31(8), 4685-4694. https://doi.org/10.3390/curroncol31080349





