Detection of HPV DNA in Saliva of Patients with HPV-Associated Oropharyngeal Cancer Treated with Radiotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Inclusion and Exclusion Criteria
2.3. Saliva Collection and DNA Extraction
2.4. Real-Time PCR
2.5. Cancer Panel Sequencing
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Treatment
3.3. Treatment Outcomes
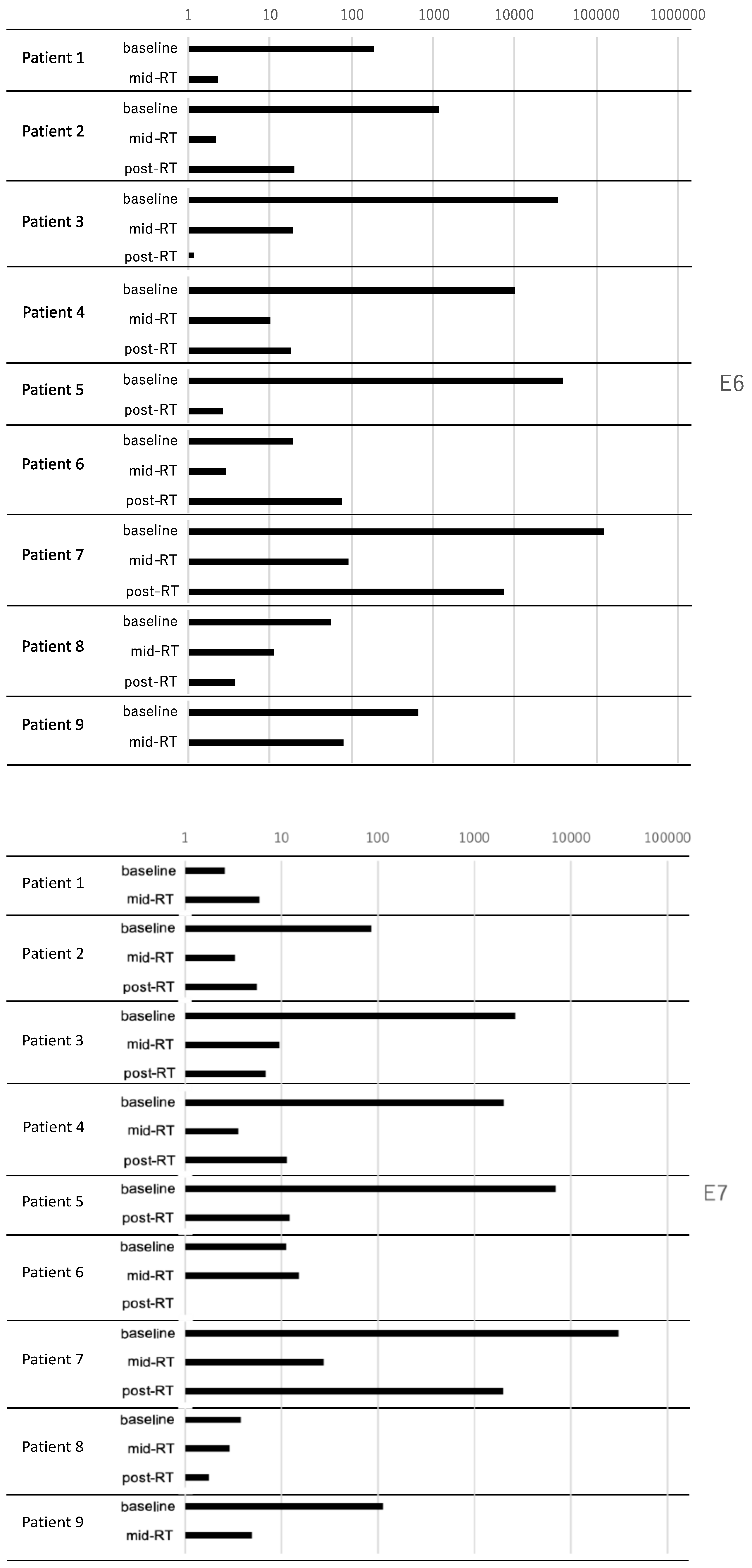
3.4. Sample Collection and Quantification of Saliva HPV DNA by RT–PCR
3.5. Cancer Panel Sequencing and HPV DNA Reads
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.A.; Turkdogan, S.; Roy, C.F.; Subramaniam, T.; Henry, M.; Sadeghi, N. De-Escalation Strategies for Human Papillomavirus-Associated Oropharyngeal Squamous Cell Carcinoma—Where Are We Now? Curr. Oncol. 2022, 29, 3668–3697. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M. De-escalated radiation for human papillomavirus virus-related oropharyngeal cancer: Evolving paradigms and future strategies. Front. Oncol. 2023, 13, 1175578. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-Y.; Zhang, X.; Cha, I.-H. Identification of human papillomavirus status specific biomarker in head and neck cancer. Head Neck 2015, 37, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.; Spruce, R.; Beggs, A.; Sharma, N.; Kong, A.; Martin, T.; Parmar, S.; Praveen, P.; Nankivell, P.; Mehanna, H. Circulating tumor DNA as a biomarker and liquid biopsy in head and neck squamous cell carcinoma. Head Neck 2018, 40, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Strickler, J.H.; Loree, J.M.; Ahronian, L.G.; Parikh, A.R.; Niedzwiecki, D.; Pereira, A.A.L.; McKinney, M.; Michael Korn, W.; Atreya, C.E.; Banks, K.C.; et al. Genomic landscape of cell-free DNA in patients with colorectal cancer. Cancer Discov. 2018, 8, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Azad, T.D.; Chaudhuri, A.A.; Fang, P.; Qiao, Y.; Esfahani, M.S.; Chabon, J.J.; Hamilton, E.G.; Yang, Y.D.; Lovejoy, A.; Newman, A.M.; et al. Circulating Tumor DNA Analysis for Detection of Minimal Residual Disease After Chemoradiotherapy for Localized Esophageal Cancer. Gastroenterology 2020, 158, 494–505.e6. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.M.; Chan, J.Y.K.; Zhang, Z.; Wang, H.; Khan, Z.; Bishop, J.A.; Westra, W.; Koch, W.M.; Califano, J.A. Saliva and plasma quantitative polymerase chain reaction-based detection and surveillance of human papillomavirus-related head and neck cancer. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Ekanayake Weeramange, C.; Liu, Z.; Hartel, G.; Li, Y.; Vasani, S.; Langton-Lockton, J.; Kenny, L.; Morris, L.; Frazer, I.; Tang, K.D.; et al. Salivary High-Risk Human Papillomavirus (HPV) DNA as a Biomarker for HPV-Driven Head and Neck Cancers. J. Mol. Diagn. 2021, 23, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Patel, S.; Patel, P.; Tanavde, V. Saliva Based Liquid Biopsies in Head and Neck Cancer: How Far Are We From the Clinic? Front. Oncol. 2022, 12, 828434. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xu, F.; Wu, J.; Schubert, J.; Li, M.M. Application of Next Generation Sequencing in Laboratory Medicine. Ann. Lab. Med. 2021, 41, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Moberg, M.; Gustavsson, I.; Gyllensten, U. Real-time pcr-based system for simultaneous quantification of human papillomavirus types associated with high risk of cervical cancer. J. Clin. Microbiol. 2003, 41, 3221–3228. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Springer, S.; Mulvey, C.L.; Silliman, N.; Schaefer, J.; Sausen, M.; James, N.; Rettig, E.M.; Guo, T.; Pickering, C.R.; et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci. Transl. Med. 2015, 7, 293ra104. [Google Scholar] [CrossRef] [PubMed]
- Lakshmipathy, D.; Prasad, A.; Fritz, C.G.; Go, B.C.; Rajasekaran, K. Accuracy of Salivary Circulating Tumor Human Papillomavirus DNA in Detecting Oropharyngeal Cancer. JAMA Otolaryngol. Neck Surg. 2024, 150, 580. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.J.; Lau, C.J.; Mahmood, U.; Supplee, J.G.; Mogili, A.R.; Haddad, R.I.; Jänne, P.A.; Paweletz, C.P. Salivary HPV DNA informs locoregional disease status in advanced HPV-associated oropharyngeal cancer. Oral Oncol. 2019, 95, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Sais, D.; Tran, N. Advances in human papillomavirus detection and molecular understanding in head and neck cancers: Implications for clinical management. J. Med. Virol. 2024, 96, e29746. [Google Scholar] [CrossRef] [PubMed]
- Eggersmann, T.K.; Baumeister, P.; Kumbrink, J.; Mayr, D.; Schmoeckel, E.; Thaler, C.J.; Dannecker, C.; Jeschke, U.; Nagler, T.; Mahner, S.; et al. Oropharyngeal HPV detection techniques in HPV-associated head and neck cancer patients. Anticancer Res. 2020, 40, 2117–2123. [Google Scholar] [CrossRef] [PubMed]

| Patient # | Stage (AJCC ver.7) | Sex | Age | Subsite | Brinkman Index | Chemotherapy |
|---|---|---|---|---|---|---|
| # 1 | T2N2c | m | 69 | tonsil | 800 | TPS f/b RT alone |
| # 2 | T4aN0 | m | 44 | tonsil | 345 | conc. CDDP |
| # 3 | T4aN2b | m | 69 | tonsil | 800 | conc. CBDCA |
| # 4 | T4aN3 | f | 74 | BOT | 0 | PCE f/b Conc.Cmab |
| # 5 | T4aN2b | m | 62 | tonsil | 672 | conc. CDDP |
| # 6 | T3N0 | m | 60 | tonsil | 90 | conc. CDDP |
| # 7 | T4aN2b | f | 64 | tonsil | 0 | conc. CDDP |
| # 8 | T1N2b | f | 66 | tonsil | 0 | conc. CDDP |
| # 9 | T2N2b | f | 54 | tonsil | 50 | conc. CDDP |
| Patient # | Baseline Relative Quantity Order of | Progressive Disease | |
|---|---|---|---|
| HPV E6 | HPV E7 | ||
| # 1 | 7 | 9 | − |
| # 2 | 5 | 6 | − |
| # 3 | 3 | 3 | + |
| # 4 | 4 | 4 | + |
| # 5 | 2 | 2 | − |
| # 6 | 9 | 8 | − |
| # 7 | 1 | 1 | + |
| # 8 | 8 | 7 | − |
| # 9 | 6 | 5 | − |
| # of Patient | Timing of Collection | HPV DNA Reads | Gene | Type of Mutation |
|---|---|---|---|---|
| # 1 | baseline | 8 | PIK3CA E707K TP53 G113D | MISSENSE MISSENSE |
| # 3 | baseline | 1266 | PIK3CA A224T PIK3CA P421Q TP53 S212T TP53 G113D | MISSENSE MISSENSE MISSENSE MISSENSE |
| Post-RT | n/d | PIK3CA G112V PIK3CA Q269K PIK3CA E418 PIK3CA W424L PIK3CA W1051 (tGf/tAg) PIK3CA W1051 (tgG/tgA) PIK3CA W1052K TP53 S212T | MISSENSE MISSENSE NONSENSE MISSENSE NONSENSE NONSENSE NONSENSE MISSENSE | |
| # 4 | baseline | 4 | PIK3CA E545K TP53 G113D | MISSENSE MISSENSE |
| Post-RT | n/d | CDKN2A D23A CDKN2A E10G | MISSENSE MISSENSE | |
| # 6 | baseline | n/d | TP53 S212T | MISSENSE |
| Post-RT | n/a | TP53 S212T | MISSENSE | |
| # 7 | baseline | 16,377 | PIK3CA E707K TP53 S212T | MISSENSE MISSENSE |
| # 8 | baseline | 1634 | PIK3CAD84Y CDKN2AV25G | MISSENSE MISSENSE |
| mid-RT | 2 | PIK3CA D84Y PIK3CA W1051 CDKN2A V25G TP53 E17K | MISSENSE NONSENSE MISSENSE MISSENSE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motegi, A.; Kageyama, S.-i.; Kashima, Y.; Hirata, H.; Hojo, H.; Nakamura, M.; Fujisawa, T.; Enokida, T.; Tahara, M.; Matsuura, K.; et al. Detection of HPV DNA in Saliva of Patients with HPV-Associated Oropharyngeal Cancer Treated with Radiotherapy. Curr. Oncol. 2024, 31, 4397-4405. https://doi.org/10.3390/curroncol31080328
Motegi A, Kageyama S-i, Kashima Y, Hirata H, Hojo H, Nakamura M, Fujisawa T, Enokida T, Tahara M, Matsuura K, et al. Detection of HPV DNA in Saliva of Patients with HPV-Associated Oropharyngeal Cancer Treated with Radiotherapy. Current Oncology. 2024; 31(8):4397-4405. https://doi.org/10.3390/curroncol31080328
Chicago/Turabian StyleMotegi, Atsushi, Shun-ichiro Kageyama, Yukie Kashima, Hidenari Hirata, Hidehiro Hojo, Masaki Nakamura, Takeshi Fujisawa, Tomohiro Enokida, Makoto Tahara, Kazuto Matsuura, and et al. 2024. "Detection of HPV DNA in Saliva of Patients with HPV-Associated Oropharyngeal Cancer Treated with Radiotherapy" Current Oncology 31, no. 8: 4397-4405. https://doi.org/10.3390/curroncol31080328
APA StyleMotegi, A., Kageyama, S.-i., Kashima, Y., Hirata, H., Hojo, H., Nakamura, M., Fujisawa, T., Enokida, T., Tahara, M., Matsuura, K., & Zenda, S. (2024). Detection of HPV DNA in Saliva of Patients with HPV-Associated Oropharyngeal Cancer Treated with Radiotherapy. Current Oncology, 31(8), 4397-4405. https://doi.org/10.3390/curroncol31080328





