Abstract
Background: Diagnostic blood tests have the potential to identify lung cancer in people at high risk. We assessed the cost-effectiveness of a lung cancer screening intervention, using the EarlyCDT®-Lung Test (ECLS) with subsequent X-ray and low-dose chest CT scans (LDCT) for patients with a positive test result, compared to both usual care and LDCT screening for the target population. Methods: We conducted a model-based lifetime analysis from a UK NHS and personal social services perspective. We estimated incremental net monetary benefit (NMB) for the ECLS intervention compared to no screening and to LDCT screening. Results: The incremental NMB of ECLS intervention compared to no screening was GBP 33,179 (95% CI: −GBP 81,396, GBP 147,180) and GBP 140,609 (95% CI: −GBP 36,255, GBP 316,612), respectively, for a cost-effectiveness threshold of GBP 20,000 and GBP 30,000 per quality-adjusted life year. The same figures compared with LDCT screening were GBP 162,095 (95% CI: GBP 52,698, GBP 271,735) and GBP 52,185 (95% CI: −GBP 115,152, GBP 219,711). Conclusions: The ECLS intervention is the most cost-effective screening alternative, with the highest probability of being cost-effective, when compared to no screening or LDCT screening. This result may change with modifications of the parameters, suggesting that the three alternatives considered in the main analysis are potentially cost-effective.
1. Introduction
Lung cancer (LC) has the highest mortality of cancers worldwide [1], with Scotland having one of the highest rates in the world; almost 4000 deaths in 2021 [2,3]. Early detection and diagnosis improve prognosis, the 5-year survival rate is approximately 60% for stage I LC but only 3% for those with stage IV [4]. Eighty-five per cent of LC cases are not diagnosed until they become symptomatic, at which point the cancer is advanced [5]. Early detection not only improves prognosis but the cost of treating early-stage LC is around half that of treating stage IV cancer; evidence shows that achieving earlier diagnosis would be highly cost-effective [6]. In 2022, the UK National Screening Committee recommended that all four nations implement targeted screening for lung cancer [7]. Whilst The Targeted Lung Health Checks programme currently running in England is a good starting point, and implementation is underway in some places, screening is not yet a national programme and more work is needed to achieve this [8].
Effective screening programmes identify asymptomatic people with cancer, achieving early diagnosis [6]. Previous research in England showed screening using low-dose chest CT (LDCT) resulted in 85% of LC cases detected at stage I or II, with over 90% of these cases being potentially curable with treatment [9]. However, CT scans are expensive and have been found to result in a high number of false positives (over 90% of tumours detected are benign), overdiagnosis and exposure to radiation [10]. Another drawback of LDCT screening programmes is capacity; there is presently not enough infrastructure to carry out large numbers of LDCT in the UK, which is why the targeted NHS lung cancer screening programme in England is not projected to be universally available until 2029 [11,12].
The EarlyCDT®-Lung Test is a blood test that identifies biomarkers useful for the prediction of LC. The test detects early and late stages of LC with sensitivity of 41% and specificity of 90% [13], and could act as the first step in a targeted approach to LC screening for early-stage detection. The Early detection of Cancer of the Lung Scotland (ECLS) trial designed a screening strategy where the target population was administered with the EarlyCDT®-Lung Test and, if tested positive, followed by chest X-ray and serial chest LDCT scanning.
The ECLS trial sought to assess the effectiveness and cost-effectiveness of this screening strategy, evaluating whether using the test, followed by chest X-ray and serial chest LDCT scanning, reduces the incidence of patients with late-stage LC (III and IV) or unclassified presentation (U) at diagnosis, compared to standard clinical practice (i.e., no-screening for LC) [14]. After the first published results, some questions were raised regarding the interpretation of the findings and the study design. First, ECLS found a difference in the prevalence of LC between arms, which may contradict the intuitive expectation that screening interventions should not affect the underlying incidence of LC in the target population [14]. Even if the reported difference in prevalence was due to chance alone, ideally, we should evaluate the screening intervention assuming equal prevalence in both treatment and comparator. Secondly, it has been suggested that the ECLS study may have underestimated LC prevalence overall, and therefore overestimated the sensitivity of the EarlyCDT®-Lung test [15]. The same source suggested that a more appropriate comparator for the new screening intervention should have been an LDCT-only screening (i.e., the same as the intervention screening without administering the EarlyCDT®-Lung Test) rather than using standard clinical practice (no screening) [15]. Third, resource-use data collected for intervention costs are only available for test-positive participants, which makes it necessary to use assumptions for participants with a negative test result.
In this paper, we use a modelling approach, which allows us to address the aforementioned issues, to analyse the cost-effectiveness of the proposed screening strategy (EarlyCDT®-Lung Test and, if tested positive, followed by chest X-ray and serial chest LDCT scanning) compared to no-screening detection, on the one hand, and LDCT scanning for the whole target population, on the other hand.
2. Materials and Methods
2.1. The ECLS Trial
Details of the ECLS study are reported in the protocol and main clinical results paper and described briefly here [5,14]. Participants between 50 and 75 years and at high risk of LC were recruited between April 2013 and July 2016. High risk was defined as current or former smokers with a minimum of 20 pack-years, or less than 20 pack-years plus a family history (parent, sibling, or child) of LC. Participants were healthy enough to undergo radical treatment either by pulmonary resection or stereotactic radiotherapy. It was expected that about 2% of participants would develop LC in the 24-month follow-up period of the trial based on a previous screening study with a similar target population [16].
Participants were recruited from targeted general practices serving patients in the lowest quintile of deprivation in Scotland (from Greater Glasgow and Clyde, Tayside and Lanarkshire), as measured by the Scottish Index of Multiple Deprivation (SIMD) [17]. Additional recruitment was attained through adverts, posters, flyers and community-based interactions. In total, 12,209 participants were randomised to either the intervention or the control group and followed up for 24 months.
The intervention (screening) arm comprised an EarlyCDT®-Lung Test administered to all subjects in the target population. The test result could be negative, in which case no additional investigation was offered, or positive, in which case immediate investigation by X-ray and LDCT imaging was offered. Participants in the screening arm with a positive test result and no evidence of LC from the X-ray and LDCT scan were invited for subsequent 6-monthly LDCT scans over the 24-month follow-up. Alternatively, if the results of the X-ray or LDCT scan were suspicious, contrast-enhanced staging CT was undertaken; depending on the results of this scan, the participant was referred to NHS care (clinically significant results) or continued with 6-monthly scans. The comparator (no-screening) arm comprised UK standard clinical practice at that time; awaiting the development of symptoms and investigation of those symptoms according to national guidelines [18,19]. For outcomes, validated data on cancer occurrence, mortality and comorbidities were obtained, with patient consent, from National Services Scotland, a high-quality health services data repository. These were deterministically linked to baseline and follow-up visit data in OpenClinica 3.1.2 Community Edition (a clinical research service provider) using Scotland’s Community Health Index number and analysed at the Dundee Health Informatics Centre Safe Haven [20]. Pathology and tumour staging reports were prepared by independent assessors, blinded to the allocation status of study participants. Staging data were taken from the Scottish Cancer Registry (SMR06) [21].
2.2. Overview of Economic Analysis
The analysis used an NHS and personal social services perspective following recommendations by the National Institute for Health and Care Excellence (NICE), including healthcare costs only [22]. A 3.5% discounting rate was applied to outcomes and costs occurring after the first year from start of screening, and 0% and 6% were used in a sensitivity analysis. Best practice methods and Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guidelines were followed as appropriate [23,24,25]. A cost-effectiveness analysis was performed to estimate the incremental costs, quality-adjusted life years (QALYs) and net monetary benefit using a decision tree model.
The model used information obtained by the ECLS trial about the LC stage at detection to extrapolate long-term QALYs and healthcare costs of LC patients. The key hypothesis behind the model is that a screening intervention which detects LC at an earlier stage will bring future benefits in terms of higher life expectancy (because the patient could benefit from earlier treatment), and lower treatment costs, whilst late-stage cancers are related to lower life expectancy and more resource use and costly treatments.
A decision analytic modelling approach is appropriate in the context of this study not only to estimate long-term benefits but also to overcome some challenges raised by the ECLS trial results. For example, the model allowed us to equalise LC prevalence in the screening intervention and the comparator as it is expected that screening interventions do not affect the underlying incidence of LC in the target population [14]. Also, model parameters like the prevalence of LC and accuracy of the test could be modified to study plausible scenarios and alternative screening interventions, e.g., LDCT screening offered to all members of the target population [15].
2.3. The Model
The model shown in Figure 1 simulated the diagnostic pathway (type of screening tests or investigations administered to the individual), and LC stage at detection (LC status and stage at detection), for a cohort of participants in the ECLS screening intervention. Lifetime costs and QALYs were assigned to participants depending on the diagnosis pathway and disease stage at detection.
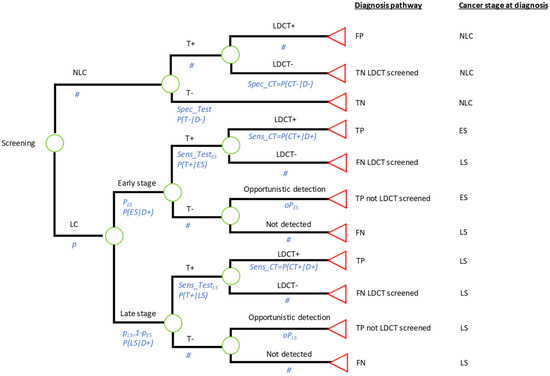
Figure 1.
Decision tree model. ECSL trial intervention, if specificity and sensitivity are those of the EarlyCDT®-Lung Test; Comparator 1: Standard care (no screening), if specificity is set at 1 and sensitivity is set at 0; Comparator 2: LDCT screening to all individuals in the target population, if specificity is set at 0 and sensitivity is set at 1. TN—true negative; TP—true positive; FN—false negative; FP—false positive; LDCT—low-dose computed tomography; LC—lung cancer; NLC—no lung cancer; ES—early stage; LS—late stage; # represents the probability of the complementary event at each chance node.
2.4. Structure and Endpoints: Disease Pathway and Disease Stage at Detection
An underlying LC prevalence (p) of LC at the start of the screening intervention was assumed to be either early stage (I/II) or late stage (III, IV or U) with conditional probabilities and , respectively. Participants undergo the EarlyCDT®-Lung test with specificity given by the probability of correctly identifying disease-negative participants, i.e., P(T−|D−). A different sensitivity can be specified for early-stage (ES) and late-stage (LS) cancers given by the probabilities P(T+|ES) and P(T+|LS). Participants with a positive test result are sent to 6-monthly LDCTs as designed in the ECLS trial, and those with a negative test result are no longer investigated. LDCT investigations could confirm an LC diagnosis or not, depending on the true disease state of the patient and the sensitivity and specificity of the 6-monthly LDCTs. Finally, LC patients who did not undertake LDCT investigations could be detected opportunistically during the screening period, for example, in a hospital visit related or unrelated to LC symptoms, with probabilities and for ES and LS, respectively.
The model classified individuals depending on their diagnosis pathway:
- True positive (TP). Individuals with LC who obtained a positive test result and were correctly identified by LDCTs.
- TP (not LDCT screened). Individuals with LC who obtained a negative test result and therefore were not offered LDCTs. These cases were opportunistically detected.
- True Negative (TN). Individuals with no LC who obtained a negative test result.
- TN (LDCT screened). Individuals with no LC who obtained a positive test result and were investigated by LDCTs with negative results.
- False Positive (FP). Those individuals with no LC but with a positive result from the test and from LDCT investigations.
- False Negative (FN). Those individuals with LC who obtained a negative test result.
- FN (LDCT screened). Those individuals with LC who obtained a positive test result but received a negative result after LDCT investigations.
The model classified individuals according to LC stage at diagnosis, into the following:
- No LC. Individuals who were disease-negative during the period of the ECLS screening intervention.
- ES LC. Individuals who had LC at ES during the screening intervention and were correctly detected during the course of the screening intervention by LDCTs. These patients were assumed to be detected at an early stage and therefore would benefit from early treatment. They were attached to a high(er) life expectancy, consistent with being detected soon(er).
- LS LC. Two subgroups of patients can be considered. First, individuals who were at LS in the screening period. Second, individuals who had ES LC during the screening period but were undetected, either because they had a negative test result with no opportunistic detection, or because they had a positive test result followed by an LDCT negative result. The LS LC patients were assumed to be diagnosed too late to benefit from early treatment. Hence, they were attached to a low(er) life expectancy.
In the analysis, all the disease-pathway groups were assigned the same cost for the administration of the test. However, the costs of LDCTs and other diagnostic imaging were different depending on the specific pathway followed by each group of individuals. For example, a TN (LDCT-screened) participant incurred LDCT screening costs while a TN individual was not investigated due to a negative test result. Also, treatment costs were dependent on the LC stage at detection to account for the differences in the type of therapies administered. Finally, lifetime and health utilities, used to construct QALYs, were conditional on the cancer stage at the moment of diagnosis to account for the fact that quality of life and life expectancy is lower for LS cases [4].
2.5. ECLS Screening Intervention and Comparators
The model depicted in Figure 1 allowed us to simulate the alternatives compared in this analysis by changing the sensitivity and specificity of the test provided before the administration of LDCT screening:
Intervention: The ECLS intervention. This strategy was simulated by using the sensitivity and specificity of the EarlyCDT®-Lung Test as estimated in the ECLS trial published results [14]. Under this alternative, only some patients will obtain a positive test result and therefore only some of them will be screened by 6-monthly LDCTs for 2 years.
Comparator 1: No screening. This strategy was simulated by assuming a test with sensitivity 0 and specificity 1. Therefore, none of the participants will obtain a positive test result and none of them will be sent to LDCT screening. The test used in this strategy would have a zero cost.
Comparator 2: LDCT screening administered 6-monthly for 2 years. This strategy was simulated by setting the sensitivity and specificity of the test to 1 and 0, respectively. In this case, all the subjects in the target population will be sent to LDCT screening. Again, this test would be administered at no cost.
2.6. Parameters: Sources and Estimation
The model parameters are listed in Table 1, including pathway probabilities, costs and outcomes for base case, probabilistic and deterministic sensitivity analysis (PSA and DSA). Details of parameter estimations are found in the Supplementary Material.

Table 1.
Value of model parameters, probabilistic sensitivity analysis and source.
The base case analysis used a prevalence (p) of LC of 2% following the study protocol expectations based on a previous study of a similar population [16]. In a sensitivity analysis, prevalence was changed to 1% and 4% to relax this assumption. All the remaining probabilities were estimated using the data collected in the ECLS trial. The relative prevalence of ES cancers () was estimated as the ratio of expected total ES cases to LS cases in the ECLS trial intervention arm using data of the cases detected and sensitivity of the EarlyCDT®-Lung Test for each LC stage. Sensitivity of the EarlyCDT®-Lung Test, for ES and LS cancers, and specificity was estimated as reported in the ECLS trial published manuscript [14]. The probability of opportunistic detection ( and ) was estimated using data on detected LC in the ECLS trial control (no-screening) arm. Finally, the 6-monthly LDCT screening for two years was assumed to have a sensitivity and specificity of 100% in the base case analysis, i.e., no false positive or false negative was allowed for participants sent to LDCT screening.
Cost parameters represented 2021/22 pounds sterling and comprised the administration of an LC test, i.e., the EarlyCDT®-Lung Test for the intervention alternative, diagnostic costs for LDCT screening and imaging, and treatment costs. Costs of the test were applied at the beginning of the screening programme. However, diagnostic costs were assumed to occur one year after (i.e., the average time for a two-year screening programme) or at the moment of diagnosis if the person was diagnosed during the course of the screening intervention. Treatment costs were assumed to happen in the same year of diagnosis. The cost of the EarlyCDT®-Lung Test kit was advised by Oncimmune (GBP 95) and its administration was assumed equivalent to 15 min of nurse time at GP surgery (GBP 11.50). The testing costs were applied only to individuals in the ECLS intervention. The diagnostic costs differed by diagnostic pathway. Average number of X-ray and CT scans were estimated from ECLS data and valued for patients with a positive test result (i.e., those sent to LDCT screening) differentiating between those with and without a confirmatory LC diagnosis. In addition, a confirmatory diagnostic cost was applied to all participants with a diagnosis of LC. Confirmatory diagnostic tests were based on the opinion of clinical experts in the ECLS study and consisted of an X-ray, a contrast CT scan and either a bronchoscopy or CT-guided biopsy, with the average of a bronchoscopy and a CT-guided biopsy cost applied. Individuals with a negative test result and no diagnosis had a zero diagnostic cost. Lung cancer treatment costs included surgery, radiotherapy, chemotherapy, immunotherapy and others as calculated in a UK National Screening Committee (UK-NSC) report authored by the Exeter Test Group and Health Economics Group [27]. Average treatment costs at diagnosis for LC stage I and II, as calculated by the UK-NSC report, were assigned to ES. In the same way, average treatment costs at diagnosis for LC stage III and IV were assigned to LS. Only 38.85% of LS LCs not detected during the screening period will be expected to receive treatment before dying according to the opportunistic detection rate estimated with the ECLS study data. Also, a proportion of ES LC was assumed to recur and incur lung cancer treatment for advanced stage (about 50% according to Cancer Research UK) [6]. Unit costs and details of the computation of each cost item are in the Supplementary Materials.
The computation of QALYs for participants in the screening programme involved the multiplication of lifetime (LT) and health utility (HE). LT for LC patients was computed as the addition of two constructs: (1) lead time, defined as the time passed between start of the screening programme and diagnosis; and (2) life expectancy (LE) from diagnosis. Lead time for TP LC detected at ES was estimated as the average for LC cases with a positive test result in the ECLS trial intervention arm, with a mean of 0.444 years. For consistency, the same value was attached to TP (not LDCT screened) early-stage LCs. Late-stage LCs were assigned the average lead time for LC cases in the control arm and LC cases with a negative test result in the intervention arm, which was 1.049 years. These lead time estimations imply an average time to progression (from early to late stage) of about 0.6 years, which lies within the range of previous estimations for Caucasian patients [32]. Weibull survival curves were estimated to compute life expectancy from diagnosis using digitised data from K–M curves published by the International Association for the Study of LC IASLC staging project [28]. Then, survival parameters were calibrated for each LC stage using five-year survival figures from an England national study that followed lung cancer patients diagnosed from 2015 to 2019 and followed up to 2020 [29]. Finally, the model assigned the average life expectancy of LC stage I and II to ES patients. Life expectancy of stage III and IV, calibrated from English data, is unlikely to capture the effect of immunotherapy on survival of advanced lung cancer, recommended from the year 2019, but rather it is the result of previous standard treatments such as chemotherapy. A modified life expectancy was computed for LS LCs receiving immunotherapy by applying a hazard ratio estimated by the KEYNOTE-24 study (HR = 0.62, 95% CI: 0.48 to 0.81; overall survival of immunotherapy vs. chemotherapy) [30]. Immunotherapy-augmented life expectancy was applied to LS LC detected during or after the screening period. On the contrary, “pre-immunotherapy” survival figures were assigned to those patients expected to die before receiving treatment. An average health utility for patients with and without LC was estimated from EQ-5D responses reported in the ECLS study at baseline. Previous literature was followed by attaching a decremental health utility to each stage [31]. Details of the estimation and computation of lead time, life expectancy and health utilities are found in the Supplementary Materials.
2.7. Sensitivity and Scenario Analysis
A PSA was conducted via 10,000 Monte Carlo simulations using the following distribution functions: beta distribution for prevalence, sensitivity, specificity, probability of opportunistic detection and health utilities; normal distribution for life expectancy and incremental health utilities; gamma distribution for lead time bias and diagnostic costs for the test positive patients. The remaining parameters based on deterministic data, assumptions or expert opinion were fixed: treatment costs; cost of EarlyCDT®-Lung Test; and diagnostic costs for test-negative patients. Also, a DSA was conducted, to check the robustness of the results to changes in key parameters of the ECLS intervention (among them: prevalence of lung cancer in the target population, relative prevalence of ES LC, cost of the test, sensitivity and specificity) and to illustrate the mechanism of the model. The minimum and maximum values used for the DSA for key parameters were chosen to make an impact on the cost-effectiveness results at the policy-relevant thresholds.
Two scenario analyses were conducted to address some challenges relating to the interpretation of the ECLS study. First, the sensitivity of the EarlyCDT®-Lung Test was set to 25%, addressing concerns about the overestimation of the sensitivity of the test for early LCs [15]. Second, the false-positive rate (1—specificity) was set equal to the true-positive rate (sensitivity) and the cost of the test to zero. The latter simulates a strategy where patients receive a completely random test; patients are sent to LDCT screening independently of their true disease state with a probability of 52%.
2.8. Cost-Effectiveness Analysis
Mean and 95% normal confidence intervals for the PSA were estimated for costs and QALYs for the intervention and the two comparators for every 1000 participants. Net Monetary Benefit (NMB) was also estimated using policy-relevant cost-effectiveness thresholds (20,000 and 30,000 GBP per QALY) [22], and cost-effectiveness acceptability curves (CEACs) were calculated. Results of the DSA were presented as mean costs, QALYs and NMBs. The scenario analysis was presented on a cost-effectiveness plane for ease of interpretation.
The statistical analysis was performed using STATA 18.0 (StataCorp, College Station, TX, USA) and the model was run in R using the package heemod [33,34].
3. Results
3.1. Base Case Analysis
Table 2 includes QALYs, costs and NMB per 1000 participants for the base case analysis. NMB figures point to the ECLS intervention as the most cost-effective alternative. The incremental NMB estimates of the ECLS intervention compared to no screening are subject to some uncertainty with GBP 33,179 (95% CI: −GBP 81,396.4, GBP 147,180) and GBP 140,609 (95% CI: −GBP 36,255.1, GBP 316,612) for a cost-effectiveness threshold of GBP 20,000 and GBP 30,000 per QALY, respectively. The ECLS intervention brings both higher costs, GBP 181,681 (95% CI: GBP 168,243, GBP 195,121), and QALYs, 10.7 (95% CI: 4.5, 17), at a ratio of less than GBP 20,000 per QALY. The use of the ECLS intervention implies 2.67 more early-stage LCs than in the no-screening alternative at the expense of 94.2 participants (out of 980 no-LC individuals) being unnecessarily investigated (see Supplementary Materials for model counts for each diagnostic pathway).

Table 2.
Base case cost-effectiveness results: costs, QALYs and NMBs per one thousand participants.
The incremental NMB compared to LDCT screening is GBP 162,095 (95% CI: GBP 52,698.3, GBP 271,735) and GBP 52,185 (95% CI: −GBP 115,152, GBP 219,711), respectively, for the lower and higher thresholds. Even though an LDCT screening would bring a gain of 11 QALYs (95% CI: 5.2, 16.8), due to more ES cancers detected, it would also incur GBP 381,915 in higher costs (95% CI: GBP 401,080, GBP 363,100) because all no-LC participants would be sent for LDCT investigation. Out of the 20 patients (2% of 1000) who would have LC in the analysis cohort, an average of 8.21 would be detected at an ES if investigated by LDCT, 2.44 more than in the case of the ECLS intervention. At the same time, a full LDCT screening would send 980 participants with no LC to unnecessary CT investigations, whereas only 94.2 would be test-positive with the EarlyCDT-Lung test. LDCT screening, compared to the ECLS intervention, would gain one QALY at a cost of GBP 34,810.
The CEACs in Figure 2 show that the ECLS intervention has the highest probability of being cost-effective for any cost-effectiveness threshold between GBP 18,000 and GBP 35,000. Below GBP 18,000 per QALY, no screening would become the alternative with a higher probability of being cost-effective. An LDCT screening would become the one with a higher probability of cost-effectiveness if we set a value of QALY above GBP 35,000. The PSA concludes that the ECLS intervention has a maximum 82.39% probability of being cost-effective at a threshold of GBP 25,000 per QALY.
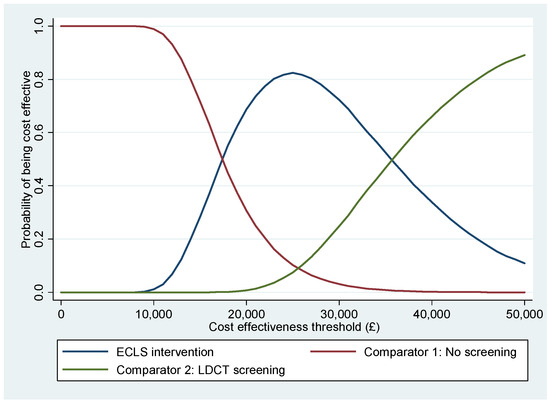
Figure 2.
Cost-effectiveness acceptability curves.
3.2. Deterministic and Scenario Analyses
The deterministic sensitivity analysis is shown in Table 3 where we see how cost-effectiveness changes with the cost of the EarlyCDT test and with the prevalence of LC in the target population. Reducing the cost of the test to half (GBP 47.5 rather than GBP 95, plus GBP 11.50 administration costs) would further improve the cost-effectiveness of the ECLS intervention. However, if we double the cost of the EarlyCDT test (GBP 190 plus GBP 11.5 administration costs) LDCT screening would stop being the most cost-effective alternative; although the 95% CI shows much uncertainty in this case. If the prevalence was set to 1%, no screening would be the most cost-effective alternative, even though the ECLS intervention would continue to be better than an LDCT screening. If the prevalence was as high as 4%, LDCT screening would be the most cost-effective alternative at the two thresholds used. The proportion of early-stage cases among all lung cancers is also a relevant factor; for example, if this proportion was 25%, then the ECLS screening would not be cost-effective at a threshold of GBP 20,000 per QALY, favouring no screening. On the other hand, a 75% relative prevalence of ES LCs would favour full LDCT screening as the best strategy at a threshold of GBP 30,000 per QALY. The ECLS intervention would not be cost-effective if the test sensitivity for ES LC was only 0.25, or if the specificity was as low as 0.5, at any policy-relevant thresholds. The DSA (in the Supplementary Materials) shows that the cost-effectiveness of the three alternatives compared could be affected by the rate of opportunistic detection for ES lung cancer (higher rate favouring no screening or ECLS intervention vs. full LCDT screening), discount rate (lower rate favouring the ECLS and LDCT screening interventions), health utilities used (lower base utilities favouring the no screening alternative), and LCDT sensitivity (lower sensitivity favouring no screening).

Table 3.
Deterministic sensitivity analysis: costs, QALYs and NMBs per one thousand participants.
Base case and scenario alternatives are represented on a cost-effectiveness plane as incremental QALYs and costs compared to no screening in Figure 3. Three lines representing three value thresholds (GBP 10,000, GBP 20,000 and GBP 30,000 per QALY) are depicted for interpretation of the results. The ECLS intervention is the most cost-effective alternative, below the GBP 20,000 threshold. The scenario with EarlyCDT®-Lung Test sensitivity set to 25% is the least cost-effective with an incremental cost-effectiveness ratio (ICER) above GBP 30,000 per QALY. A random test, sending 52% of participants to LDCT screening independently on LC state, would be cost-effective (compared to no screening) at the GBP 30,000 threshold, even though it would be much less costly than a full LDCT screening (i.e., sending 100% of participants to LDCT screening). Notice that a zero-cost random test would necessarily be at the same cost-effectiveness threshold as a full LDCT screening strategy, only the scale of costs and QALYs would be modified.
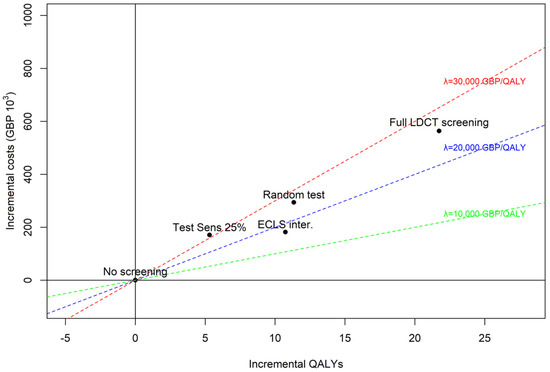
Figure 3.
Scenario vs. base case alternatives (ECLS intervention, no screening, and LDCT screening). Scenario 1: sensitivity of early-stage LC set to 25%. Scenario 2: “random test”, sensitivity and 1-specificity set to 52% and cost of test set to zero.
4. Discussion
The ECLS trial identified a statistically insignificant higher number of LC cases in the no-screening arm compared to the screening arm; however, a larger proportion of cases were ES in the screening arm compared to the no-screening arm. To address this surprising result, we used modelling techniques to conduct the economic evaluation. The base case analysis estimated a cost per QALY gained of less than GBP 20,000 when comparing the ECLS intervention to a no-screening strategy. Only reducing the prevalence to 1% or setting the cost of the EarlyCDT®-Lung Test above GBP 190 could make no screening the highest NMB alternative at the policy-relevant cost-effectiveness threshold. The base case analysis also concluded that a full LDCT screening programme is not cost-effective unless the prevalence of LC among the target population is assumed to be 4%. The scenario analysis showed that a screening policy using the EarlyCDT®-Lung Test performs better than a random test given its capacity to differentiate between LC patients and healthy individuals, e.g., 90% of healthy participants will save unnecessary LDCT. These results suggest that the ECLS intervention has the potential to be cost-effective when considering the detection of ES LC.
4.1. Comparison to Other Studies
As this is the first health-economic evaluation of a diagnostic blood test for LC screening, it is not possible to compare results to previous studies, and we were limited to comparing studies reporting LDCT screening strategies. The National Lung Screening Trial (NLST) in the United States reported a cost of USD 81,000 per QALY for an annual three-year LDCT screening compared to no-screening [35]. The UK Lung-cancer Screening (UKLS) trial reported an estimated ICER of GBP 8466 (95% CI GBP 5516 to GBP 12,634) per QALY gained for once-only screening with follow-up if necessary, compared to no screening (follow-up 12–15 months). (9) An analysis based on the methods used in the UKLS trial and evidence from an LC screening pilot in Manchester UK reported an ICER of GBP 10,069. A model also based on the UKLS results demonstrated a lifetime ICER of GBP 28,784 for single screening and GBP 95,292 for triple screening (baseline, 12- and 24-months) [36]. These results compare to an average incremental cost per QALY of GBP 25,972 for LDCT screening vs. no screening in this study; however, this is for 6-monthly LDCT scans compared to once-only screening in the UKLS trial and annual three-year screening in NLST. The cost-effectiveness of LDCT screening has been estimated to change with the frequency of screening, age and risk of the target population; in this sense, the estimated cost per QALY gain of an every-year, for ages 55–65, screening program in Spain is closer to the estimates for the 6-monthly screening intervention designed in the ECLS study [37,38].
4.2. Strengths
The large sample of over 12,000 participants allowed us the best opportunity to find differences in the distribution of LC stages in the screening and no-screening arms. The analysis captured the long-term consequences in terms of costs and outcomes of detecting LC at an ES compared to LS. The results highlighted these consequences in terms of lower treatment costs and improved survival and quality of life. The NMB measure allows a comparison between alternatives at the policy-relevant thresholds. Modelling allows us to explore the scenarios under which the ECLS intervention will be (most) cost-effective by varying the prevalence or the cost of the blood test. The population chosen was slightly younger and had fewer pack years compared to previous cost-effectiveness analyses of LC screening strategies [35], suggesting that the present study results are a conservative estimate of cost-effectiveness. Specific criticisms of the ECLS study design by Baldwin et al. have been addressed by including no screening and LCDT screening as comparators, as well as including additional alternatives in the scenario analyses [15]. The main criticism was that regular CT scans were offered to test-positive participants only; Baldwin et al. suggest that, therefore, generating a test result at random would result in an earlier diagnosis in the screening group. To address this criticism, we mimicked random test results using our model; the ECLS study proved to be a more cost-effective alternative than a zero-cost random test. The second criticism was that the sensitivity of the EarlyCDT®-Lung Test was overestimated; to address this, we reduced the sensitivity of the test to more than half (25% vs. 52% estimated in the ECLS trial); the resulting cost per QALY of this alternative (compared to no screening) was just above GBP 30,000. The final criticism was that the EarlyCDT®-Lung Test should be compared to CT scan screening; this was not chosen as a comparator in the ECLS trial as CT scanning was not the standard of care in Scotland when the trial was designed and approved, nor was there capacity to conduct this number of scans. To address this criticism in the economic evaluation, we mimicked all participants in the screening arm receiving 6-monthly LDCT scans, again the resulting ICER (vs. no screening) was GBP 25,972 for the base case scenario.
4.3. Limitations
The main limitation was the lack of resource-use data, which led us to use a model-based approach, populating it with a mix of trial data and expert opinion. This may have underestimated the resource use of confirmatory diagnoses as some participants may have had more than one confirmatory diagnostic test, receiving initial negative diagnostic results but needing additional testing after that. Participants may have modified their smoking behaviour due to participation in the study; participants in the no-screening arm may have been more aware of LC symptoms and more likely to seek medical help than had they not been in the study, participants in the test-negative group may have felt able to engage in risky behaviour if they felt they were ‘invincible’, and those in the test-positive group, without a confirmatory diagnosis, may have changed their smoking behaviour if they felt a positive result was an indication of increased risk of LC diagnosis [39]. However, it is noteworthy that a subgroup analysis of ECLS patients who had nodules detected showed no change in smoking behaviour [40]. We did not include the cost to the NHS of identifying high-risk patients and inviting them to screening; the UKLS trial estimated the cost per person of selection and invitation to be GBP 10 [9].
The ECLS study is continuing to monitor participants and has five- and ten-year analyses planned.
5. Conclusions
The base case analysis results estimated that the ECLS intervention is the most cost-effective alternative, with the highest probability, when compared to no screening or LDCT screening. This result may change with modifications to the prevalence of lung cancer and EarlyCDT®-Lung Test cost, suggesting that the three alternatives considered in the main analysis are potentially cost-effective depending on the disease risk of the target population and the cost of testing.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/curroncol31060261/s1, Table S1: Description and estimation of model parameters; Table S2: Unit costs (2021-22 pounds sterling); Table S3: Weibull survival function estimation; Table S4: 5-year age-standardised survival () for LC. England NHS-Digital; Table S5: Calibration of Weibull scale parameter (); Table S6: Life expectancy for each LC stage; Table S7: Treatment costs (£) at diagnosis from the UK National Screening Committee (UK-NSC) report; Table S8: Utility decrements based on Grutters et al. (2010) [31]; Table S9: Cohort counts for each model pathway. Baseline results. 1000 patients; Table S10. Deterministic sensitivity analysis.
Author Contributions
F.S., F.S.M. and A.B. contributed to study inception and design. Data analysis was undertaken by N.M. Model design and analysis were undertaken by J.A.R.-Z. and A.B. The first draft of the manuscript was written by J.A.R.-Z. and N.M., and all authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for the ECLS study was received from Oncimmune Ltd. and the Scottish Government Health & Social Care Directorate of the Chief Scientist Office (CSO).
Institutional Review Board Statement
The study was conducted in accordance with the principles of Good Clinical Practice and the UK National Research Governance Framework. The University of Dundee and Tayside Health Board co-sponsored the trial, which was registered at ClinicalTrials.gov with identifier NCT01925625. Institutional Review Board approval was provided by the East of Scotland Research Ethics Committee (REC 13/ES/0024, on 16 April 2013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Acknowledgments
ECLS trial team, particularly Joana Rocha, Petra Rauchhaus and Pauline Armory.
Conflicts of Interest
All authors report grants from Oncimmune Ltd., and grants from the Scottish Government Health & Social Care Directorate of the Chief Scientist Office (CSO), during the conduct of the study.
References
- World Health Organisation. Cancer. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 22 March 2024).
- Public Health Scotland. Cancer Mortality in Scotland—Annual Update to 2021. 2024. Available online: https://publichealthscotland.scot/publications/cancer-mortality/cancer-mortality-in-scotland-annual-update-to-2021/ (accessed on 22 March 2024).
- Public Health Information for Scotland. Lung Cancer: Key Points. 2024. Available online: https://www.scotpho.org.uk/health-conditions/cancer-lung/key-points/ (accessed on 22 March 2024).
- Office of National Statistics. Cancer Survival in England—Adults Diagnosed—Office for National Statistics. 2019. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/cancersurvivalratescancersurvivalinenglandadultsdiagnosed (accessed on 22 March 2024).
- Sullivan, F.M.; Farmer, E.; Mair, F.S.; Treweek, S.; Kendrick, D.; Jackson, C.; Robertson, C.; Briggs, A.; McCowan, C.; Bedford, L.; et al. Detection in blood of autoantibodies to tumour antigens as a case-finding method in lung cancer using the EarlyCDT((R))-Lung Test (ECLS): Study protocol for a randomized controlled trial. BMC Cancer 2017, 17, 187. [Google Scholar] [CrossRef] [PubMed]
- Cancer Research UK. Saving Lives, Averting Costs. 2019. Available online: https://news.cancerresearchuk.org/2014/09/22/saving-lives-and-averting-costs-the-case-for-earlier-diagnosis-just-got-stronger/ (accessed on 22 March 2024).
- UK-NSC. UK NSC Screening Recommendation. Available online: https://view-health-screening-recommendations.service.gov.uk/lung-cancer/ (accessed on 22 March 2024).
- O’Dowd, E.L.; Lee, R.W.; Akram, A.R.; Bartlett, E.C.; Bradley, S.H.; Brain, K.; Callister, M.E.J.; Chen, Y.; Devaraj, A.; Eccles, S.R.; et al. Defining the road map to a UK national lung cancer screening programme. Lancet Oncol. 2023, 24, e207–e218. [Google Scholar] [CrossRef]
- Field, J.K.; Duffy, S.W.; Baldwin, D.R.; Whynes, D.K.; Devaraj, A.; Brain, K.E.; Eisen, T.; Gosney, J.; Green, B.A.; Holemans, J.A.; et al. UK Lung Cancer RCT Pilot Screening Trial: Baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016, 71, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Bach, P.B.; Mirkin, J.N.; Oliver, T.K.; Azzoli, C.G.; Berry, D.A.; Brawley, O.W.; Byers, T.; Colditz, G.A.; Gould, M.K.; Jett, J.R.; et al. Benefits and Harms of CT Screening for Lung Cancer A Systematic Review. JAMA-J. Am. Med. Assoc. 2012, 307, 2418–2429. [Google Scholar] [CrossRef] [PubMed]
- The Royal College of Radiologists. Clinical Radiology UK Workforce Census 2020 Report. 2020. Available online: https://www.rcr.ac.uk/media/3gjdr23o/clinical_radiology_census_report_2020.pdf (accessed on 22 March 2024).
- National Health Service. Who Is Eligible for a Lung Health Check. Available online: https://www.nhs.uk/conditions/lung-health-checks/#:~:text=Who%20is%20eligible%20for%20a,be%20available%20everywhere%20by%202029 (accessed on 22 March 2024).
- Chapman, C.J.; Healey, G.F.; Murray, A.; Boyle, P.; Robertson, C.; Peek, L.J.; Allen, J.; Thorpe, A.J.; Hamilton-Fairley, G.; Parsy-Kowalska, C.B.; et al. EarlyCDTA (R)-Lung test: Improved clinical utility through additional autoantibody assays. Tumor Biol. 2012, 33, 1319–1326. [Google Scholar] [CrossRef]
- Sullivan, F.M.; Mair, F.S.; Anderson, W.; Armory, P.; Briggs, A.; Chew, C.; Dorward, A.; Haughney, J.; Hogarth, F.; Kendrick, D.; et al. Earlier diagnosis of lung cancer in a randomised trial of an autoantibody blood test followed by imaging. Eur. Respir. J. 2021, 57, 2000670. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, D.R.; Callister, M.E.; Crosbie, P.A.; O’Dowd, E.L.; Rintoul, R.C.; Robbins, H.A.; Steele, R.J.C. Biomarkers in lung cancer screening: The importance of study design. Eur. Respir. J. 2021, 57, 2004367. [Google Scholar] [CrossRef] [PubMed]
- Swensen, S.J.; Jett, J.R.; Hartman, T.E.; Midthun, D.E.; Mandrekar, S.J.; Hillman, S.L.; Sykes, A.M.; Aughenbaugh, G.L.; Bungum, A.O.; Allen, K.L. CT screening for lung cancer: Five-year prospective experience. Radiology 2005, 235, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Scottish Government. Scottish Index of Multiple Deprivation 2020. Available online: https://www.gov.scot/collections/scottish-index-of-multiple-deprivation-2020/ (accessed on 6 October 2021).
- Baldwin, D.R.; White, B.; Schmidt-Hansen, M.; Champion, A.R.; Melder, A.M.; Guideline Development Group. Diagnosis and treatment of lung cancer: Summary of updated NICE guidance. BMJ 2011, 342, d2110. [Google Scholar] [CrossRef] [PubMed]
- Scottish Intercollegiate Guidelines Network. Management of Patients with Lung Cancer. A National Clinical Guideline (SIGN 80). 2005, 80. Available online: https://www.sign.ac.uk/media/1075/sign137.pdf (accessed on 22 March 2024).
- University of Dundee. HIC Trusted Research Environment—Safe Haven. Available online: https://www.dundee.ac.uk/hic/safe-haven (accessed on 22 March 2024).
- Public Health Scotland. Scottish Cancer Registry. Available online: https://www.isdscotland.org/Health-Topics/Cancer/Scottish-Cancer-Registry/ (accessed on 3 February 2023).
- National Institute for Health and Care Excellence, NICE Health Technology Evaluations: The Manual. 2022. Available online: https://www.nice.org.uk/process/pmg36/resources/nice-health-technology-evaluations-the-manual-pdf-72286779244741 (accessed on 22 March 2024).
- Ramsey, S.D.; Willke, R.J.; Glick, H.; Reed, S.D.; Augustovski, F.; Jonsson, B.; Briggs, A.; Sullivan, S.D. Cost-effectiveness analysis alongside clinical trials II—An ISPOR Good Research Practices Task Force report. Value Health 2015, 18, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Sanders, G.D.; Neumann, P.J.; Basu, A.; Brock, D.W.; Feeny, D.; Krahn, M.; Kuntz, K.M.; Meltzer, D.O.; Owens, D.K.; Prosser, L.A.; et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA 2016, 316, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Husereau, D.; Drummond, M.; Augustovski, F.; de Bekker-Grob, E.; Briggs, A.H.; Carswell, C.; Caulley, L.; Chaiyakunapruk, N.; Greenberg, D.; Loder, E.; et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: Updated reporting guidance for health economic evaluations. Int. J. Technol. Assess. Health Care 2022, 38, e13. [Google Scholar] [CrossRef] [PubMed]
- Curtis, L.; Burns, A.; Unit Costs of Health and Social Care 2017. Personal Social Services Research Unit (PSSRU). 2017. Available online: https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2017/ (accessed on 22 March 2024).
- Exeter-Test-Group-and-Health-Economics-Group. Interim Report on the Cost-Effectiveness of Low Dose Computed Tomography (LDCT) Screening for Lung Cancer in High Risk Individuals. 2022. Available online: https://view-health-screening-recommendations.service.gov.uk/document/586/download (accessed on 22 March 2024).
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- NHS-Digital, Cancer Survival in England, Cancers Diagnosed 2015 to 2019, Followed Up to 2020, in National Statistics. 2022. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/cancer-survival-in-england/cancers-diagnosed-2015-to-2019-followed-up-to-2020 (accessed on 22 March 2024).
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non–Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50%. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Grutters, J.P.; Joore, M.A.; Wiegman, E.M.; Langendijk, J.A.; De Ruysscher, D.; Hochstenbag, M.; Botterweck, A.; Lambin, P.; Pijls-Johannesma, M. Health-related quality of life in patients surviving non-small cell lung cancer. Thorax 2010, 65, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Cao, J.L.; Rustam, A.; Zhang, C.; Yuan, X.S.; Bao, F.C.; Lv, W.; Hu, J. Time-to-Progression of NSCLC from Early to Advanced Stages: An Analysis of data from SEER Registry and a Single Institute. Sci. Rep. 2016, 6, 28477. [Google Scholar] [CrossRef] [PubMed]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Filipović-Pierucci, A.; Zarca, K.; Durand-Zaleski, I. Markov Models for Health Economic Evaluations: The R Package Heemod. 2017. Available online: https://arxiv.org/abs/1702.03252 (accessed on 14 November 2022).
- Black, W.C.; Gareen, I.F.; Soneji, S.S.; Sicks, J.D.; Keeler, E.B.; Aberle, D.R.; Naeim, A.; Church, T.R.; Silvestri, G.A.; Gorelick, J.; et al. Cost-Effectiveness of CT Screening in the National Lung Screening Trial. N. Engl. J. Med. 2014, 371, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Griffin, E.; Hyde, C.; Long, L.; Varley-Campbell, J.; Coelho, H.; Robinson, S.; Snowsill, T. Lung cancer screening by low-dose computed tomography: A cost-effectiveness analysis of alternative programmes in the UK using a newly developed natural history-based economic model. Diagn. Progn. Res. 2020, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; Garcia, M.; Vidal, C.; Santiago, A.; Gnutti, G.; Gómez, D.; Trapero-Bertran, M.; Fu, M.; Lung Cancer Prevention LUCAPREV Research Group. Health and economic impact at a population level of both primary and secondary preventive lung cancer interventions: A model-based cost-effectiveness analysis. Lung Cancer 2021, 159, 153–161. [Google Scholar] [CrossRef]
- Tomonaga, Y.; Ten Haaf, K.; Frauenfelder, T.; Kohler, M.; Kouyos, R.D.; Shilaih, M.; Lorez, M.; de Koning, H.J.; Schwenkglenks, M.; Puhan, M.A. Cost-effectiveness of low-dose CT screening for lung cancer in a European country with high prevalence of smoking—A modelling study. Lung Cancer 2018, 121, 61–69. [Google Scholar] [CrossRef]
- Young, B.; Vedhara, K.; Kendrick, D.; Littleford, R.; Robertson, J.F.R.; Sullivan, F.M.; Schembri, S.; das Nari, R.; Collaboration with the ECLS Study Team. Determinants of motivation to quit in smokers screened for the early detection of lung cancer: A qualitative study. BMC Public Health 2018, 18, 1276. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.E.; Young, B.; Bedford, L.E.; das Nair, R.; Robertson, J.F.R.; Vedhara, K.; Sullivan, F.; Mair, F.S.; Schembri, S.; Littleford, R.C.; et al. Lung cancer screening: Does pulmonary nodule detection affect a range of smoking behaviours? J. Public Health 2019, 41, 600–608. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).