Outcome of an Accelerated Treatment Algorithm for Patients Developing Diarrhea as a Complication of Ipilimumab-Based Cancer Immunotherapy in a Community Practice
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Collection
2.2. Clinical Characteristics and Outcomes of Patients
2.3. Treatment Regimens
2.4. Accelerated Diarrhea Treatment Schema
2.5. Statistical Analysis
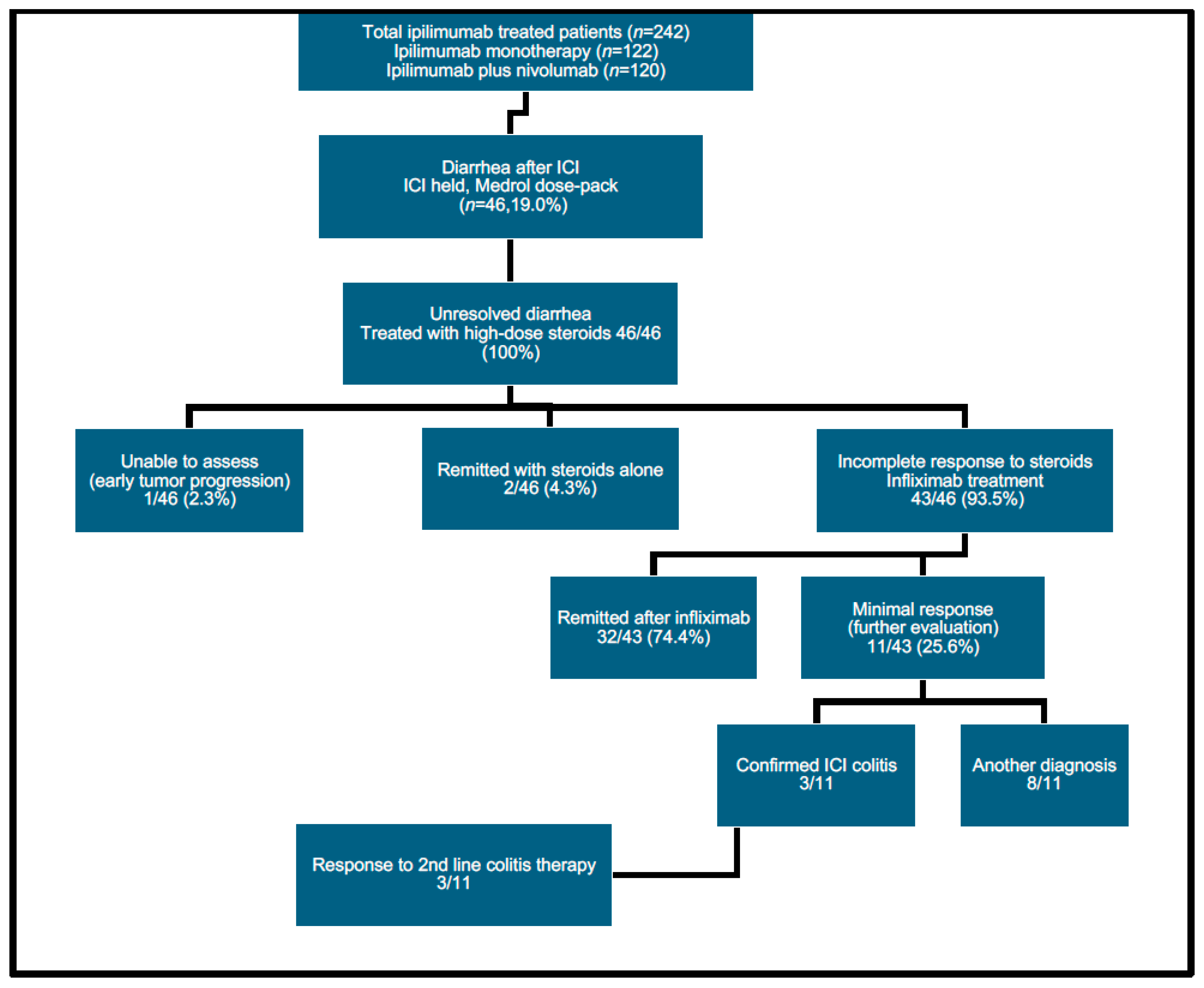
3. Results
3.1. Patient Characteristics
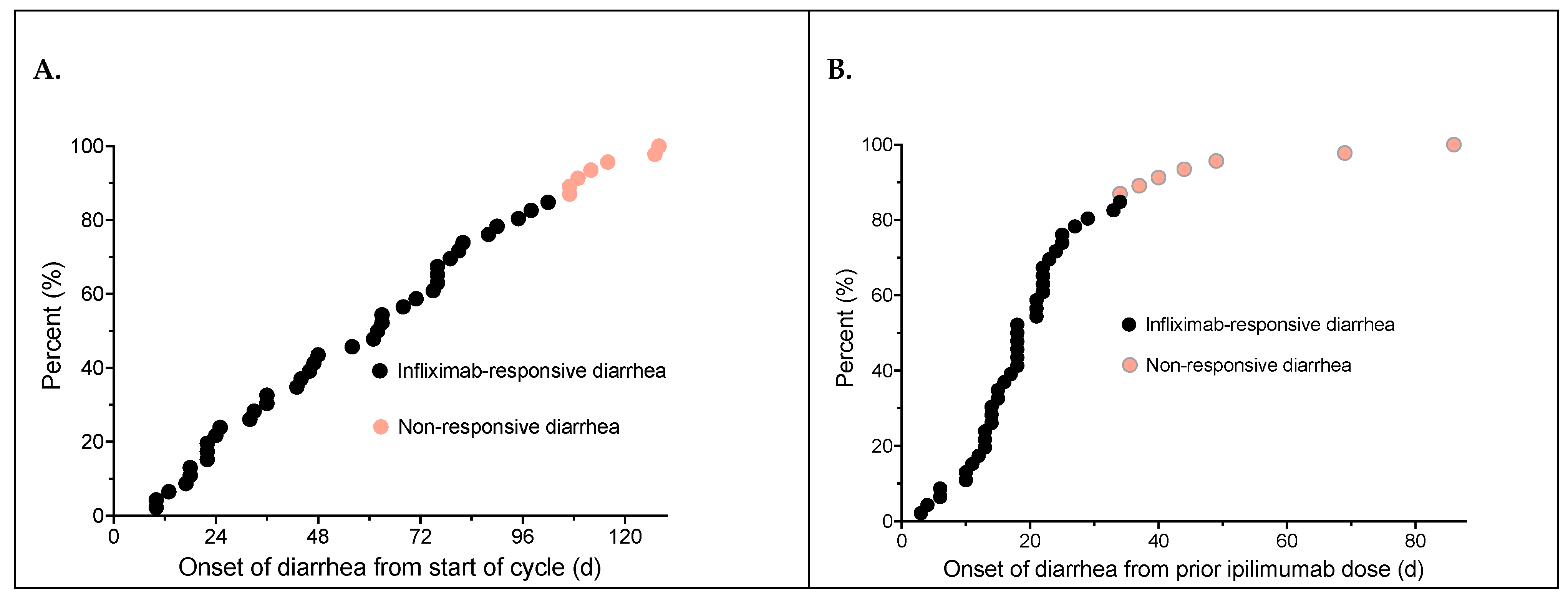
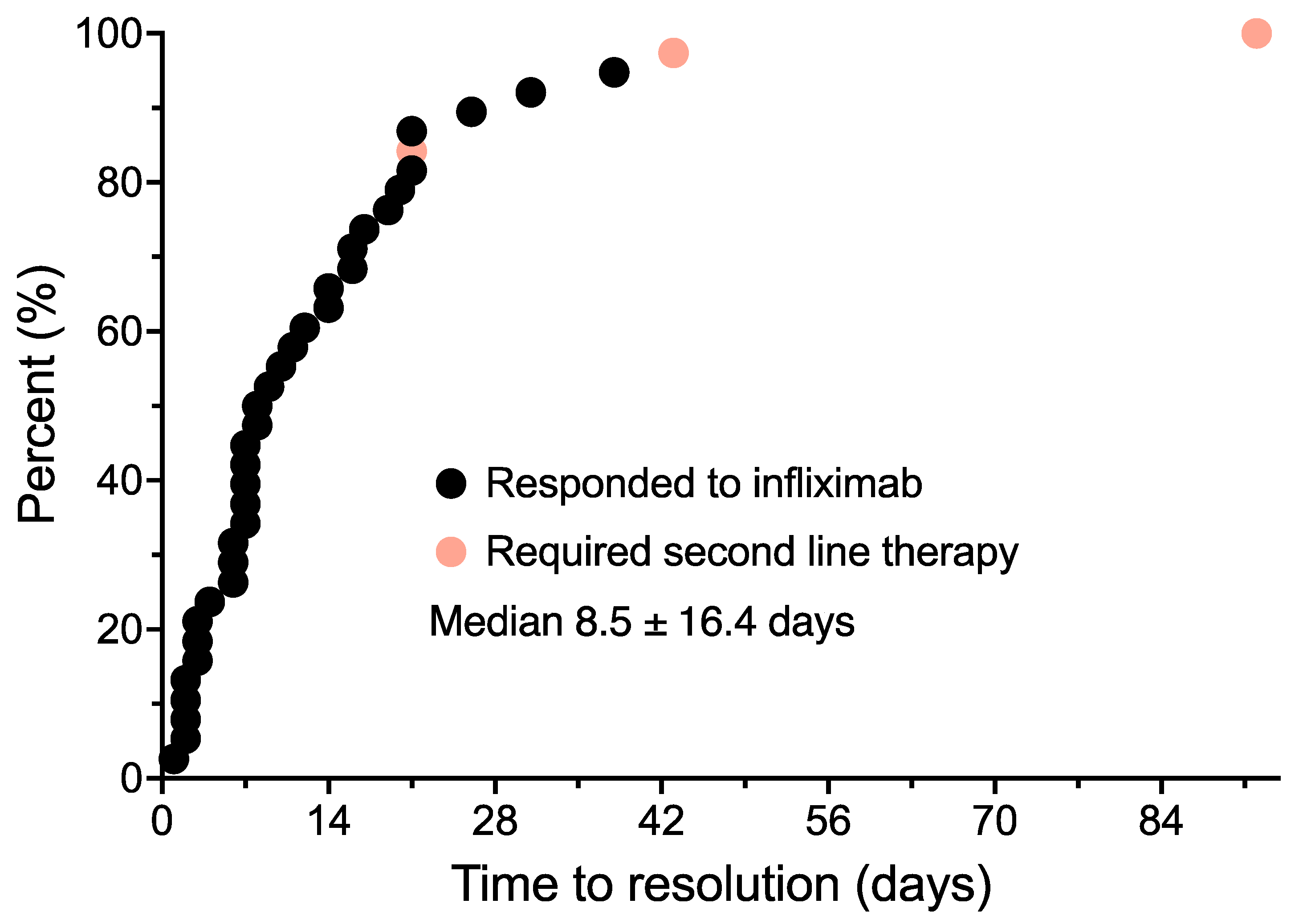
3.2. Diarrhea Onset and Treatment
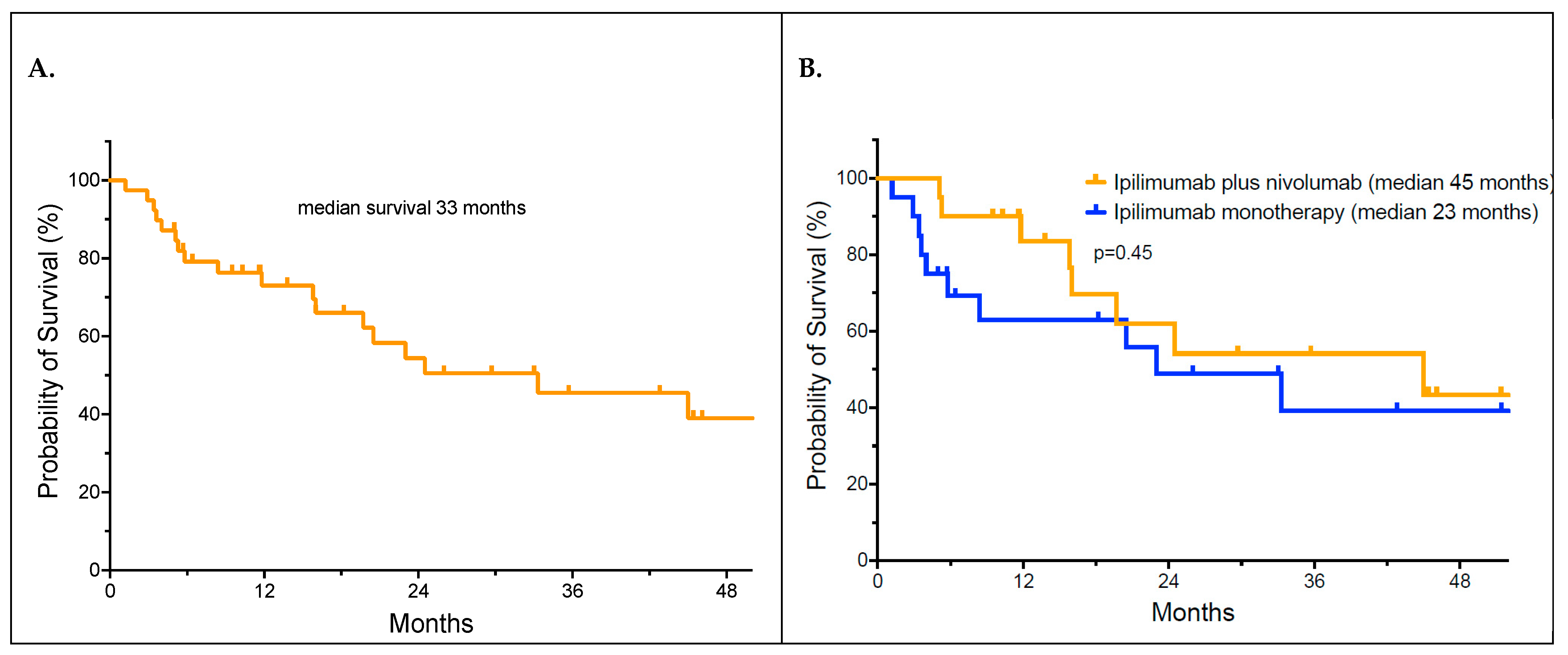
3.3. Outcomes and Analysis of Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Middleton, M.R.; Grob, J.J.; Aaronson, N.; Fierlbeck, G.; Tilgen, W.; Seiter, S.; Gore, M.; Aamdal, S.; Cebon, J.; Coates, A.; et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J. Clin. Oncol. 2000, 18, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Korn, E.L.; Liu, P.Y.; Lee, S.J.; Chapman, J.A.; Niedzwiecki, D.; Suman, V.J.; Moon, J.; Sondak, V.K.; Atkins, M.B.; Eisenhauer, E.A.; et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J. Clin. Oncol. 2008, 26, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; Hodi, F.S.; Robert, C.; Weber, J.S.; Margolin, K.; Hamid, O.; Patt, D.; Chen, T.T.; Berman, D.M.; Wolchok, J.D. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J. Clin. Oncol. 2015, 33, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.W.; Weber, J.S.; et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 2013, 369, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014, 32, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. 2021, 40, 127–137. [Google Scholar] [CrossRef]
- Atkins, M.B.; Lee, S.J.; Chmielowski, B.; Tarhini, A.A.; Cohen, G.I.; Truong, T.G.; Moon, H.H.; Davar, D.; O'Rourke, M.; Stephenson, J.J.; et al. Combination Dabrafenib and Trametinib Versus Combination Nivolumab and Ipilimumab for Patients with Advanced BRAF-Mutant Melanoma: The DREAMseq Trial-ECOG-ACRIN EA6134. J. Clin. Oncol. 2022, 41, 186–197. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Chaudhary, N.; Garg, M.; Floudas, C.S.; Soni, P.; Chandra, A.B. Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front. Pharmacol. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Achufusi, A.; Armand, P.; Berkenstock, M.K.; Bhatia, S.; Budde, L.E.; Chokshi, S.; Davies, M.; et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Dougan, M. Checkpoint Blockade Toxicity and Immune Homeostasis in the Gastrointestinal Tract. Front. Immunol. 2017, 8, 1547. [Google Scholar] [CrossRef]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Asher, N.; Ben-Betzalel, G.; Lev-Ari, S.; Shapira-Frommer, R.; Steinberg-Silman, Y.; Gochman, N.; Schachter, J.; Meirson, T.; Markel, G. Real World Outcomes of Ipilimumab and Nivolumab in Patients with Metastatic Melanoma. Cancers 2020, 12, 2329. [Google Scholar] [CrossRef]
- Singh, B.P.; Marshall, J.L.; He, A.R. Workup and Management of Immune-Mediated Colitis in Patients Treated with Immune Checkpoint Inhibitors. Oncologist 2020, 25, 197–202. [Google Scholar] [CrossRef]
- O'Day, S.J.; Maio, M.; Chiarion-Sileni, V.; Gajewski, T.F.; Pehamberger, H.; Bondarenko, I.N.; Queirolo, P.; Lundgren, L.; Mikhailov, S.; Roman, L.; et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: A multicenter single-arm phase II study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol./ESMO 2010, 21, 1712–1717. [Google Scholar] [CrossRef]
- Mitchell, K.A.; Kluger, H.; Sznol, M.; Hartman, D.J. Ipilimumab-induced perforating colitis. J. Clin. Gastroenterol. 2013, 47, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Witt, D.; Asif, T.; Mir, F.F. Ipilimumab as a Cause of Severe Pan-Colitis and Colonic Perforation. Cureus 2017, 9, e1182. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.W.; Shillington, A.C.; Lee, T.A.; Macahilig, C.P.; Diede, S.J.; Dave, V.; Harshaw, Q.; Scherrer, E.; Liu, F.X. Hospitalization and emergency department utilization in patients with advanced melanoma receiving pembrolizumab versus ipilimumab plus nivolumab in US academic centers. J. Med. Econ. 2020, 23, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Som, A.; Mandaliya, R.; Alsaadi, D.; Farshidpour, M.; Charabaty, A.; Malhotra, N.; Mattar, M.C. Immune checkpoint inhibitor-induced colitis: A comprehensive review. World J. Clin. Cases 2019, 7, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services; National Institutes of Health; National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. 2017; pp. 1–155. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 12 June 2024).
- Lebbe, C.; Meyer, N.; Mortier, L.; Marquez-Rodas, I.; Robert, C.; Rutkowski, P.; Menzies, A.M.; Eigentler, T.; Ascierto, P.A.; Smylie, M.; et al. Evaluation of Two Dosing Regimens for Nivolumab in Combination with Ipilimumab in Patients with Advanced Melanoma: Results from the Phase IIIb/IV CheckMate 511 Trial. J. Clin. Oncol. 2019, 37, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Hilts, A.; Samlowski, W. Cautious Addition of MEK Inhibitors to PD-1 Antibody Treatment in Patients with NRAS or NF1 Mutant Metastatic Melanoma Failing Initial Immunotherapy. Ann. Case Rep. 2022, 7, 795–805. [Google Scholar]
- Samlowski, W.; Adajar, C. Cautious addition of targeted therapy to PD-1 inhibitors after initial progression of BRAF mutant metastatic melanoma on checkpoint inhibitor therapy. BMC Cancer 2021, 21, 1187. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. The logrank test. BMJ 2004, 328, 1073. [Google Scholar] [CrossRef]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Abu Zaid, M.; Achufusi, A.; Armand, P.; Bermas, B.; Braaten, T.; Budde, L.E.; Chokshi, S.; et al. Management of Immunotherapy-Related Toxicities, Version 1.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2024, 20, 387–405. [Google Scholar]
- Dougan, M.; Wang, Y.; Rubio-Tapia, A.; Lim, J.K. AGA Clinical Practice Update on Diagnosis and Management of Immune Checkpoint Inhibitor Colitis and Hepatitis: Expert Review. Gastroenterology 2021, 160, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, G.; Angelillo, D.; Favia, N.; Sergi, M.C.; Di Leo, A.; Triggiano, G.; Tucci, M. Checkpoint Inhibitor-Induced Colitis: An Update. Biomedicines 2023, 11, 1496. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wang, J.; Lin, N.; Zhou, Y.; He, W.; Liu, J.; Ma, X. Immune Checkpoint Inhibitor-Associated Colitis: From Mechanism to Management. Front. Immunol. 2021, 12, 800879. [Google Scholar] [CrossRef] [PubMed]
- Badran, Y.R.; Cohen, J.V.; Brastianos, P.K.; Parikh, A.R.; Hong, T.S.; Dougan, M. Concurrent therapy with immune checkpoint inhibitors and TNFalpha blockade in patients with gastrointestinal immune-related adverse events. J. Immunother. Cancer 2019, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- Shoushtari, A.N.; Friedman, C.F.; Navid-Azarbaijani, P.; Postow, M.A.; Callahan, M.K.; Momtaz, P.; Panageas, K.S.; Wolchok, J.D.; Chapman, P.B. Measuring Toxic Effects and Time to Treatment Failure for Nivolumab Plus Ipilimumab in Melanoma. JAMA Oncol. 2018, 4, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Panning, A.; Samlowski, W.; Allred, G. Lack of Influence of Non-Overlapping Mutations in BRAF, NRAS, or NF1 on 12-Month Best Objective Response and Long-Term Survival after Checkpoint Inhibitor-Based Treatment for Metastatic Melanoma. Cancers 2023, 15, 3527. [Google Scholar] [CrossRef] [PubMed]
- Hussaini, S.; Chehade, R.; Boldt, R.G.; Raphael, J.; Blanchette, P.; Maleki Vareki, S.; Fernandes, R. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors—A systematic review and meta-analysis. Cancer Treat. Rev. 2021, 92, 102134. [Google Scholar] [CrossRef]

| UPN | Age | Sex | Primary Site | Stage | Regimen | Comorbidities | Potential Contributory Medications | ICI Doses Prior to Colitis | Time from First ICI Dose (d) | Time from Preceding ICI Dose (d) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | F | IVC | I + N | None | 4 | 109 | 25 | ||
| 2 | 56 | M | Axilla | IVA | I + N | HTN, hypercholesteremia | 2 | 43 | 22 | |
| 3 | 74 | F | Neck | IVB | I + N | Arthritis, back pain, bronchitis/asthma, diabetes, HTN, hypercholesterolemia, GERD, chronic pain syndrome | 4 | 76 | 13 | |
| 4 | 63 | M | Scalp | III—adjuvant; IVB | I | Low-back pain, SCC on left upper chest | 1 * | 22 | 22 | |
| 5 | 75 | F | Scalp | IVC | I | HTN, GERD | 3 | 62 | 12 | |
| 6 | 65 | M | Mid-back | IVC | I + N | Sleep apnea, pulmonary embolism, DVT | 2 | 25 | 4 | |
| 7 | 75 | M | Extr | IVA | I | HTN, hypercholesteremia, COPD, atrial fibrillation, spinal spondylosis, history of prostate cancer | 4 | 112 | 49 | |
| 8 | 85 | M | Scalp | IVB | I + N | Atopic dermatitis, hypotensive heart disease, CKD, COPD, CAD, diabetes, diabetic neuropathy, duodenal ulcer, esophageal reflux, HLD, HTN, early Alzheimer’s, metabolic syndrome, peripheral neuropathy, PVD, degenerative joint disease | 4 | 95 | 18 | |
| 9 | 66 | M | RCC | IV | I + N | Hypothyroidism, HTN, GERD | 3 | 63 | 21 | |
| 10 | 63 | M | Extr | IV | I + N | HTN | 1 | 22 | 22 | |
| 11 | 49 | M | Trunk | IVC | I | Inflammatory arthritis | D | 4 | 107 | 44 |
| 12 | 59 | M | Trunk | IIB—adjuvant | I | History of testicular cancer, HTN, hypercholesterolemia, chronic back pain, arthritis | 4 | 128 | 37 | |
| 13 | 61 | M | Neck | IVB | I | Diabetes (Type II), arthritis, HTN | 1 | 17 | 17 | |
| 14 | 71 | M | RCC | IV | I + N | Hypothyroidism, HTN | 2 | 44 | 23 | |
| 15 | 80 | F | Vulva | IIIC (unresectable) | I | HTN, thyroid nodules, hypercholesterolemia, arthritis | 1 | 18 | 18 | |
| 16 | 51 | F | Extr | IVC | I | None | 1 | 24 | 24 | |
| 17 | 64 | M | Face | IIIC (adjuvant) | I | Back pain, CAD, hypercholesteremia, BPH | 1 | 18 | 18 | |
| 18 | 80 | M | Trunk | IVC | I | Hypercholesterolemia, GERD, CAD, peptic ulcer disease | 2 | 36 | 15 | |
| 19 | 52 | M | Trunk | IVA | I + N | Arthritis | 4 ** | 127 | 40 | |
| 20 | 70 | M | Ear | IVC | I | HTN, myocardial infarction hypercholesterolemia, history of prostate cancer, history of chronic lymphocytic leukemia | 2 | 33 | 13 | |
| 21 | 76 | M | Face | IVB | I | HTN, diabetes, myocardial infarction, atrial fibrillation, actinic keratoses, history of squamous cell/basal cell carcinoma, benign bowel tumor | 4 | 116 | 34 | |
| 22 | 67 | M | Sinonasal | IVC | I | BPH, HTN | 2 | 90 | 69 | |
| 23 | 49 | F | Vulva | IVA | I + N | Endometriosis, depression | 2 | 46 | 18 | |
| 24 | 73 | F | Extr | IVC | I + N | HTN, hypothyroidism, restless leg syndrome, cyst disease in bilateral breasts | 4 | 88 | 21 | |
| 25 | 56 | M | Extr | IVD | I | Unilateral kidney | 6 * | 47 | 14 | |
| 26 | 62 | F | Rectal | IVC | I + N | None | 4 | 98 | 14 | |
| 27 | 60 | F | Unknown | IV | I | Hypothyroidism, hypercholesteremia, DVT, pulmonary embolism, uterine ablation, tubal ligation, scalp cyst excision, reversible posterior leukoencephalopathy | 3 | 75 | 33 | |
| 28 | 67 | M | Prostate Ca | IVA | I | HTN | S | 1 * | 10 | 16 |
| 29 | 85 | F | Extr | IVC | I + N | HTN, atrial fibrillation, cataracts, emphysema, Parkinsonism, pulmonary HTN, mitral regurgitation | 3 | 63 | 2 | |
| 30 | 62 | F | Unknown | IV | I + N | None | 3 * | 48 | 6 | |
| 31 | 61 | F | Extr | IV | I + N | HTN, asthma/chronic bronchitis | 3 | 82 | 9 | |
| 32 | 25 | M | Trunk | I + N | None | 4 | 76 | 6 | ||
| 33 | 55 | M | Extr | IIIC—unresectable | I | Myocardial infarction, HTN, history of acute renal failure | S | 4 | 102 | 7 |
| 34 | 43 | M | Extr | IVB | I | Chronic bladder inflammation, glaucoma | D, T | 4 | 81 | 4 |
| 35 | 42 | M | Unknown | IV | I + N | HTN, GERD, chronic back pain, chronic ITP | 4 | 68 | 21 | |
| 36 | 66 | F | Extr | IVC | I | Morbid obesity, HTN, history of rheumatic fever, arthritis, endometrial hyperplasia | 3 | 56 | 2 | |
| 37 | 65 | M | Trunk | IV | I + N | Depression, rheumatoid arthritis, low-back pain, HTN, hyperlipidemia | 1 | 10 | 10 | |
| 38 | 66 | M | Scalp | IVC | I + N | History of prostate cancer | 4 | 79 | 8 | |
| 39 | 52 | F | Unknown | IV | I | History of breast cancer | D, T | 2 | 36 | 15 |
| 40 | 57 | F | Extr | IV | I + N | None | N/A | N/A | N/A | |
| 41 | 65 | F | Left groin | IVB | I | HTN, hypercholesterolemia, hypothyroidism, chronic left leg edema, small pulmonary nodules | N/A | N/A | N/A | |
| 42 | 61 | M | Extr | IVB | I | Degenerative back problems, HTN, BPH, hypothyroidism, hypercholesteremia | N/A | N/A | N/A | |
| 43 | 58 | F | Ear | IV | I + N | Chemical hyperthyroidism | N/A | N/A | N/A | |
| 44 | 60 | M | Scalp | IVA | I | Asthma, depression, arthritis, BPH | N/A | N/A | N/A | |
| 45 | 44 | M | Extr | IIB | I | Anxiety | N/A | N/A | N/A | |
| 46 | 64 | F | Sinonasal | IVC | I | Degenerative joint disease, HTN, asthma | N/A | N/A | N/A |
| UPN | Infliximab Doses | Time to Resolution (d) | Additional ICI Doses Post-Colitis | Hospitalization | Additional Treatment | OS (mo) | Current Status |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 7 | 0 | 5.1 | DOD | ||
| 2 | 1 | 9 | Entyvio | 45.4 | NED | ||
| 3 | 1 | 6 | 10 | 10.3 | DOD | ||
| 4 * | 1 | 8 | 1 | Diarrhea | 11.8 | DOD | |
| 5 | 3 | 0 | 2.9 | DOD | |||
| 6 | 1 | 3 | Diarrhea; diverticulitis | Entyvio | 9.5 | NED | |
| 7 | 1 | 14 | 0 * | Metabolic encephalopathy; diarrhea | 26.0 | NED | |
| 8 | 1 | 2 | 2 | 5.3 | Died—other | ||
| 9 | 1 | 2 | 0 | 13.8 | NED | ||
| 10 | * | 2 | 51.4 | NED | |||
| 11 | 1 | 7 * | 0 * | 4.0 | DOD | ||
| 12 | 1 | 19 | 0 * | Infliximab * | 20.5 | Died—other | |
| 13 | 1 | 2 | 2 | 23.0 | DOD | ||
| 14 | 1 | 7 | 33 | 35.7 | NED | ||
| 15 | 1 | 2 | 0 | Infliximab * | 5.0 | DOD | |
| 16 | 1 | 17 | 3 | 42.8 | NED | ||
| 17 | 2 | 14 | 0 | 3.6 | DOD | ||
| 18 | 1 | 3 | 0 | Dehydration, infliximab * | 5.7 | DOD | |
| 19 | 1 | 14 | 2 | Colitis (atypical presentation) | 11.6 | DOD | |
| 20 | 1 | 12 | 0 | 3.4 | DOD | ||
| 21 | 1 | 1 | 1 | Colitis (atypical presentation) | 8.4 | Died—other | |
| 22 | 1 | 7 * | 2 | 5.8 | DOD | ||
| 23 | 1 | 7 * | 5 | 16.0 | DOD | ||
| 24 | 2 | 2 | 5 | 46.1 | NED | ||
| 25 * | 3 | 14 | 2 | 33.3 | DOD | ||
| 26 | 2 | 14 | 5 | Colitis (delayed management) | 16.0 | DOD | |
| 27 | 1 | 33 | 1 | 91.7 | NED | ||
| 28 * | 1 | 16 | 1 | 57.0 | DOD | ||
| 29 | 1 | 2 | 1 | 15.8 | DOD | ||
| 30 * | * | 4 | 11.7 | DOD | |||
| 31 | 1 | 9 | 1 | Colitis, hypopituitarism | 19.7 | DOD | |
| 32 | 1 | 6 | 9 | 29.7 | NED | ||
| 33 | 1 | 7 | 0 * | 33.0 | NED | ||
| 34 | 1 | 4 | 0 * | 18.2 | NED | ||
| 35 | 1 | 21 | 6 | 52.8 | NED | ||
| 36 | 1 | 2 | 1 | 6.4 | DOD | ||
| 37 | 1 | 1 | Entyvio | 24.5 | NED | ||
| 38 | 1 | 8 | 12 | 45.0 | NED | ||
| 39 | 1 | 3 | 0 | Progressive melanoma | 1.2 | DOD | |
| 40 | 2 | 0 | Targeted therapy held | 6.2 | NED | ||
| 41 | 2 | 2 | Etiology unclear | 7.1 | DOD | ||
| 42 | 1 | 0 | Ischemic colitis | 5.7 | DOD | ||
| 43 | 4 | 2 | C. diff colitis | 25.0 | NED | ||
| 44 | 1 | 1 | Lymphocytic colitis | 88.9 | NED | ||
| 45 | 2 | 0 | C. diff colitis | 53.5 | NED | ||
| 46 | 2 | 5 | C. diff colitis | 41.4 | NED |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, C.; Samlowski, W. Outcome of an Accelerated Treatment Algorithm for Patients Developing Diarrhea as a Complication of Ipilimumab-Based Cancer Immunotherapy in a Community Practice. Curr. Oncol. 2024, 31, 3529-3545. https://doi.org/10.3390/curroncol31060260
Ho C, Samlowski W. Outcome of an Accelerated Treatment Algorithm for Patients Developing Diarrhea as a Complication of Ipilimumab-Based Cancer Immunotherapy in a Community Practice. Current Oncology. 2024; 31(6):3529-3545. https://doi.org/10.3390/curroncol31060260
Chicago/Turabian StyleHo, Clarice, and Wolfram Samlowski. 2024. "Outcome of an Accelerated Treatment Algorithm for Patients Developing Diarrhea as a Complication of Ipilimumab-Based Cancer Immunotherapy in a Community Practice" Current Oncology 31, no. 6: 3529-3545. https://doi.org/10.3390/curroncol31060260
APA StyleHo, C., & Samlowski, W. (2024). Outcome of an Accelerated Treatment Algorithm for Patients Developing Diarrhea as a Complication of Ipilimumab-Based Cancer Immunotherapy in a Community Practice. Current Oncology, 31(6), 3529-3545. https://doi.org/10.3390/curroncol31060260





