Discrepancy in the Location of Prostate Cancer Indicated on Biparametric Magnetic Resonance Imaging and Pathologically Diagnosed Using Surgical Specimens
Abstract
1. Introduction
2. Materials and Methods
2.1. Enrolled Patients
2.2. bpMRI
2.3. Protocol for Prostate Biopsy
2.4. Tumor Staging and the Assessment of Biopsy and Surgical Specimens
2.5. Statistical Analysis
3. Results
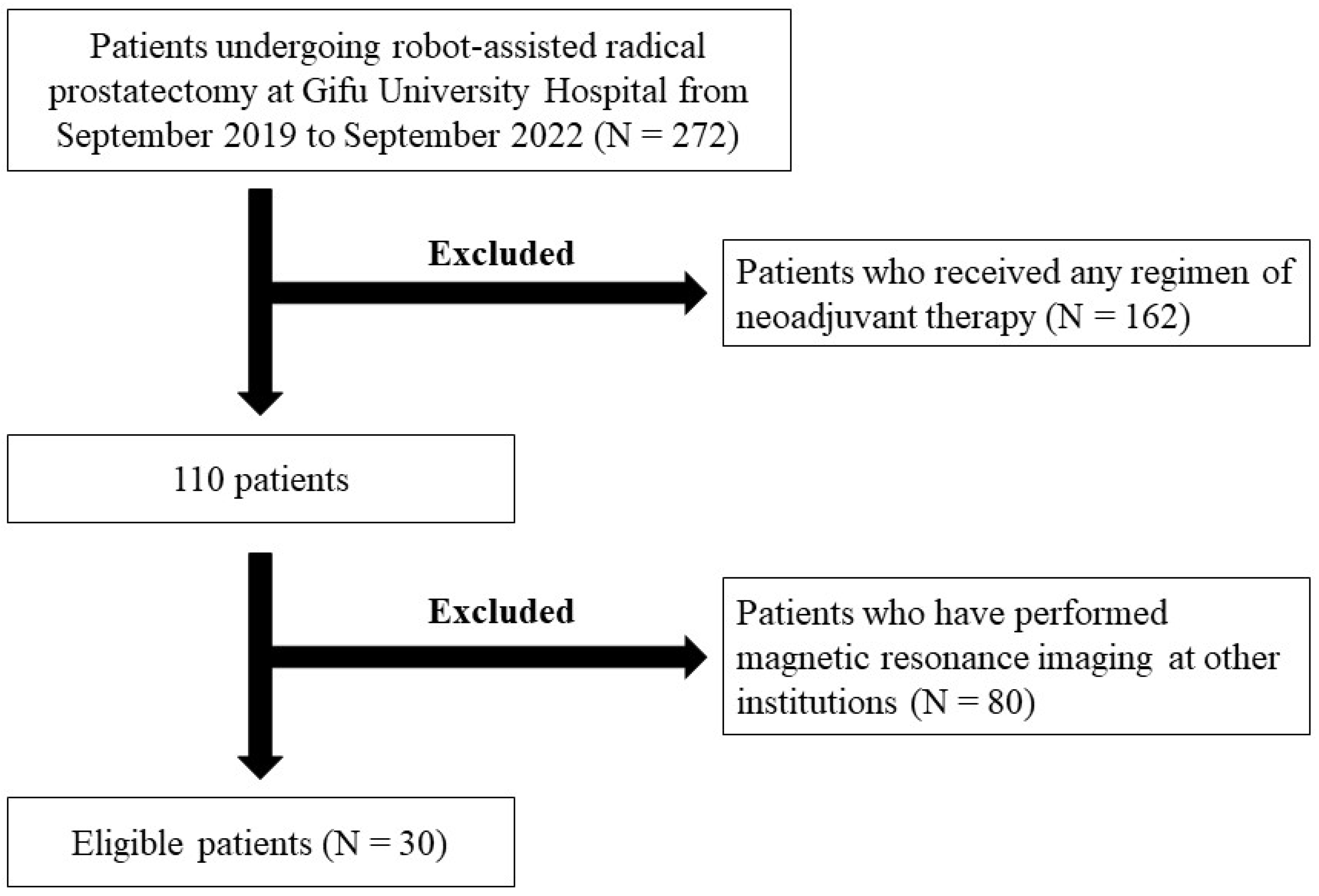
Selection of Eligible Patients and Patients’ Backgrounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berenguer, C.V.; Pereira, F.; Câmara, J.S.; Pereira, J.A.M. Underlying Features of Prostate Cancer-Statistics, Risk Factors, and Emerging Methods for Its Diagnosis. Curr. Oncol. 2023, 30, 2300–2321. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Djulbegovic, M.; Jung, J.H.; Hwang, E.C.; Zhou, Q.; Cleves, A.; Agoritsas, T.; Dahm, P. Prostate cancer screening with prostate-specific antigen (PSA) test: A systematic review and meta-analysis. BMJ 2018, 362, k3519. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Rajesh, A.; Rosenkrantz, A.B.; Choyke, P.L.; Turkbey, B. PI-RADS version 2.1: One small step for prostate MRI. Clin. Radiol. 2019, 74, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Mohler, J.L.; Antonarakis, E.S. NCCN Guidelines Updates: Management of Prostate Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 583–586. [Google Scholar]
- Prostate Cancer (2022) NCCN Guidelines®. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 27 February 2024).
- Sanda, M.G.; Cadeddu, J.A.; Kirkby, E.; Chen, R.C.; Crispino, T.; Fontanarosa, J.; Freedland, S.J.; Greene, K.; Klotz, L.H.; Makarov, D.V.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part II: Recommended Approaches and Details of Specific Care Options. J. Urol. 2018, 199, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Danneman, D.; Drevin, L.; Delahunt, B.; Samaratunga, H.; Robinson, D.; Bratt, O.; Loeb, S.; Stattin, P.; Egevad, L. Accuracy of prostate biopsies for predicting Gleason score in radical prostatectomy specimens: Nationwide trends 2000–2012. BJU Int. 2017, 119, 50–56. [Google Scholar] [CrossRef]
- Carroll, P.H.; Mohler, J.L. NCCN Guidelines Updates: Prostate Cancer and Prostate Cancer Early Detection. J. Natl. Compr. Cancer Netw. 2018, 16, 620–623. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Kato, D.; Ozawa, K.; Takeuchi, S.; Kawase, M.; Kawase, K.; Nakai, C.; Takai, M.; Iinuma, K.; Nakane, K.; Kato, H.; et al. The Utility of Combined Target and Systematic Prostate Biopsies in the Diagnosis of Clinically Significant Prostate Cancer Using Prostate Imaging Reporting and Data System Version 2 Based on Biparametric Magnetic Resonance Imaging. Curr. Oncol. 2021, 287, 1294–1301. [Google Scholar] [CrossRef]
- Uno, H.; Taniguchi, T.; Seike, K.; Kato, D.; Takai, M.; Iinuma, K.; Horie, K.; Nakane, K.; Koie, T. The accuracy of prostate cancer diagnosis in biopsy-naive patients using combined magnetic resonance imaging and transrectal ultrasound fusion-targeted prostate biopsy. Transl. Androl. Urol. 2021, 10, 2982–2989. [Google Scholar] [CrossRef]
- Caglic, I.; Sushentsev, N.; Shah, N.; Warren, A.Y.; Lamb, B.W.; Barrett, T. Comparison of biparametric versus multiparametric prostate MRI for the detection of extracapsular extension and seminal vesicle invasion in biopsy naïve patients. Eur. J. Radiol. 2021, 141, 109804. [Google Scholar] [CrossRef] [PubMed]
- Quentin, M.; Boschheidgen, M.; Radtke, J.P.; Spohn, F.; Ullrich, T.; Drewes, L.; Valentin, B.; Lakes, J.; Al-Monajjed; Arsov, C.; et al. MRI in-bore biopsy following MRI/US fusion-guided biopsy in patients with persistent suspicion of clinically significant prostate cancer. Eur. J. Radiol. 2024, 175, 111436. [Google Scholar] [CrossRef] [PubMed]
- Kato, D.; Namiki, S.; Ueda, S.; Takeuchi, Y.; Takeuchi, S.; Kawase, M.; Kawase, K.; Nakai, C.; Takai, M.; Iinuma, K.; et al. Validation of standardized training system for robot-assisted radical prostatectomy: Comparison of perioperative and surgical outcomes between experienced surgeons and novice surgeons at a low-volume institute in Japan. Minim. Invasive Ther. Allied Technol. 2022, 31, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Namiki, S.; Kawase, M.; Ebara, S.; Tatenuma, T.; Sasaki, T.; Ikehata, Y.; Nakayama, A.; Toide, M.; Yoneda, T.; Sakaguchi, K.; et al. Pelvic Lymphadenectomy May Not Improve Biochemical Recurrence-Free Survival in Patients with Prostate Cancer Treated with Robot-Assisted Radical Prostatectomy in Japan (The MSUG94 Group). Cancers 2022, 14, 5803. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.; Humphrey, P.A.; Grading Committee. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Buyyounouski, M.K.; Choyke, P.L.; McKenney, J.K.; Sartor, O.; Sandler, H.M.; Amin, M.B.; Kattan, M.W.; Lin, D.W. Prostate cancer—Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Washino, S.; Okochi, T.; Saito, K.; Konishi, T.; Hirai, M.; Kobayashi, Y.; Miyagawa, T. Combination of prostate imaging reporting and data system (PI-RADS) score and prostate-specific antigen (PSA) density predicts biopsy outcome in prostate biopsy naïve patients. BJU Int. 2016, 119, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Hosseiny, M.; Shakeri, S.; Felker, E.R.; Lu, D.; Sayre, J.; Ahuja, P.; Raman, S.S. 3-T Multiparametric MRI Followed by In-Bore MR-Guided Biopsy for Detecting Clinically Significant Prostate Cancer After Prior Negative Transrectal Ultrasound-Guided Biopsy. AJR Am. J. Roentgenol. 2020, 215, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Perrin, A.; Venderink, W.; Patak, M.A.; Möckel, C.; Fehr, J.L.; Jichlinski, P.; Porcellini, B.; Lucca, I.; Futterer, J.; Valerio, M. The utility of in-bore multiparametric magnetic resonance-guided biopsy in men with negative multiparametric magnetic resonance-ultrasound software-based fusion targeted biopsy. Urol. Oncol. 2021, 39, 297.e9–297.e16. [Google Scholar] [CrossRef]
- Gillessen, S.; Bossi, A.; Davis, I.D.; de Bono, J.; Fizazi, K.; James, N.D.; Mottet, N.; Shore, N.; Small, E.; Smith, M.; et al. Management of Patients with Advanced Prostate Cancer. Part I: Intermediate-/High-risk and Locally Advanced Disease, Biochemical Relapse, and Side Effects of Hormonal Treatment: Report of the Advanced Prostate Cancer Consensus Conference 2022. Eur. Urol. 2023, 83, 267–293. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Uehara, S.; Yoshida, S.; Tanaka, H.; Tanaka, H.; Kijima, T.; Yokoyama, M.; Ishioka, J.; Saito, K.; Fujii, Y. Value of extra-target prostate biopsy for the detection of magnetic resonance imaging-missed adverse pathology according to the Prostate Imaging Reporting and Data System scores: Spatial analysis using magnetic resonance-ultrasound fusion images. Int. J. Urol. 2020, 27, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.U.; El-Shater Bosaily, A.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef]
- Günzel, K.; Magheli, A.; Busch, J.; Baco, E.; Cash, H.; Heinrich, S.; Edler, D.; Schostak, M.; Borgmann, H.; Schlegel, J.; et al. Evaluation of systematic prostate biopsies when performing transperineal MRI/TRUS fusion biopsy with needle tracking-what is the additional value? Int. Urol. Nephrol. 2022, 54, 2477–2483. [Google Scholar] [CrossRef]
- Johnson, D.C.; Reiter, R.E. Focal Therapy Should Not Be Considered for Men with Gleason Grade Group 3-5 Prostate Cancer. Eur. Urol. Focus 2020, 6, 203–204. [Google Scholar] [CrossRef]
- Klingebiel, M.; Arsov, C.; Ullrich, T.; Quentin, M.; Al-Monajjed, R.; Mally, D.; Sawicki, L.M.; Hiester, A.; Esposito, I.; Albers, P.; et al. Reasons for missing clinically significant prostate cancer by targeted magnetic resonance imaging/ultrasound fusion-guided biopsy. Eur. J. Radiol. 2021, 137, 109587. [Google Scholar] [CrossRef] [PubMed]
- Noh, T.I.; Shim, J.S.; Kang, S.G.; Cheon, J.; Lee, J.G.; Lee, J.H.; Kang, S.H. Concordance between biparametric MRI, transperineal targeted plus systematic MRI-ultrasound fusion prostate biopsy, and radical prostatectomy pathology. Sci. Rep. 2022, 12, 6964. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Farolfi, A.; Fanti, S.; Iagaru, A. Prostate cancer: Molecular imaging and MRI. Eur. J. Radiol. 2021, 143, 109893. [Google Scholar] [CrossRef]
- Mena, E.; Black, P.C.; Rais-Bahrami, S.; Gorin, M.; Allaf, M.; Choyke, P. Novel PET imaging methods for prostate cancer. World J. Urol. 2021, 39, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Tourinho-Barbosa, R.R.; de la Rosette, J.; Sanchez-Salas, R. Prostate cancer multifocality, the index lesion, and the microenvironment. Curr. Opin. Urol. 2018, 28, 499–505. [Google Scholar] [CrossRef]
- Kam, J.; Yuminaga, Y.; Krelle, M.; Gavin, D.; Koschel, S.; Aluwihare, K.; Sutherland, T.; Skinner, S.; Brennan, J.; Wong, L.M.; et al. Evaluation of the accuracy of multiparametric MRI for predicting prostate cancer pathology and tumour staging in the real world: An multicentre study. BJU Int. 2019, 124, 297–301. [Google Scholar] [CrossRef]
- Truong, M.; Hollenberg, G.; Weinberg, E.; Messing, E.M.; Miyamoto, H.; Frye, T.P. Impact of Gleason Subtype on Prostate Cancer Detection Using Multiparametric Magnetic Resonance Imaging: Correlation with Final Histopathology. J. Urol. 2017, 198, 316–321. [Google Scholar] [CrossRef]
- van Houdt, P.J.; Ghobadi, G.; Schoots, I.G.; Heijmink, S.W.T.P.J.; de Jong, J.; van der Poel, H.G.; Pos, F.J.; Rylander, S.; Bentzen, L.; Haustermans, K.; et al. Histopathological Features of MRI-Invisible Regions of Prostate Cancer Lesions. J. Magn. Reson. Imaging 2020, 51, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Junker, D.; Steinkohl, F.; Fritz, V.; Bektic, J.; Tokas, T.; Aigner, F.; Herrmann, T.R.W.; Rieger, M.; Nagele, U. Comparison of multiparametric and biparametric MRI of the prostate: Are gadolinium-based contrast agents needed for routine examinations? World J. Urol. 2019, 37, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Greer, M.D.; Shih, J.H.; Lay, N.; Barrett, T.; Kayat Bittencourt, L.; Borofsky, S.; Kabakus, I.M.; Law, Y.M.; Marko, J.; Shebel, H.; et al. Validation of the Dominant Sequence Paradigm and Role of Dynamic Contrast-enhanced Imaging in PI-RADS Version 2. Radiology 2017, 285, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Tamada, T.; Kido, A.; Yamamoto, A.; Takeuchi, M.; Miyaji, Y.; Moriya, T.; Sone, T. Comparison of Biparametric and Multiparametric MRI for Clinically Significant Prostate Cancer Detection with PI-RADS Version 2.1. J. Magn. Reson. Imaging 2021, 53, 283–291. [Google Scholar] [CrossRef]
| Variables | Patients (N = 30) |
|---|---|
| Age, years (median, IQR) | 73 (68–75) |
| Body mass index, kg/m2 (median, IQR) | 23.1 (21.2–24.8) |
| Initial prostate-specific antigen level, ng/mL (median, IQR) | 6.36 (4.73–10.38) |
| Prostate volume, mL (median, IQR) | 31.0 (24.3–43.6) |
| Clinical T stage (number, %) | |
| 1c | 4 (13.3) |
| 2a | 20 (66.7) |
| 2b | 1 (3.3) |
| 2c | 5 (16.7) |
| Biopsy grade group according to ISUP criteria (number, %) | |
| 1 | 7 (23.3) |
| 2 | 14 (46.7) |
| 3 | 8 (26.7) |
| 4 | 0 (0.0) |
| 5 | 1 (3.3) |
| NCCN risk classification (number, %) | |
| Very Low | 1 (3.3) |
| Low | 5 (16.7) |
| Intermediate | 23 (76.7) |
| High | 1 (3.3) |
| Pathological T stage (number, %) | |
| 2a | 7 (23.3) |
| 2b | 2 (6.7) |
| 2c | 16 (53.3) |
| 3a | 3 (10.0) |
| 3b | 2 (6.7) |
| Surgical specimens grade group according to ISUP criteria (number, %) | |
| 1 | 12 (14.1) |
| 2 | 45 (52.9) |
| 3 | 22 (25.9%) |
| 4 | 0 (0.0%) |
| 5 | 6 (7.1%) |
| Follow-up period, months (median, IQR) | 25 (12–30) |
| Variables | Positive Group (n = 42) | Negative Group (n = 43) | p-Value |
|---|---|---|---|
| MRI findings using PI-RADS score version 2.1 (number, %) | <0.001 | ||
| 1 | 1 (2.4) | 20 (46.5) | |
| 2 | 1 (2.4) | 9 (20.9) | |
| 3 | 12 (28.6) | 8 (18.6) | |
| 4 | 26 (61.9) | 6 (14.0) | |
| 5 | 2 (4.8) | 0 (0.0) | |
| Biopsy grade group according to ISUP criteria (number, %) | 0.288 | ||
| 1 | 11 (26.2) | 10 (23.3) | |
| 2 | 20 (47.6) | 18 (41.9) | |
| 3 | 11 (26.2) | 11 (25.6) | |
| 4 | 0 (0.0) | 0 (0.0) | |
| 5 | 0 (0.0) | 4 (9.3) | |
| Surgical specimen grade group according to ISUP criteria (number, %) | 0.112 | ||
| 1 | 6 (14.3) | 6 (14.0) | |
| 2 | 20 (47.6) | 25 (58.1) | |
| 3 | 15 (35.7) | 7 (16.3) | |
| 4 | 0 (0.0) | 0 (0.0) | |
| 5 | 1 (2.4) | 5 (11.6) | |
| Maximum cancer diameter on MRI lesions, mm (median, IQR) | 10.0 (6.50–13.0) | 8.0 (8.0–8.0) | 0.662 |
| Maximum cancer diameter on surgical specimens, mm (median, IQR) | 13.5 (10.0–19.5) | 6.0 (4.0–12.5) | <0.001 |
| Localization of prostate cancer (number, %) | |||
| Ventral side of the prostate | 29 (69.0) | 23 (53.5) | 0.327 |
| Dorsal side of the prostate | 13 (31.0) | 20 (46.5) | 0.139 |
| Apex of the prostate gland | 10 (23.8) | 12 (27.9) | 0.763 |
| Middle of the prostate gland | 32 (76.2) | 24 (55.8) | 0.186 |
| Base of the prostate gland | 0 (0.0) | 7 (16.3) | <0.001 |
| Clinically significant prostate cancer (%) | 41 (97.6) | 38 (88.4) | 0.202 |
| Ventral side of the prostate | 29 (100) | 21 (91.3) | 0.191 |
| Dorsal side of the prostate | 12 (92.3) | 17 (85.0) | >0.999 |
| Apex of the prostate gland | 9 (90.0) | 11 (91.7) | >0.999 |
| Middle of the prostate gland | 32 (100) | 20 (83.3) | 0.029 |
| Base of the prostate gland | 0 | 7 (100) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomioka, M.; Nakane, K.; Kawase, M.; Iinuma, K.; Kato, D.; Kawase, K.; Taniguchi, T.; Tobisawa, Y.; Sugino, F.; Kaga, T.; et al. Discrepancy in the Location of Prostate Cancer Indicated on Biparametric Magnetic Resonance Imaging and Pathologically Diagnosed Using Surgical Specimens. Curr. Oncol. 2024, 31, 2846-2855. https://doi.org/10.3390/curroncol31050216
Tomioka M, Nakane K, Kawase M, Iinuma K, Kato D, Kawase K, Taniguchi T, Tobisawa Y, Sugino F, Kaga T, et al. Discrepancy in the Location of Prostate Cancer Indicated on Biparametric Magnetic Resonance Imaging and Pathologically Diagnosed Using Surgical Specimens. Current Oncology. 2024; 31(5):2846-2855. https://doi.org/10.3390/curroncol31050216
Chicago/Turabian StyleTomioka, Masayuki, Keita Nakane, Makoto Kawase, Koji Iinuma, Daiki Kato, Kota Kawase, Tomoki Taniguchi, Yuki Tobisawa, Fumiya Sugino, Tetsuro Kaga, and et al. 2024. "Discrepancy in the Location of Prostate Cancer Indicated on Biparametric Magnetic Resonance Imaging and Pathologically Diagnosed Using Surgical Specimens" Current Oncology 31, no. 5: 2846-2855. https://doi.org/10.3390/curroncol31050216
APA StyleTomioka, M., Nakane, K., Kawase, M., Iinuma, K., Kato, D., Kawase, K., Taniguchi, T., Tobisawa, Y., Sugino, F., Kaga, T., Kato, H., Matsuo, M., Kito, Y., Saigo, C., Suzui, N., Ito, T., Miyazaki, T., Takeuchi, T., & Koie, T. (2024). Discrepancy in the Location of Prostate Cancer Indicated on Biparametric Magnetic Resonance Imaging and Pathologically Diagnosed Using Surgical Specimens. Current Oncology, 31(5), 2846-2855. https://doi.org/10.3390/curroncol31050216






