Real-World Analysis of Post-Progression Treatment Patterns and Outcomes for EGFR Mutation-Positive Patients Treated with First-Line Osimertinib
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
Clinical Response and Outcome to Systemic Therapies
2.2. Statistical Methods
3. Results
3.1. Baseline Characteristics and Response to Osimertinib
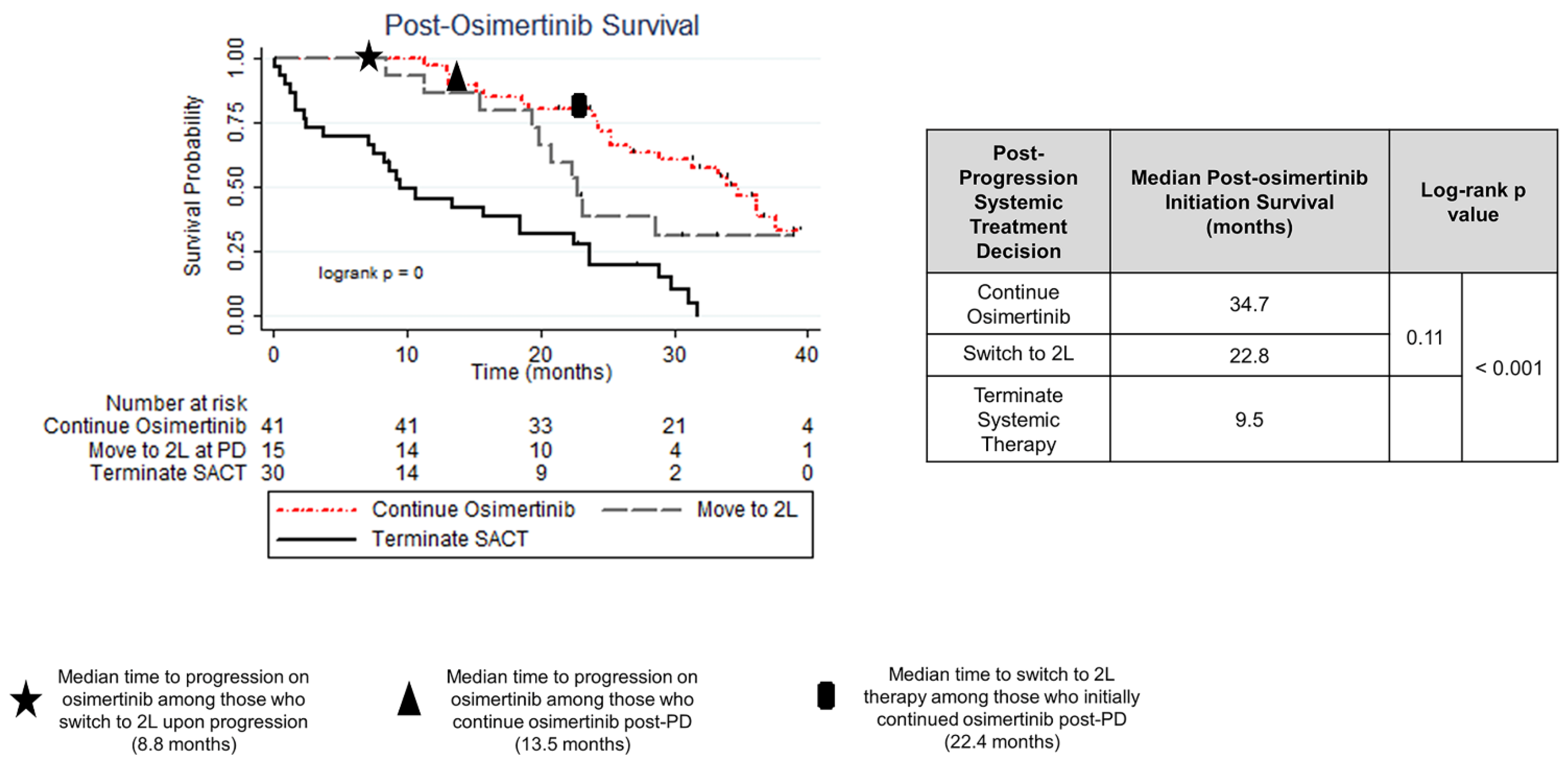
3.2. Post-Osimertinib Treatment Patterns
- Group A (n = 41): upon progression, 1L osimertinib was continued >90 days.
- Group B (n = 15): upon progression, 1L osimertinib was terminated and 2L systemic therapy was initiated.
- Group C (n = 30): upon progression, 1L osimertinib was terminated, and no further systemic therapy was received.

3.3. CNS Disease and Non-Systemic Modes of Disease Control
3.4. Systemic Therapy Regimens Post-Progression
3.4.1. Osimertinib Therapy > 90 Day Post Progression
3.4.2. Second-Line Systemic Therapy Regimens Post-Progression
3.5. Treatment Strategy and Outcome
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Del Rivero, J.; Enewold, L.; Thomas, A. Metastatic lung cancer in the age of targeted therapy: Improving long-term survival. Transl. Lung Cancer Res. 2016, 5, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Tudor, R.A.; D’Silva, A.; Tremblay, A.; MacEachern, P.; Morris, D.; Kopciuk, K.; Bebb, D.G. Beyond disease-progression: Clinical outcomes after EGFR-TKIs in a cohort of EGFR mutated NSCLC patients. PLoS ONE 2017, 12, e0181867. [Google Scholar] [CrossRef] [PubMed]
- Recondo, G.; Facchinetti, F.; Olaussen, K.A.; Besse, B.; Friboulet, L. Making the first move in EGFR-driven or ALK-driven NSCLC: First-generation or next-generation TKI? Nat. Rev. Clin. Oncol. 2018, 15, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cardarella SLydon, C.; Dahlberg, S.; Jackman, D.; Jänne, P.; Johnson, B. Five-Year Survival in EGFR-Mutant Metastatic Lung Adenocarcinoma Treated with EGFR-TKIs. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2016, 11, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Yoshida, K.; Morita, S.; Imamura, F.; Seto TOkamoto, I.; Nakagawa, K.; Yamamoto, N.; Muto, S.; Fukuoka, M. Characteristics and overall survival of EGFR mutation-positive non-small cell lung cancer treated with EGFR tyrosine kinase inhibitors: A retrospective analysis for 1660 Japanese patients. Jpn. J. Clin. Oncol. 2016, 46, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lim, S.H.; Kim, M.; Kim, S.; Jung, H.A.; Chang, W.J.; Choi, M.K.; Lee, S.Y.; Sun, J.M.; Ahn, J.S.; et al. Is there any predictor for clinical outcome in EGFR mutant NSCLC patients treated with EGFR TKIs? Cancer Chemother. Pharmacol. 2014, 73, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Kerr, K.M.; Bubendorf, L.; Edelman, M.J.; Marchetti, A.; Mok, T.; Novello, S.; O’Byrne, K.; Stahel, R.; Peters, S.; Felip, E. Second ESMO consensus conference on lung cancer: Pathology and molecular biomarkers for non-small-cell lung cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv192–iv237. [Google Scholar] [CrossRef]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskylyong, B.; Kee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N. Eng. J. Med. 2017, 378, 113–125. [Google Scholar] [CrossRef]
- Lorenzi, M.; Ferro, A.; Cecere, F.; Scattolin, D.; Del Conte, A.; Follador, A.; Pilotto, S.; Polo, V.; Santarpis, M.; Chiari, R.; et al. First-Line Osimertinib in Patients with EGFR-Mutant Advanced Non-Small Cell Lung Cancer: Outcome and Safety in the Real World: FLOWER Study. Oncologist 2022, 47, e87–e115. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Ahmed, I.; Miao, E.; Chung, S.; Patel, K.; Kohn, N.; Seetharamu, N. A real world analysis of first line treatment of advanced EGFR mutated non-small cell lung cancer: A multi-center, retrospective study. J. Oncol. Pharm. Pract. 2022, 28, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Xie, F.; Wang, F.; Fu, L. Therapeutic strategies for EGFR-mutated non-small cell lung cancer patients with osimertinib resistance. J. Hematol. Oncol. 2022, 15, 173. [Google Scholar] [CrossRef] [PubMed]
- Melosky, B.; Banerji, S.; Blais, N.; Chu, Q.; Juergens, R.; Leighl, N.B.; Liu, G.; Cheema, P. Canadian consensus: A new systemic treatment algorithm for advanced EGFR-mutated non-small-cell lung cancer. Curr. Oncol. 2020, 27, e146–e155. [Google Scholar] [CrossRef] [PubMed]
- Janne, P. WCLC 2023—Osimertinib with/without Platinum-Based Chemotherapy as First-line Treatment in Patients with EGFRm Advanced NSCLC (FLAURA2). Plenary Session 3. In Proceedings of the World Conference on Lung Cancer, Singapore, 11 September 2023; Available online: https://cattendee.abstractsonline.com/meeting/10925/presentation/2752 (accessed on 30 October 2023).
- Passaro, A.; Wang, J.; Wang, Y.; Lee, S.-H.; Melosky, B.; Shih, J.-Y.; Wang, J.; Azuma, K.; Juan-Vidal, O.; Cono, M.; et al. Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: Primary results from the phase 3 MARIPOSA-2 study. Ann. Oncol. 2023, 35, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Cho, B. Amivantamab plus lazertinib vs osimertinib as first-line treatment in patients with EGFR-mutated, advanced non-small cell lung cancer (NSCLC): Primary results from MARIPOSA, a phase III, global, randomized, controlled trial. Presidential Symposium 3. In Proceedings of the ESMO Congress, Madrid, Spain, 23 October 2023. [Google Scholar]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.; Greene, F.; Bryd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: Berlin/Heidelberg, Germany; American Joint Commision on Cancer: Chicago, IL, USA, 2017. [Google Scholar]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; HIrsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment with Targeted Tyrosine Kinase Inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med. 2018, 142, 321–346. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Izano, M.A.; Tran, N.; Fu, A.; Toland, L.; Idryo, D.; Hilbelink, R.; Tu, H.; Hsu, H.; Sommers, C.; Riothe, M.; et al. Implementing Real-World RECIST-based Tumor Response Assessment in Patients With Metastatic Non-small Cell Lung Cancer. Clin. Lung Cancer 2022, 23, 191–194. [Google Scholar] [CrossRef]
- Fleming, T.R.; Rothmann, M.D.; Lu, H.L. Issues in Using Progression-Free Survival When Evaluating Oncology Products. J. Clin. Oncol. 2009, 27, 2874–2880. [Google Scholar] [CrossRef] [PubMed]
- Stata Statistical Software, Release 12 [Computer program]; StataCorp: College Station, TX, USA, 2011.
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Eng. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Griesinger, F.; Roeper, J.; Pöttgen, C.; Willborn, K.C.; Eberhardt, W.E.E. Brain metastases in ALK-positive NSCLC—Time to adjust current treatment algorithms. Oncotarget 2018, 9, 35181–35194. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bi, J.; Moreira, A.; Chachoua Am Velcheti, V.; Lay, S.C.M.; Puneka, S.R.; Sabri, J.K.; Shum, E. Real-world clinical outcomes in a U.S. Asian population with stage IV NSCLC treated with osimertinib (Osi) stratified by EGFR subtype. JCO 2023, 41 (Suppl. S16), e21128. [Google Scholar] [CrossRef]
- Winfree, K.B.; Sheffield, K.M.; Cui, Z.L.; Sugihara, T.; Feliciano, J. Study of patient characteristics, treatment patterns, EGFR testing patterns and outcomes in real-world patients with EGFRm+ non-small cell lung cancer. Curr. Med. Res. Opin. 2022, 38, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, F.; Liu, Z.; Xiong, A.; Jia, Y.; Zhou, S.; Zhou, C.; Li, X.; Jian, R.; Han, R.; et al. Predictive and prognostic significance of M descriptors of the 8th TNM classification for advanced NSCLC patients treated with immune checkpoint inhibitors. Transl. Lung Cancer Res. 2020, 9, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Nieva, J.; Reckamp, K.L.; Potter, D.; Taylor, A.; Sun, P. Retrospective Analysis of Real-World Management of EGFR-Mutated Advanced NSCLC, After First-Line EGFR-TKI Treatment: US Treatment Patterns, Attrition, and Survival Data. Drugs-Real World Outcomes 2022, 9, 333–345. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Yu, H.A. The Evolving Landscape of Resistance to Osimertinib. J. Thorac. Oncol. 2020, 15, 18–21. [Google Scholar] [CrossRef]
- Frisone, D.; Friedlaender, A.; Malapelle, U.; Banna, G.; Addeo, A. A BRAF new world. Crit. Rev. Oncol./Hematol. 2020, 152, 103008. [Google Scholar] [CrossRef]
- Marcoux, N.; Gettinger, S.N.; O’Kane, G.; Arbour, K.C.; Neal, J.W.; Husain, H.; Evans, T.L.; Brahmer, J.R.; Muzikanski, A.; Bonomi, P.D.; et al. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas: Clinical Outcomes. J. Clin. Oncol. 2019, 37, 278–285. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Chan, J.M.; Kubota, D.; Sato, H.; Rizvi, H.; Daneshbod, Y.; Chang, J.C.; Paik, P.K.; Offin, M.; Arcila, M.E.; et al. Tumor Analyses Reveal Squamous Transformation and Off-Target Alterations As Early Resistance Mechanisms to First-line Osimertinib in EGFR-Mutant Lung Cancer. Clin. Cancer Res. 2020, 26, 2654–2663. [Google Scholar] [CrossRef] [PubMed]
- Population Statistics|Alberta.ca. Available online: https://www.alberta.ca/population-statistics (accessed on 23 October 2023).
| Characteristic | Entire Cohort (n = 150) | Progressive Disease Noted during 1L Osimertinib Therapy (n = 86) | ||
|---|---|---|---|---|
| Osimertinib ≥ 90 Days Post-PD Group A (n = 41) | 2L Systemic Therapy at PD Group B (n = 15) | No additional Systemic Therapy at PD Group C (n = 30) | ||
| Sex | ||||
| Female Male | 93 (62) 57 (38) | 24 (58) 17 (42) | 9 (60) 6 (40) | 22 (73) 8 (27) |
| Smoking history | ||||
| Ever Never | 66 (44) 84 (56) | 22 (54) 19 (46) | 8 (53) 7 (47) | 18 (60) 12 (40) |
| Age (years) | ||||
| Median (IQR) | 68.3 (61.3–78.4) | 64.3 (54.9–77.6) | 69.2 (59.4–75.3) | 71.5 (62.4–80.4) |
| Asian ancestry | ||||
| No Yes | 104 (69) 46 (31) | 25 (61) 16 (49) | 12 (80) 3 (20) | 19 (63) 11 (37) |
| EGFR mutation | ||||
| Exon 19 deletion Exon 21L858R | 87 (58) 63 (42) | 23 (56) 18 (44) | 5 (33) 10 (67) | 17 (57) 13 (43) |
| ECOG at 1L osimertinib initiation | ||||
| ECOG < 2 ECOG ≥ 2 | 100 (67) 50 (33) | 34 (83) 7 (17) | 12 (80) 3 (20) | 10 (33) 20 (67) |
| TNM 8th Edition M-stage at 1L osimertinib initiation | ||||
| M0 M1a M1b M1c | 16 (11) 37 (24) 48 (32) 49 (33) | 5 (13) 10 (24) 16 (39) 10 (24) | 0 (0) 4 (26) 4 (26) 7 (48) | 2 (7) 6 (20) 10 (33) 12 (40) |
| ≥3 sites of metastatic disease at 1L osimertinib initiation | ||||
| No Yes | 112 (81) 29 (19) | 36 (88) 5 (12) | 8 (54) 7 (46) * | 24 (60) 6 (20) |
| Metastatic disease in liver, bone or CNS present upon 1L osimertinib initiation | ||||
| Liver Bone Central Nervous System | 28 (19) 72 (48) 35 (23) | 7 (17) 19 (46) 8 (20) | 2 (13) 10 (67) 5 (33) | 10 (67) * 15 (100) * 6 (40) |
| Concurrent mutations (known at time of osimertinib initiation) | ||||
| None BRAF KRAS PIK3CA ERBB2 | 141 (94) 1 (<1) 1 (<1) 7 (5) 0 (0) | 40 (98) 1 (2) 0 (0) 0 (0) 0 (0) | 15 0 (0) 0 (0) 0 (0) 0 (0) | 26 (87) 0 (0) 1 (3) 3 (10) 0 (0) |
| Duration of treatment (months) Median (IQR) | 15.6 (6.0–26.9) | 26.8 * (18.7–33.8) | 9.4 (6.9–16.0) | 8.0 (2.7–25.7) |
| Tolerability | ||||
| Adverse events—no intervention required | 71% | 83% | 67% | 57% |
| Toxicity | ||||
| Adverse events—intervention required (dose reduction, treatment break, hospitalization, treatment termination) | 35% | 29% | 40% | 17% |
| Real-world objective response rate (rwORR) | 49% | 54% | 60% | 56% |
| Real-world disease control rate (rwDCR) | 82% | 100% | 100% | 76% * |
| Primary resistance | 5% | 0% | 0% | 3% |
| Time to progression | ||||
| Median [95% confidence interval] | 17.4 [14.7–23.3] | 13.5 [10.0–17.2] | 8.8 [5.6–11.9] | 8.6 [5.8–16.2] |
| Nature of progression | ||||
| Unknown (rapid clinical decline) Thoracic progression only Distant progression only Thoracic + distant progression | - | 0 (0) 22 (54) 11 (27) 8 (19) | 0 (0) 6 (40) 3 (20) 6 (40) | 15 (50) 6 (20) 3 (10) 6 (20) |
| Metastatic disease in liver, bone or CNS present upon progression | ||||
| Liver Bone Central Nervous System | - | 7 (17) 25 (61) 10 (24) | 6 (40) 10 (67) 5 (33) | 4 (13) 17 (57) 5 (17) |
| ≥3 Unique sites of metastatic disease upon progression | ||||
| No Yes | - | 29 (71) 12 (29) | 7 (46) 8 (53) | 22 (73) 8 (27) |
| Development or progression of CNS disease | ||||
| No Yes | - | 38 (93) 3 (7) | 13 (7) 2 (13) | 29 (97) 1 (3) |
| Survival post-progression on with osimertinib | ||||
| Median [95% Confidence interval] | - | 14.9 [8.7–24.9] | 11.8 [4.9–17.0] | 1.9 * [0.9–3.7] |
| Survival following osimertinib initiation | ||||
| Median [95% Confidence interval] | 28.9 [25.9–NR] | 34.7 [25.2–45.9] | 22.8 [15.4–NR] | 9.5 * [7.1–18.5] |
| 3-year survival rate | 27% | 46% | 33% | 0% * |
| Characteristic | Osimertinib ≥ 90 Days Post-PD Group A (n = 41) n (%) |
|---|---|
| Sex | |
| Female Male | 24 (58) 17 (42) |
| Age (years) at 1L osimertinib continuation | |
| Median (IQR) | 65.1 (56.4–78.1) |
| Asian ancestry | |
| No Yes | 25 (61) 16 (39) |
| EGFR mutation | |
| Exon 19 deletion Exon 21L858R | 23 (56) 18 (44) |
| TNM 8th Edition M-stage at 1L osimertinib continuation | |
| M0 M1a M1b M1c | 4 (10) 11 (27) 11 (27) 15 (36) |
| ≥3 sites of metastatic disease | |
| No Yes | 29 (71) 12 (29) |
| Metastatic disease in liver, bone or CNS present upon 1L osimertinib continuation | |
| Liver Bone Central Nervous System | 7 (17) 25 (61) 10 (24) |
| CNS present (but controlled) at 1L osimertinib continuation | 7/10 (70%) |
| Osimertinib continued on reduced dose | |
| No Yes (50% reduction to 40mg/day) | 37 (82) 7 (17) |
| Adverse events post-progression (requiring intervention) | |
| Treatment break (Grade 2 Fatigue; 14 days) | 1 (2) |
| Duration of treatment post-progression (months) | |
| Median (IQR) | 6.9 (4.3–14.3) |
| Reason for osimertinib termination | |
| Toxicity Death New treatment identified Further progressive disease Osimertinib ongoing | 1 (2) 12 (29) 1 (2) 15 (37) 12 (29) |
| Second-Line (non-osimertinib) therapy received | 11 (38) |
| Clinical Measure | By Post-Osimertinib Systemic Therapy Line | ||
|---|---|---|---|
| 2L n (%) (n = 26) | 3L n (%) (n = 7) | 4L n (%) (n = 2) | |
| Cytotoxic chemotherapy | 17 (65) | 6 (86) | 0 (0) |
| Platinum-doublet NSCLC regimen Single-agent regimen | (n = 17) (n = 0) | (n = 0) (n = 6) | |
| Immune checkpoint inhibitor | 0 (0) | 0 (0) | 1 (50) |
| Targeted therapy | 7 (27) | 1 (14) | 1 (50) |
| Second generation EGFR TKI Osimertinib rechallenge after treatment break SAVANNAH (osimertinib + savolitinib) trial Tepotinib | (n = 2) (n = 1) (n = 4) (n = 0) | (n = 0) (n = 1) (n = 0) (n = 0) | (n = 0) (n = 0) (n = 0) (n = 1) |
| Cytotoxic chemotherapy and targeted therapy | 2 (8) | 0 (0) | 0 (0) |
| Platinum-doublet + osimertinib MARIPOSA-2 trial (Arm C: platinum doublet + amivantamab + lazertinib) | (n = 1) (n = 1) | ||
| ECOG at initiation | |||
| ECOG < 2 ECOG ≥ 2 | 19 (73) 7 (27) | 4 (57) 3 (43) | 1 (50) 1 (50) |
| M-stage at initiation | |||
| M0 M1a M1b M1c | 1 (4) 4 (15) 7 (27) 14 (54) | 0 (0) 1 (15) 1 (15) 5 (70) | 0 (0) 0 (0) 0 (0) 2 (100) |
| Duration of treatment (months) Median (IQR) | 3.7 (1.4–11.4) | 1.8 (0–4.2) | 3.8 (2.5–5.1) |
| Real-world progression-free survival (months) Median [95% confidence interval] | 7.0 [5.2–9.0] | 5.0 [0.7–NR] | NR |
| Nature of initial progression | (n = 18) | (n = 6) | (n = 2) |
| Thoracic progression only Distant progression only Both thoracic and distant progression | 7 (39) 3 (17) 8 (44) | 2 (33) 2 (33) 2 (33) | 0 (0) 1 (50) 1 (50) |
| Progressive disease in the brain upon initial progression | (n = 18) | (n = 6) | (n = 2) |
| No Yes | 9 (50) 9 (50) | 6 (100) 0 (0) | 1 (50) 1 (50) |
| Reason for treatment termination | |||
| Treatment ongoing Completed as planned Progressive disease ECOG decline/death Adverse events | 6 (23) 1 (3) 12 (44) 4 (15) 4 (15) | 1 (14) 1 (14) 4 (58) 1 (14) 0 (0) | 1 (50) 0 (0) 0 (0) 1 (50) 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibson, A.J.W.; Dean, M.L.; Litt, I.; Box, A.; Cheung, W.Y.; Navani, V. Real-World Analysis of Post-Progression Treatment Patterns and Outcomes for EGFR Mutation-Positive Patients Treated with First-Line Osimertinib. Curr. Oncol. 2024, 31, 2427-2440. https://doi.org/10.3390/curroncol31050182
Gibson AJW, Dean ML, Litt I, Box A, Cheung WY, Navani V. Real-World Analysis of Post-Progression Treatment Patterns and Outcomes for EGFR Mutation-Positive Patients Treated with First-Line Osimertinib. Current Oncology. 2024; 31(5):2427-2440. https://doi.org/10.3390/curroncol31050182
Chicago/Turabian StyleGibson, Amanda Jane Williams, Michelle Liane Dean, Ishjot Litt, Adrian Box, Winson Y. Cheung, and Vishal Navani. 2024. "Real-World Analysis of Post-Progression Treatment Patterns and Outcomes for EGFR Mutation-Positive Patients Treated with First-Line Osimertinib" Current Oncology 31, no. 5: 2427-2440. https://doi.org/10.3390/curroncol31050182
APA StyleGibson, A. J. W., Dean, M. L., Litt, I., Box, A., Cheung, W. Y., & Navani, V. (2024). Real-World Analysis of Post-Progression Treatment Patterns and Outcomes for EGFR Mutation-Positive Patients Treated with First-Line Osimertinib. Current Oncology, 31(5), 2427-2440. https://doi.org/10.3390/curroncol31050182






