Preoperative Direct Puncture Embolization of Castleman Disease of the Parotid Gland: A Case Report
Abstract
1. Introduction
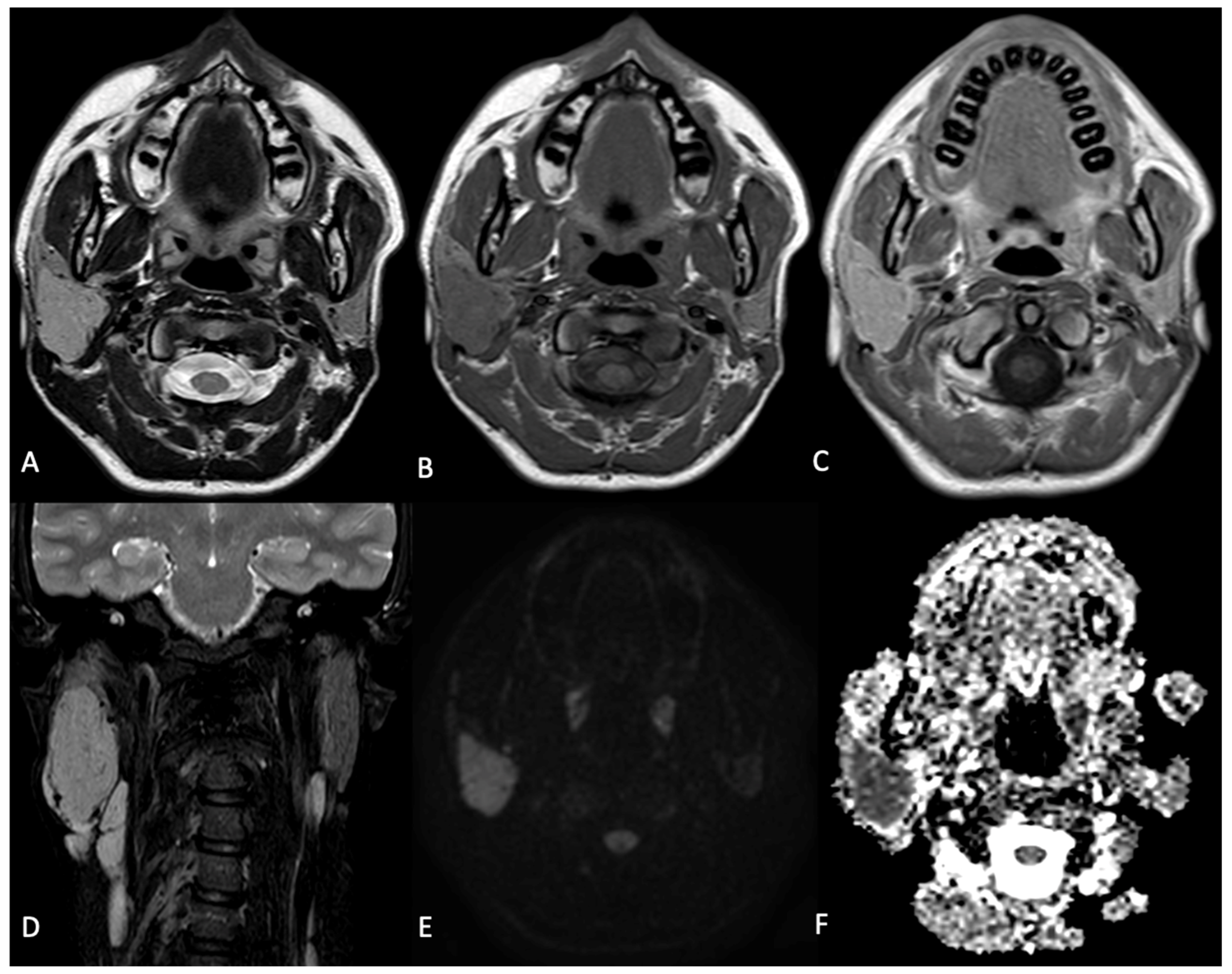
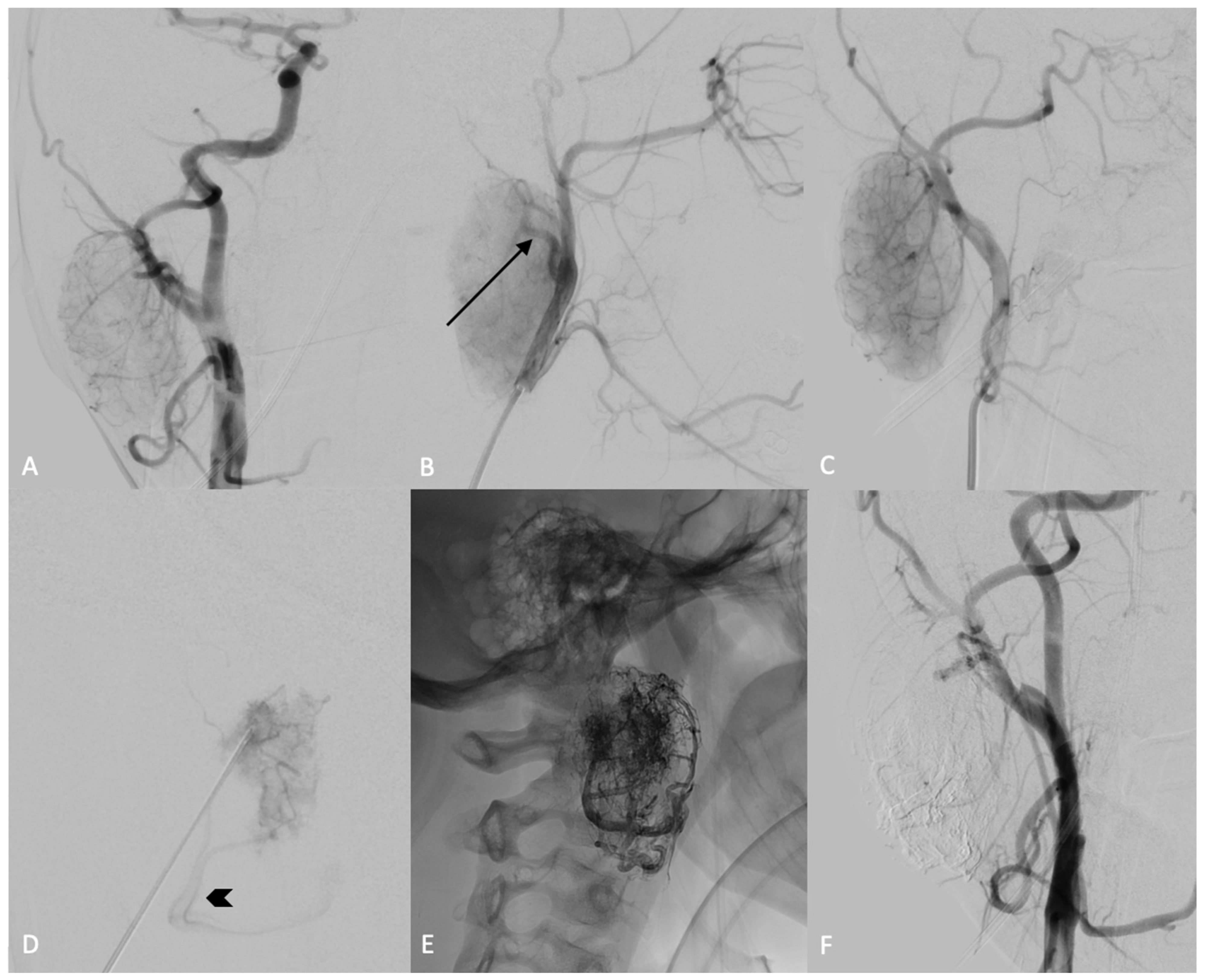
2. Case
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castleman, B.; Iverson, L.; Menendez, V.P. Localized mediastinal lymph-node hyperplasia resembling thymoma. Cancer 1956, 9, 822–830. [Google Scholar] [CrossRef]
- Munshi, N.; Mehra, M.; Van De Velde, H.; Desai, A.; Potluri, R.; Vermeulen, J. Use of a claims database to characterize and estimate the incidence rate for Castleman disease. Leuk. Lymphoma 2014, 56, 1252–1260. [Google Scholar] [CrossRef]
- Yu, G.; Cao, F.; Gong, H.; Liu, P.; Sun, G.; Zhang, W. Embolization of blood-supply artery followed by surgery for treatment of mesorectal Castleman’s disease: Case report and literature review. Gastroenterol. Rep. 2017, 7, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.R.; Hochholzer, L.; Castleman, B. Hyaline-vascular and plasma-cell types of giant lymph node hyperplasia of the mediastinum and other locations. Cancer 1972, 29, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Temirbekov, D.; Yazici, Z.M.; Ergelen, R.; Turgut, H.; Kayhan, F.T. Castelman disease of the parotid gland: An unusual entity. Otolaryngol. Polska 2014, 68, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Talat, N.; Belgaumkar, A.P.; Schulte, K.M. Surgery in castlemans disease: A systematic review of 404 published cases. Ann. Surg. 2012, 255, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Lee, J.H.; Lee, Y.M.; Lee, H.J. Castleman Disease of Parotid Gland. J. Craniofacial Surg. 2016, 27, 1373–1374. [Google Scholar] [CrossRef] [PubMed]
- Abo-Alhassan, F.; Faras, F.; Bastaki, J.; Al-Sihan, M.K. Castleman Disease of the Parotid Gland: A Report of a Case. Case Rep. Otolaryngol. 2015, 2015, 265187. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-D.; Wang, Q.-X.; Liu, W.-X. Castleman Disease of the Parotid Gland: A Case Report. J. Oral Maxillofac. Surg. 2019, 78, 400.e1–400.e6. [Google Scholar] [CrossRef]
- Newlon, J.L.; Couch, M.; Brennan, J. Castleman’s Disease: Three Case Reports and a Review of the Literature. Ear Nose Throat J. 2007, 86, 414–418. [Google Scholar] [CrossRef]
- Denenberg, S.; Levine, P. Castleman’s disease—The lymphoma impostor. Laryngoscope 1984, 94, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Tartaglione, T.; Botto, A.; Sciandra, M.; Gaudino, S.; Danieli, L.; Parrilla, C.; Paludetti, G.; Colosimo, C. Differential diagnosis of parotid gland tumours: Which magnetic reso-nance findings should be taken in account? Acta Otorhinolaryngol. Ital. 2015, 35, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Rzepakowska, A.; Osuch-Wójcikiewicz, E.; Sobol, M.; Cruz, R.; Sielska-Badurek, E.; Niemczyk, K. The differential diagnosis of parotid gland tumors with high-resolution ultrasound in otolaryngological practice. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 3231–3240. [Google Scholar] [CrossRef] [PubMed]
- Alvi, S.; Chudek, D.; Limaiem, F. Parotid Cancer. In StatPearls; Updated 2023 May 19 [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538340/ (accessed on 19 May 2023).
- Bewley, A.F.; Azhdam, A.M.; Borrelli, M. Intraparotid Facial Nerve Schwannoma Mimicking Primary Parotid Neoplasm. Ear Nose Throat J. 2021, 100 (Suppl. 6), 881S–883S. [Google Scholar] [CrossRef] [PubMed]
- Topuz, M.F.; Genç, Ö.; Kadioglu, N.; Osma, Ü. Intraparotid Facial Nerve Schwannoma: A Report of Two Cases. Int. J. Otorhinolaryngol. Clin. 2020, 12, 4–7. [Google Scholar] [CrossRef]
- Vora, A.A.; Lai, C.K.; Rao, J.Y.; Apple, S.K.; Moatamed, N.A. Paraganglioma with unusual presentation in parotid gland: A diagnostic dilemma in fine needle aspiration. Cytojournal 2012, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singla, R.; Chand, P.; Singh, R. Carotid body tumor presenting as parotid swelling misdiagnosed as pleomorphic adenoma: A rare presentation. Niger. J. Surg. 2015, 21, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, Z.; Khajavi, M.; Bazgir, N.; Kordjazi, M. A rare presentation of head and neck Paragangliomas in parotid gland: A case report. Otolaryngol. Case Rep. 2022, 24, 100441. [Google Scholar] [CrossRef]
- Tan, T.Y.; Pang, K.P.; Goh, H.K.C.; Teo, E.L.H.; Abhilash, B.; Walford, N. Castleman’s disease of the neck: A description of four cases on contrast-enhanced CT. Br. J. Radiol. 2004, 77, 253–256. [Google Scholar] [CrossRef]
- Takayama, F.; Takashima, S.; Wang, J.; Ishiyama, T.; Kadoya, M. Castleman disease of the parotid gland: MR imaging findings with pathologic correlation. Eur. J. Radiol. Extra 2003, 45, 118–121. [Google Scholar] [CrossRef]
- van Rhee, F.; Oksenhendler, E.; Srkalovic, G.; Voorhees, P.; Lim, M.; Dispenzieri, A.; Ide, M.; Parente, S.; Schey, S.; Streetly, M.; et al. International evidence-based consensus diagnostic and treatment guidelines for unicentric Castleman disease. Blood Adv. 2020, 4, 6039–6050. [Google Scholar] [CrossRef]
- Safford, S.D.; Lagoo, A.S.; A Mahaffey, S. Preoperative embolization as an adjunct to the operative management of mediastinal castleman disease. J. Pediatr. Surg. 2003, 38, E21–E23. [Google Scholar] [CrossRef] [PubMed]
- Sato, A. Castleman’s disease in the pelvic retroperitoneum: A case report and review of the Japanese literature. Int. J. Surg. Case Rep. 2013, 4, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Amano, Y.; Takai, D.; Ohishi, N.; Shinozaki-Ushiku, A.; Fukayama, M.; Akahane, M.; Nakajima, J.; Nagase, T. Successful Treatment of Mediastinal Unicentric Castleman’s Disease Using Video-Assisted Thoracoscopic Surgery with Preoperative Embolization. Case Rep. Med. 2013, 2013, 354507. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, B.; Okay, T.; Imamoglu, O.; Sahin, S.; Dogusoy, I. Preoperative Embolization in Mediastinal Castleman’s Disease. Thorac. Cardiovasc. Surg. 2010, 58, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.H.; Sgourdos, G.; Kritikos, N.; Didier, D.; Terraz, S. Preoperative Embolization of Hypervascular Castleman’s Disease of the Mediastinum. Cardiovasc. Interv. Radiol. 2007, 31, 186–188. [Google Scholar] [CrossRef]
- Swee, W.; Housseini, A.M.; Angle, J.F.; Jones, D.R.; Daniel, T.M.; Turba, U.C.; Abdel-Gawad, E.A.; Hagspiel, K.D. Preoperative Embolization of Castleman’s Disease Using Microspheres. Ann. Thorac. Surg. 2009, 88, 1999–2001. [Google Scholar] [CrossRef]
- Lorenz, J.M.; Zangan, S.M.; Leef, J.A. Mediastinal Castleman Disease: Embolization without Surgery. J. Vasc. Interv. Radiol. 2009, 20, 1393–1394. [Google Scholar] [CrossRef]
- Sanchez-Ros-Sanchez, A.; Infante-Cossio, P.; Gonzalez-Garcia, A.; Borrero-Martin, J.-J. Preoperative Embolization for the Treatment of Cervical Castleman Disease. J. Craniofacial Surg. 2012, 23, e257–e259. [Google Scholar] [CrossRef]
- Casasco, A.; Herbreteau, D.; Houdart, E.; George, B.; Huy, P.T.B.; Deffresne, D.; Merland, J.J. Devascularization of craniofacial tumors by percutaneous tumor puncture. Am. J. Neuroradiol. 1994, 15, 1233–1239. [Google Scholar]
- Chaloupka, J.C.; Mangla, S.; Huddle, D.C.; Roth, T.C.; Mitra, S.; Ross, D.A.; Sasaki, C.T.; Od, D.C.H. Evolving Experience With Direct Puncture Therapeutic Embolization for Adjunctive and Palliative Management of Head and Neck Hypervascular Neoplasms. Laryngoscope 1999, 109, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Pedicelli, A.; Lozupone, E.; Valente, I.; Snider, F.; Rigante, M.; D’argento, F.; Alexandre, A.; Garignano, G.; Chiumarulo, L.; Paludetti, G.; et al. Pre-operative direct puncture embolization of head and neck hypervascular tumors using SQUID 12. Interv. Neuroradiol. 2019, 26, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, A.M.; Scarcia, L.; Clarençon, F.; Camilli, A.; Bartolo, A.; Incandela, F.; Mele, D.A.; Rigante, M.; Natola, M.; Valente, I.; et al. Preoperative Direct Puncture Embolization Using a Nonadhesive Ethylene Vinyl Alcohol (EVOH) Liquid Embolic Agent for Head and Neck Paragangliomas. Clin. Neuroradiol. 2023, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lamin, S.; Chew, H.; Chavda, S.; Thomas, A.; Piano, M.; Quilici, L.; Pero, G.; Holtmannspolter, M.; Cronqvist, M.; Casasco, A.; et al. Embolization of Intracranial Dural Arteriovenous Fistulas Using PHIL Liquid Embolic Agent in 26 Patients: A Multicenter Study. Am. J. Neuroradiol. 2016, 38, 127–131. [Google Scholar] [CrossRef]
- Lozupone, E.; Bracco, S.; Trombatore, P.; Milonia, L.; D’argento, F.; Alexandre, A.; Valente, I.; Semeraro, V.; Cioni, S.; Pedicelli, A. Endovascular treatment of cerebral dural arteriovenous fistulas with SQUID 12. Interv. Neuroradiol. 2020, 26, 651–657. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedicelli, A.; Trombatore, P.; Bartolo, A.; Camilli, A.; Rossi, E.D.; Scarcia, L.; Alexandre, A.M. Preoperative Direct Puncture Embolization of Castleman Disease of the Parotid Gland: A Case Report. Curr. Oncol. 2024, 31, 2047-2056. https://doi.org/10.3390/curroncol31040151
Pedicelli A, Trombatore P, Bartolo A, Camilli A, Rossi ED, Scarcia L, Alexandre AM. Preoperative Direct Puncture Embolization of Castleman Disease of the Parotid Gland: A Case Report. Current Oncology. 2024; 31(4):2047-2056. https://doi.org/10.3390/curroncol31040151
Chicago/Turabian StylePedicelli, Alessandro, Pietro Trombatore, Andrea Bartolo, Arianna Camilli, Esther Diana Rossi, Luca Scarcia, and Andrea M. Alexandre. 2024. "Preoperative Direct Puncture Embolization of Castleman Disease of the Parotid Gland: A Case Report" Current Oncology 31, no. 4: 2047-2056. https://doi.org/10.3390/curroncol31040151
APA StylePedicelli, A., Trombatore, P., Bartolo, A., Camilli, A., Rossi, E. D., Scarcia, L., & Alexandre, A. M. (2024). Preoperative Direct Puncture Embolization of Castleman Disease of the Parotid Gland: A Case Report. Current Oncology, 31(4), 2047-2056. https://doi.org/10.3390/curroncol31040151




