Abstract
Using digitized data from progression-free survival (PFS) and overall survival Kaplan–Meier curves, one can assess population survival kinetics through exponential decay nonlinear regression analyses. To demonstrate their utility, we analyzed PFS curves from published curative-intent trials of non-small cell lung cancer (NSCLC) adjuvant chemotherapy, adjuvant osimertinib in resected EGFR-mutant NSCLC (ADAURA trial), chemoradiotherapy for inoperable NSCLC, and limited small cell lung cancer (SCLC). These analyses permit assessment of log–linear curve shape and estimation of the proportion of patients cured, PFS half-lives for subpopulations destined to eventually relapse, and probability of eventual relapse in patients remaining progression-free at different time points. The proportion of patients potentially cured was 41% for adjuvant controls, 58% with adjuvant chemotherapy, 17% for ADAURA controls, not assessable with adjuvant osimertinib, 15% with chemoradiotherapy, and 12% for SCLC. Median PFS half-life for relapsing subpopulations was 11.9 months for adjuvant controls, 17.4 months with adjuvant chemotherapy, 24.4 months for ADAURA controls, not assessable with osimertinib, 9.3 months with chemoradiotherapy, and 10.7 months for SCLC. For those remaining relapse-free at 2 and 5 years, the cure probability was 74%/96% for adjuvant controls, 77%/93% with adjuvant chemotherapy, 51%/94% with chemoradiation, and 39%/87% with limited SCLC. Relatively easy population kinetic analyses add useful information.
1. Introduction
Progression-free survival (PFS) and overall survival (OS) Kaplan–Meier plots have several potential uses in the analysis and reporting of clinical trials, including estimations of medians and calculation of hazard ratios. We have also used clinical trial data from digitized PFS and OS Kaplan–Meier curves for exponential decay nonlinear regression analyses to assess population survival kinetics.
Population survival kinetics model the disappearance rate of patients from a population, analogous to pharmacokinetics modeling the rate of drug disappearance from the blood [1]. These analyses permit the calculation of PFS and OS half-lives (time to progression or death of half the remaining patients) and assessment of log–linear curve shape. For PFS and OS, the half-lives and medians correlate strongly (R2 ≥ 0.96) with each other [2].
Importantly, the PFS log–linear curve shape varies significantly with therapy type [3,4]. These curves generally follow first-order kinetics, approximating a straight line on log–linear plots, unless factors that impact therapy efficacy cause deviations from straight lines. Consequently, log–linear curve shapes provide insight into factors impacting therapy efficacy. For example, many combination chemotherapy PFS curves demonstrate convexity starting at 3–4 months since progression accelerates following therapy interruption upon the completion of induction [4].
Distinct subpopulations with differing progression rates can result in a rightward inflection on PFS log–linear curves, with curve data fitting two-phase decay nonlinear regression models. The probability of PFS curve two-phase decay is low for some therapies but high for others (e.g., EGFR inhibitors in unselected patients, where EGFR wild-type patients progress rapidly, while EGFR-mutant patients progress slowly) [4]. PFS 2-phase decay should prompt a search for a present-vs-absent factor predicting high-vs-low therapy benefit.
Population survival kinetics models also make PFS gain a good predictor of OS gain [2]. Drug approval can be delayed when using OS endpoints since the assessment of OS gain is compromised by crossover and because more patients and longer follow-ups are needed for adequate statistical power with OS endpoints [5]. Consequently, PFS endpoints are increasingly used for drug approval [6]. While PFS hazard ratios are weak predictors of OS hazard ratios [2,7,8,9], PFS half-life gain reliably predicts OS half-life gain. In 124 solid tumor trials with low crossover rates, a statistically significant PFS half-life gain ≥1.5 months predicted an OS half-life gain ≥2 months, with positive and negative predictive values of ≥80% [2]. Higher PFS gains reliably predicted even higher OS gains [2].
However, PFS gain underpredicted OS gain for immune checkpoint inhibitors [2]. PFS gain was also unreliable for prostate cancer (since prostate cancer metastasizes predominantly to bone, and bone metastases are difficult to measure) or for toxic high-dose therapies (where residual toxicity from first-line therapy can preclude effective second-line therapy). If prostate cancer (11 trials) and high-dose therapies (two trials) were excluded, then a PFS gain of ≥1.5 months predicted an OS gain of ≥2 months with positive and negative predictive values of 90% and 86%, respectively (DJ Stewart, unpublished data).
Population kinetic analyses can also guide the optimal frequency of follow-up scans [10] and reveal that, on average, 4% of the remaining patients with metastatic non-small cell lung cancer (NSCLC) die each week that therapy initiation is delayed [11].
In this paper, we present population survival kinetics analyses of PFS from clinical trials of lung cancer patients treated with curative intent. We assessed the use of this approach to estimate the proportion of the population that is cured, the PFS half-life for patients destined to eventually relapse, and the probability of future relapse for patients remaining relapse-free at different time points. In an online appendix, we provide a tutorial for clinicians, trainees, and others on how to use readily available tools for these analyses.
2. Materials and Methods
We identified relevant papers in PubMed. (See below.) For a publication to be included, it had to present a Kaplan–Meier PFS curve, relapse-free survival curve, or disease-free survival curve. Below, we use “PFS” to also refer to relapse-free and disease-free survival. As previously described [2,4,10,11], we used the application https://apps.automeris.io/wpd/ (accessed on 19 January 2024) to digitize the PFS curves from these publications.
The use of Kaplan–Meier curves as the source for our data adjusted for data censoring. We excluded curves derived from <50 patients to reduce potential variability arising solely from low patient numbers. Survival curves based on small sample sizes are subject to greater error, and the exclusion of these small trials could potentially improve the comparability of the remaining studies. The decision to use this particular cut point was arbitrary and could potentially be a source of bias. We have not tested the impact of using a higher or lower cut point.
We employed GraphPad Prism7 (GraphPad Software, La Jolla, CA, USA) built-in options for 1-phase and 2-phase exponential decay nonlinear regression analysis of digitized data and to replot data on log–linear scales. In these analyses, we applied the constraints “Y0 = 100” and “plateau = 0” since all curves start at 100% PFS and since all would eventually reach 0% survival if followed long enough.
The models provide an R2 value as an indicator of goodness of fit of the model for a particular curve. They also provide 95% confidence intervals and standard errors for the model parameters. However, the method has no specific control for experimental error that might be intrinsic within data for a given curve. To reduce the potential impact of experimental error associated with an individual curve, we calculated medians and ranges for model parameters across curves for a given treatment setting.
As in our earlier publications on population survival kinetics [2,4,10,11], we defined a curve as fitting a 2-phase decay model if the model identified two subgroups, each comprising ≥1% of the total population, with the PFS half-life for the group with longer PFS being more than twice as long as the half-life for the group with shorter PFS. Curves fitting 2-phase decay models typically demonstrate rightward inflection points on log–linear plots.
As previously described [10], we calculated the proportion of patients that we projected would remain progression-free at specific future time points using the Excel spreadsheet formula “=EXP(− tn × 0.693/t1/2)” where “tn” is the time interval of interest from therapy initiation, “*” signifies multiplication, “0.693” is the natural log of 2, and “t1/2” is the subpopulation’s PFS half-life.
A different way of expressing this formula would be “x = 2^(− tn/t1/2)” [10].
In this analysis, we assessed PFS curves for postoperative platinum-based adjuvant chemotherapy or control groups not receiving adjuvant chemotherapy for the 4 clinical trials included in the NSCLC LACE meta-analysis (Supplementary Online Table S1) [12,13,14,15,16], adjuvant osimertinib and the control group not receiving adjuvant osimertinib in resected epidermal growth factor receptor-mutant NSCLC (the ADAURA trial) [17], curative-intent chemoradiotherapy trials published from 2010 to February 2022 for inoperable NSCLC (57 curves from 37 clinical trials) (Supplementary Online Table S2) [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52], and limited small cell lung cancer (SCLC) trials published 1990–2021 (55 curves) (Supplementary Online Table S3) [53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87].
Publications for chemoradiotherapy were identified in PubMed searches with the filter “Clinical Trial” that included the terms “radiotherapy or radiation or irradiation”, “non-small cell lung OR adenocarcinoma of the lung OR squamous cell carcinoma of the lung”, “locally advanced OR stage III OR stage 3”, “NOT metastatic”, “NOT stereotactic”, and “NOT neoadjuvant”. We identified 379 trials and excluded 143 that had fewer than 50 patients per trial arm, 2 with mixed populations of small cell and non-small cell lung cancer, 11 with no published PFS curves, 4 that did not include both chemotherapy and radiotherapy, and 132 that did not have PFS, relapse-free survival or disease-free survival as an endpoint. From the eligible trials, we also excluded 6 curves with the longest follow-up of less than 25 months.
Publications relating to limited small cell lung cancer were identified in PubMed searches with the filter “Clinical Trial” that included the terms “small cell lung”, “NOT non-small cell lung”, and “limited”. We identified 754 trials and excluded 218 with fewer than 50 patients per trial arm, 10 with no published PFS curves, 55 that included previously treated patients, 125 that included mixed populations of limited and extensive small cell lung cancer or small cell lung cancer plus non-small cell lung cancer, and 236 that did not have PFS as an endpoint.
For comparisons between the groups, we used GraphPad Prism7 to perform nonparametric statistical analyses.
3. Results
3.1. Overall PFS Half-Lives
We used one-phase decay models to estimate the overall trial arm PFS half-lives (Table 1). The median value for overall PFS half-lives was 50.0 months for control arms on adjuvant chemotherapy trials, 61.1 months for adjuvant chemotherapy arms, 25.0 months for the ADAURA control arm, 109.1 months for adjuvant osimertinib, 13.9 months for NSCLC chemoradiation, and 16.2 months for limited SCLC. Note that for this outcome and for outcomes below, we combined data from trials that included patients with different characteristics and treatment details. These differences across trials will have impacted the outcomes and contributed to the heterogeneity seen across trials. Future trials using individual patient data and/or more homogeneous populations would improve the reliability of the conclusions.

Table 1.
Medians (ranges) for population survival kinetics PFS variables for potentially cured lung cancer.
3.2. 2-Phase Decay Modeling
All curves except seven SCLC curves [54,58,61,66,73,79], one chemoradiation curve [88] and the ADAURA osimertinib curve [17] fit two-phase decay models, in keeping with distinct potentially cured (“slow t1/2”) vs. relapsing (“fast t1/2”) subpopulations (for the ADAURA control population, the curve’s terminal vertical drop was excluded for two-phase decay assessment).
Curves were less likely to fit two-phase decay models if the curve length (maximum follow-up) was short. Only two of the seven SCLC curves that failed to fit two-phase models had a maximum follow-up of >42 months compared to 45 of 48 curves fitting two-phase models (p = 0.0003). Similarly, the chemoradiotherapy curve that did not fit a two-phase model had a maximum follow-up of 36 months, while 52 of 56 curves fitting two-phase models had follow-ups of >36 months. All curves would probably fit two-phase decay models with longer follow-ups since all would have cured vs. relapsing subpopulations.
3.3. Models “Hitting Constraints”
GraphPad specified that the nonlinear regression models “hit constraints” if calculations were unreliable near the constraint values. For models “hitting constraints”, upper and/or lower boundaries of 95% confidence intervals may not be calculated for the relevant model parameter(s).
Of the 113 PFS curves meeting our definition of fitting two-phase models, 62 “hit constraints”. In exploratory analyses, removing the constraint “Y = 100” generally did not alter “hit constraint” notifications. Removing the constraint “plateau = 0” generally did eliminate “hit constraint” notifications. However, when this constraint was removed, data generally no longer fit two-phase models, despite there being compelling evidence of two-phase decay (rightward inflection on the log–linear curves and the presence of distinct cured vs. relapsing subpopulations). Hence, we elected to maintain this constraint, recognizing that confidence intervals might not be provided for some parameters.
Shorter PFS curves increased the probability of hitting the constraints (Table 2).

Table 2.
Two-phase decay models and hitting the constraint “plateau = 0”, across all series.
3.4. Proportion of Patients in Potentially Cured Subpopulations
Via two-phase decay analyses, the median value across trial arms for the proportion of the population in the potentially cured subpopulation was 41% for the adjuvant control groups, 58% with adjuvant chemotherapy, 17% for the ADAURA controls, not yet assessable for the ADAURA osimertinib arm, 15% for chemoradiotherapy, and 12% for SCLC (Table 1). Longer follow-up will be needed before we can assess this for the ADAURA osimertinib arm.
Across all series, the proportion of patients in the potentially cured subpopulation was 12% for curves hitting constraints vs. 19% for other curves (p = 0.006). The upper 95% confidence interval boundaries could not be calculated for percent cured for 3 of the 62 curves hitting constraints vs. 3 of 48 curves not hitting the constraints. The maximum PFS curve length was a median of 65 months when 95% confidence interval upper boundaries could be calculated and 50 months where they could not be calculated (p = 0.0003).
3.5. PFS Half-Lives for Relapsing Subpopulations
The median PFS half-life for the relapsing subpopulation was 11.9 months for the adjuvant trial control patients, 17.4 months with adjuvant chemotherapy, not definable for the ADAURA osimertinib arm, 24.4 months for the ADAURA control patients, 9.3 months with chemoradiotherapy, and 10.7 months for SCLC (Table 1). Across the series, the PFS half-life for the relapsing subpopulations was 10.3 vs. 9.9 months for curves hitting vs. not hitting constraints (p = 0.09).
For the PFS half-life for relapsing subpopulations, 95% confidence interval upper boundaries could not be calculated for 45 of 62 curves hitting constraints vs. 13 of 49 curves not hitting constraints. The maximum curve length was a median of 72 months where 95% confidence interval upper boundaries could be calculated and 60 months where they could not be (p = 0.06).
3.6. PFS Half-Lives for Potentially Cured Subpopulations
The median PFS half-life of the potentially cured subpopulation was 396.2 months with adjuvant chemotherapy, 1.1 × 1012 months for adjuvant controls, 3.3 × 1015 months for chemoradiotherapy, and 3.7 × 1015 months for SCLC (Table 1).
The upper boundaries of the 95% confidence intervals for long half-lives could not be calculated for any of the 62 curves hitting constraints, and lower boundaries were non-calculable for 61 of these 62 curves. For curves not hitting the constraints, the upper boundaries could not be calculated for 31 of 49 curves, but lower boundaries could be calculated for all 49. Very wide or indeterminate confidence intervals would be expected for this parameter since the duration of patient follow-up was much shorter than the cured subpopulation’s projected PFS half-life. The maximum curve length was a median of 98 months where 95% confidence interval upper boundaries could be calculated and 60 months where they could not be (p < 0.0001).
In many cases, PFS half-lives for potentially cured subpopulations were much longer than human life expectancy. Hence, two-phase decay models frequently overestimated true PFS half-lives for potentially cured subpopulations. True PFS half-lives were probably long, but we cannot reliably estimate how long. We need a much longer follow-up to narrow the 95% confidence intervals. For curves for which both upper and lower boundaries of 95% confidence intervals could be calculated, the median PFS half-life value for potentially cured subpopulations across trials was 112.7 months (9.4 years).
The observation that the PFS half-life for the potentially cured subpopulation was shorter with adjuvant chemotherapy than for controls might be due to the imprecision of the analytical method. Alternatively, we might hypothesize that chemotherapy substantially delays relapse in some patients (e.g., by inducing reversible senescence [89]) without killing all of the remaining tumor cells. If so, we might eventually expect three distinct subgroups on three-phase decay exponential decay nonlinear regression analysis: a rapidly relapsing subgroup, a slowly relapsing subgroup, and a cured subgroup. Three of four adjuvant chemotherapy PFS curves could be fit using three-phase decay models, but the 95% confidence intervals were very wide. The assessment of individual patient data might provide added insight.
3.7. Early PFS Curve Convexity on Log–Linear Plots
All except one curve had initial convexity, with the curve steepening after an initial brief plateau or shallow decline (Figure 1). For adjuvant osimertinib, this convexity started at 33 months, in keeping with therapy being discontinued at 36 months (Table 1). The convexity began at a median of 3.3 months for control arms in adjuvant chemotherapy and ADAURA trials, in keeping with the convexity probably being an artefact due to lack of detection of progression until the first follow-up scan. Convexity onset was at a median of 6.1 months for adjuvant chemotherapy trial arms, 4.6 months with chemoradiotherapy, and 6.2 months with SCLC, in keeping with therapy delaying tumor progression in some patients who are not cured by therapy. Across the series, the time of convexity onset was similar for curves hitting vs. not hitting constraints (4.6 vs. 4.4 months, p = 0.29).
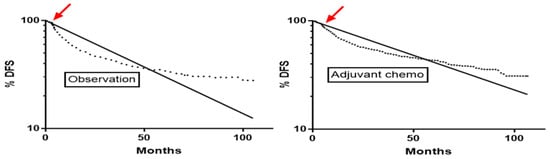
Figure 1.
Onset of convexity (arrow) on disease-free survival curves at 3.3 months in the observation arm and at 4.8 months in the adjuvant chemotherapy arm of the ANITA trial [12]. Curves after this early convexity follow 2-phase decay. The dotted line is the Kaplan-Meier curve and the solid line is the log linear one-phase decay nonlinear regression line.
For 59 of the 115 PFS curves with a late convexity point, there was also an earlier convexity point at a median (range) of 2.9 (1.0–7.1) months. We hypothesized that the early convexity point was generally based on an artefactual delay in identifying early progression due to the timing of the first follow-up scans, while the later convexity point, with further steepening of log–linear curves, was due to tumor growth acceleration in non-cured patients following therapy discontinuation.
3.8. Proportion of Remaining Patients Who Would Continue to Be Progression-Free at Different Time Points
The Excel formula “=Exp(− tn × 0.693/t1/2)” permitted estimation of the proportion of patients in relapsing subpopulations who would remain progression-free at different time points (Table 3). With adjuvant chemotherapy (both treated and control groups), chemoradiotherapy and SCLC, our analyses suggest that 11–20% of the rapidly progressing subpopulations would already have progressed by 3 months post therapy initiation.

Table 3.
A proportion of patients remaining progression-free at different time points.
Due to early convexities on the PFS curves, these calculations might overestimate or underestimate the proportion progressing by 3 months, but these curve convexities would have less impact on calculations for later time points since half-life estimates included the deviation caused by early convexities. The estimates indicate that frequent follow-up scans would initially be important to detect early relapses.
For the calculations of the percent of patients destined to eventually progress shown in Table 3, we coded the size of the potentially cured subpopulations as being constant for the adjuvant trial controls and chemoradiotherapy and SCLC groups. This assumption was based on the long PFS half-lives for these subpopulations. This would somewhat overestimate the size of this subpopulation at later times since at least some patients would have died from other causes, even if none relapsed.
For those receiving adjuvant chemotherapy, we coded the size of the potentially cured subpopulation as decreasing slowly over the time of interest, based on the subpopulations’ half-lives. It is unknown if the gradual decrease in size of this adjuvant chemotherapy subpopulation was due to eventual tumor progression vs. deaths from other causes. In addition, these calculations might change for very late time points if there were a second rightward inflection point on the PFS log–linear plots, as discussed above.
Acknowledging the limitations previously noted, the data presented in Table 3 can be used to estimate the risk of future progression for patients who remain progression-free at different time points. Across these different patient groups, the probability of cure would be 39–77% for those remaining relapse-free at 2 years, 75–91% for patients remaining relapse-free at 4 years and 87–96% for those remaining relapse-free at 5 years. There would continue to be a small possibility of relapse (but <1%) at 10 years and beyond.
3.9. Optimum Frequency of Follow-Up Scans
For all groups, the relapsing subpopulation would become a lower proportion of the entire remaining population over time, and it would be reasonable to perform scans progressively less frequently over time (Table 4). For example, in the “adjuvant controls” group in Table 4, 17% of the patients would be found to have progressed on a scan undertaken 6 months after surgery, but for patients who remained progression-free 24 months after surgery, only 8% would subsequently be found to have progressed on a scan performed 6 months later at 30 months after surgery. Hence, across these different curative therapy situations, relatively frequent scans might be considered early following therapy initiation, with no further follow-up scans at later time points except for investigation of concerning new symptoms or for screening for new primaries.

Table 4.
The percent of patients who remain progression-free at different time intervals (0–120 months) after therapy initiation who would be found to have progressed on a subsequent scan performed 2, 4, 6, or 12 months later.
3.10. Changes in SCLC Outcomes over Time
We also performed additional exploratory assessments. In SCLC, the outcome was better for studies published 2011–2021 than for studies published 1990–2010, with the median value for PFS half-lives increasing to 19.5 months from 15.0 months (p = 0.003), with the proportion in the potentially cured subpopulation increasing to 22% from 12% (p = 0.006), and with 92% vs. 87% potentially cured among those progression-free at 5 years. This apparent improvement might be due to stage migration (with increasing staging with PET/CT scanning) or to improved radiotherapy approaches.
3.11. Durvalumab Addition to Chemoradiation
For the PACIFIC trial comparing durvalumab to placebo following chemoradiotherapy [25], the durvalumab arm had an overall PFS half-life of 23.6 months, and the proportion of the patients in the potentially cured arm was high, at 57%. This favorable outcome was due in part to patient selection since patients could only receive durvalumab if they completed chemoradiotherapy without progressing. In keeping with this, the proportion of potentially cured patients in the control group was also relatively high, at 26%.
The PFS half-life for the potentially cured durvalumab subpopulation was shorter than for many other trials, at 61.4 months. Overall, durvalumab substantially prolonged PFS, but further follow-up will be required to determine if it is increasing the probability of true cure. If it is only delaying progression, then PFS curves with longer follow-up may eventually demonstrate three-phase decay, with a relapsing subpopulation, a cured subpopulation, and an intermediate group with delayed progression, as discussed earlier for adjuvant chemotherapy.
PFS half-lives in the relapsing subpopulation were similar on the durvalumab and placebo arms. In our earlier assessments of PFS curves for different therapy types, immune checkpoint inhibitors appeared to either have a marked beneficial effect or else almost no benefit in patients with metastatic disease [4]. The same may be happening in this post chemoradiation setting.
3.12. Chemotherapy Regimen Added to Curative Radiotherapy
Acknowledging the limitations of cross-study comparisons, we found no evidence of benefit from induction chemotherapy prior to concurrent chemoradiotherapy or of consolidation chemotherapy following chemoradiotherapy (Table 5), in keeping with published meta-analyses [90]. We also saw no evidence of a meaningful difference in outcome between pemetrexed, a topoisomerase inhibitor or a vinca alkaloid when used concurrently with radiation.

Table 5.
PFS for chemotherapy used concurrently with radiotherapy in locally advanced NSCLC.
However, we noted some variability in the efficacy of different chemotherapy regimens, in keeping with prior publications [91]. Specifically, the use of taxane plus platinum concurrently with radiation was associated with a shorter overall PFS half-life than the use of another agent with platinum (11.5 months vs. 13.7 months, p = 0.03) and with a slight reduction in the percent of patients in the potentially cured subpopulation (10.5% vs. 13%, p = 0.049). The overall PFS half-life was 11.4 months for a carboplatin regimen concurrently with radiation vs. 13.7 months with a cisplatin regimen (p = 0.04), and the proportion of patients in the potentially cured subpopulation was 11% vs. 12.5% (p = 0.14).
Ten of the thirteen trials using carboplatin also used taxane, and ten of the fourteen trials using taxane also used carboplatin. Hence, if drug choice has a true impact, it is unclear if the major impact is taxanes vs. other agents or if it is carboplatin vs. cisplatin. Nevertheless, while some differences were statistically significant, they were small and of limited clinical significance.
4. Discussion
We have previously published work on the use of population survival kinetics analyses in relation to incurable solid tumors [2,3,4,10,11], with limited prior data presented on their use in potentially cured populations [3]. Here, we expand on the use of the methodology in potentially cured populations. These analyses permit estimation of the proportion of patients potentially cured, the PFS half-life for the subpopulation destined to eventually relapse, and the probability of eventual progression in patients remaining progression-free at different time points after therapy initiation.
Similar assessments could also potentially be undertaken using other available methods, but this method is convenient since it can be easily and rapidly performed using readily available tools. Within minutes, published PFS and OS curves can be digitized, converted to log–linear plots, and analyzed. Here, and in earlier publications [2,3,4,10,11], we have discussed how population survival kinetics analyses might offer insights from clinical trial data over and above the insights typically gained from the calculation of medians, hazard ratios, p values, etc.
The premise of this approach is that PFS and OS curves generally follow first-order kinetics, with log–linear plots that approximate straight lines, and that major deviations from a straight log–linear line may offer useful insights. For example, early curve convexity at 1–3 months is seen since early progression is generally not identified until a patient’s first follow-up scan. Later curve convexity, typically starting at 3.5 months or longer after therapy initiation, can occur due to the late acceleration of progression or death. This might arise from drug dose reduction or discontinuation prior to tumor progression, from late initiation of an additional agent or measure that reduced drug absorption or escalated drug elimination, or from the late acceleration of the development of a resistance mechanism. The assessment of individual patient data could elucidate potential causes. Rightward log–linear curve deviation at an inflection point suggests the presence of two distinct subpopulations based on a dichotomous present vs. absent factor that impacts prognosis or therapy efficacy.
Population survival kinetics analyses can be used to assess if two subpopulations or trial arms differ with respect to the overall slope of their PFS or OS curves, the extent of late acceleration of progression or death, the relative size of a favorable subpopulation, and the rapidity of progression of the rapidly progressing subpopulation. One can estimate the proportion of the population that is potentially cured, the probability of future relapse at any time point, and the proportion of remaining patients who will remain progression-free or alive at different time points in the future. These analyses would permit the easy incorporation of these parameters into standard reporting of clinical trial outcomes.
The shape of the PFS and OS curves can be interpreted in different ways. For example, if two curves cross or initially overlap and then separate, one common explanation is that “therapy benefit occurs late”. Conversely, the population survival kinetics explanation is that the better curve has two distinct subpopulations: one subpopulation derives little or no added benefit from the new therapy and has a PFS similar to that of the control group. Conversely, the second subpopulation derives substantial benefit from the new therapy and is doing much better than the control group.
When curves initially separate and then come together, one potential explanation is that a treatment is losing its effect over time. Population survival kinetics assessments suggest that it instead often means that for a therapy that was controlling a tumor, therapy interruption is followed by rapid progression.
Since we first undertook population survival kinetics assessments, our approaches and perspectives have evolved, and we anticipate that they will continue to change as we and others further test this approach.
With respect to future applications, population survival kinetics methods could be applied to a wide range of both oncology data and non-oncology biological states. Artificial intelligence approaches could be explored to rapidly retrieve and analyze very large amounts of clinical and research data. As noted above, using individual patient data could facilitate the use of approaches such as nonlinear mixed effects modeling to assess the impact of relevant clinical variables on outcomes.
5. Limitations
Our proposed population survival kinetics methods have some potential limitations. As noted previously, early PFS curve convexities could result in overestimation or underestimation of the proportion of patients progressing at 3 months but would be expected to have less of an impact on estimates of progression at later time points. In addition, based on the long PFS half-lives for the adjuvant trial controls and chemoradiotherapy and SCLC groups, we coded the size of the potentially cured subpopulations as being constant. This somewhat overestimates the size of this subpopulation at later times since, even in the absence of relapse, at least some patients would die from other causes.
As noted previously, the method has no specific control for experimental error that might be intrinsic within data for a given curve. However, in the online Supplementary Tables S1–S3, we included the sample size for each trial to help the reader assess the strength of the evidence.
We also recognize that conclusions from across-study comparisons (e.g., with respect to the induction or consolidation chemotherapy with radiotherapy) have to be interpreted cautiously.
Furthermore, we have not yet tested this methodology with individual patient data and real-world evidence. Using individual patient data could facilitate the assessment of the role of dose reductions, therapy interruptions and other factors on curve convexity. It could also facilitate the assessment of the impact on outcomes of various patient and biological characteristics, such as tumor size, tumor differentiation, PD-L1 expression, tumor mutations, gender, age, race/ethnicity, etc. Methods such as nonlinear mixed effects modeling [92] might prove useful in this. This could facilitate increased personalization of optimal scan frequency, prediction of the probability of future relapse, etc.
Another limitation is that short patient follow-up is as limiting for these analyses as for any other approach used in assessing clinical trial data. In our two-phase decay analyses, 95% confidence intervals were generally narrow for the proportion of patients in the relapsing subpopulation, but they were typically very wide or not calculable for PFS half-lives for potentially cured subpopulations. As would be expected, more mature data with longer patient follow-ups were associated with a greater probability that 95% confidence intervals could be calculated.
Low patient numbers at the terminal portion of a survival curve mean that the loss of one patient might lead to a large drop in the curve. Conversely, the presence of a single long survivor might impact assessment by producing a very long tail on the curve. In some of our earlier analyses [2,4,10], we truncated curves if there were less than an estimated 10 remaining patients. We have not yet adequately assessed the impact of this truncation, but in preliminary assessments, it generally had minimal impact on the overall curve half-life but did somewhat reduce the probability of a curve fitting two-phase decay models (D. Stewart, unpublished data). Our requirement that each subpopulation had to be ≥1% of the entire population for a curve to be designated as fitting a two-phase decay model also means that the method would miss very small favorable subpopulations.
As with any methodology, there is at least some risk of incorrect conclusions due to misinterpretation of data. As much caution is required in the assessment of results using these methods as with any analytical method. One advantage of this approach is that these analyses can be reassessed easily by others since they use accessible published data and readily available analytical tools.
6. Conclusions
In summary, population survival kinetics analyses can be performed easily and rapidly using readily available tools. These analyses may provide useful insights into clinical trial data, including PFS log–linear curve shape, PFS half-life for the overall population, the relative size of distinct subpopulations, PFS half-lives for subpopulations, and the probability of future relapse for patients remaining relapse-free at a given time point.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/curroncol31030122/s1, Table S1: PFS population survival kinetics assessment for NSCLC postoperative adjuvant platinum regimens vs controls, Table S2: PFS population survival kinetics assessment of chemoradiation for locally advanced NSCLC, Table S3: PFS population survival kinetics assessment of limited small cell lung cancer, Tutorial S1 on Population Survival Kinetics Methodology.
Author Contributions
Conceptualization, D.J.S.; methodology, D.J.S. and D.B.; formal analysis, D.J.S.; writing—original draft preparation, D.J.S.; writing—review and editing, D.J.S., K.C., D.B., S.B., D.F. and T.R.; funding acquisition, not applicable All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available in Tables S1–S3.
Acknowledgments
Presented in part and/or abstract published in the proceedings of the World Conference on Lung Cancer 2022, the annual meetings of the American Society of Clinical Oncology 2022, and the annual meetings of the American Association for Cancer Research 2022.
Conflicts of Interest
D.J.S. has received honoraria or advisory board or consulting fees from Merck Canada, AstraZeneca Canada, Abbvie Canada, and Amgen Canada (none directly relevant to this work) and owns a 3% interest in US Patent no. 9.675.663 (a test to predict response to TUSC2/FUS1 gene therapy). The Ottawa Hospital receives research support from a broad range of pharmaceutical companies. D.B. received honoraria or advisory board or speaker fees from Ipsen, Amgen, AstraZeneca Canada, BMS, Merck, Knights Pharmaceutical, Pfizer, Janssen, EMD Serono, Bayer (none directly relevant to this work). K.C., S.B., D.F. and T.R. have no conflicts to declare.
References
- Hill, S.A. Pharmacokinetics of drug infusions. Contin. Educ. Anaesth. Crit. Care Pain 2004, 4, 76–80. [Google Scholar] [CrossRef]
- Stewart, D.J.; Bosse, D.; Goss, G.; Hilton, J.F.; Jonker, D.; Fung-Kee-Fung, M. A novel, more reliable approach to use of progression-free survival as a predictor of gain in overall survival: The Ottawa PFS Predictive Model. Crit. Rev. Oncol. Hematol. 2020, 148, 102896. [Google Scholar] [CrossRef]
- Stewart, D.J.; Behrens, C.; Roth, J.; Wistuba, I.I. Exponential decay nonlinear regression analysis of patient survival curves: Preliminary assessment in non-small cell lung cancer. Lung Cancer 2011, 71, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J.; Bosse, D.; Robinson, A.; Ong, M.; Fung-Kee-Fung, M.; Brule, S.; Hilton, J.F.; Ocana, A. Potential insights from population kinetic assessment of progression-free survival curves. Crit. Rev. Oncol. Hematol. 2020, 153, 103039. [Google Scholar] [CrossRef] [PubMed]
- Broglio, K.R.; Berry, D.A. Detecting an overall survival benefit that is derived from progression-free survival. J. Natl. Cancer Inst. 2009, 101, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Del Paggio, J.C.; Berry, J.S.; Hopman, W.M.; Eisenhauer, E.A.; Prasad, V.; Gyawali, B.; Booth, C.M. Evolution of the Randomized Clinical Trial in the Era of Precision Oncology. JAMA Oncol. 2021, 7, 728–734. [Google Scholar] [CrossRef]
- Booth, C.M.; Eisenhauer, E.A. Progression-free survival: Meaningful or simply measurable? J. Clin. Oncol. 2012, 30, 1030–1033. [Google Scholar] [CrossRef]
- Gyawali, B.; Hey, S.P.; Kesselheim, A.S. Evaluating the evidence behind the surrogate measures included in the FDA’s table of surrogate endpoints as supporting approval of cancer drugs. EClinicalMedicine 2020, 21, 100332. [Google Scholar] [CrossRef]
- Prasad, V.; Kim, C.; Burotto, M.; Vandross, A. The Strength of Association Between Surrogate End Points and Survival in Oncology: A Systematic Review of Trial-Level Meta-analyses. JAMA Intern. Med. 2015, 175, 1389–1398. [Google Scholar] [CrossRef]
- Stewart, D.J.; Macdonald, D.B.; Awan, A.A.; Thavorn, K. Optimal frequency of scans for patients on cancer therapies: A population kinetics assessment. Cancer Med. 2019, 8, 6871–6886. [Google Scholar] [CrossRef]
- Stewart, D.J.; Maziak, D.E.; Moore, S.M.; Brule, S.Y.; Gomes, M.; Sekhon, H.; Dennie, C.; Lo, B.; Fung-Kee-Fung, M.; Bradford, J.P.; et al. The need for speed in advanced non-small cell lung cancer: A population kinetics assessment. Cancer Med. 2021, 10, 9040–9046. [Google Scholar] [CrossRef]
- Douillard, J.Y.; Rosell, R.; De Lena, M.; Carpagnano, F.; Ramlau, R.; Gonzales-Larriba, J.L.; Grodzki, T.; Pereira, J.R.; Le Groumellec, A.; Lorusso, V.; et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): A randomised controlled trial. Lancet Oncol. 2006, 7, 719–727. [Google Scholar] [CrossRef]
- Arriagada, R.; Dunant, A.; Pignon, J.P.; Bergman, B.; Chabowski, M.; Grunenwald, D.; Kozlowski, M.; Le Pechoux, C.; Pirker, R.; Pinel, M.I.; et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J. Clin. Oncol. 2010, 28, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Strauss, G.M.; Herndon, J.E., 2nd; Maddaus, M.A.; Johnstone, D.W.; Johnson, E.A.; Harpole, D.H.; Gillenwater, H.H.; Watson, D.M.; Sugarbaker, D.J.; Schilsky, R.L.; et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J. Clin. Oncol. 2008, 26, 5043–5051. [Google Scholar] [CrossRef]
- Butts, C.A.; Ding, K.; Seymour, L.; Twumasi-Ankrah, P.; Graham, B.; Gandara, D.; Johnson, D.H.; Kesler, K.A.; Green, M.; Vincent, M.; et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: Updated survival analysis of JBR-10. J. Clin. Oncol. 2010, 28, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef]
- Ahn, J.S.; Ahn, Y.C.; Kim, J.H.; Lee, C.G.; Cho, E.K.; Lee, K.C.; Chen, M.; Kim, D.W.; Kim, H.K.; Min, Y.J.; et al. Multinational Randomized Phase III Trial with or Without Consolidation Chemotherapy Using Docetaxel and Cisplatin After Concurrent Chemoradiation in Inoperable Stage III Non-Small-Cell Lung Cancer: KCSG-LU05-04. J. Clin. Oncol. 2015, 33, 2660–2666. [Google Scholar] [CrossRef]
- Atagi, S.; Kawahara, M.; Yokoyama, A.; Okamoto, H.; Yamamoto, N.; Ohe, Y.; Sawa, T.; Ishikura, S.; Shibata, T.; Fukuda, H.; et al. Thoracic radiotherapy with or without daily low-dose carboplatin in elderly patients with non-small-cell lung cancer: A randomised, controlled, phase 3 trial by the Japan Clinical Oncology Group (JCOG0301). Lancet Oncol. 2012, 13, 671–678. [Google Scholar] [CrossRef]
- Bradley, J.D.; Hu, C.; Komaki, R.R.; Masters, G.A.; Blumenschein, G.R.; Schild, S.E.; Bogart, J.A.; Forster, K.M.; Magliocco, A.M.; Kavadi, V.S.; et al. Long-Term Results of NRG Oncology RTOG 0617: Standard- Versus High-Dose Chemoradiotherapy With or Without Cetuximab for Unresectable Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 706–714. [Google Scholar] [CrossRef]
- Butts, C.; Socinski, M.A.; Mitchell, P.L.; Thatcher, N.; Havel, L.; Krzakowski, M.; Nawrocki, S.; Ciuleanu, T.E.; Bosquee, L.; Trigo, J.M.; et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 59–68. [Google Scholar] [CrossRef]
- Carter, D.L.; Garfield, D.; Hathorn, J.; Mundis, R.; Boehm, K.A.; Ilegbodu, D.; Asmar, L.; Reynolds, C. A randomized phase III trial of combined paclitaxel, carboplatin, and radiation therapy followed by weekly paclitaxel or observation for patients with locally advanced inoperable non-small-cell lung cancer. Clin. Lung Cancer 2012, 13, 205–213. [Google Scholar] [CrossRef]
- Chang, J.Y.; Verma, V.; Li, M.; Zhang, W.; Komaki, R.; Lu, C.; Allen, P.K.; Liao, Z.; Welsh, J.; Lin, S.H.; et al. Proton Beam Radiotherapy and Concurrent Chemotherapy for Unresectable Stage III Non-Small Cell Lung Cancer: Final Results of a Phase 2 Study. JAMA Oncol. 2017, 3, e172032. [Google Scholar] [CrossRef]
- Choy, H.; Schwartzberg, L.S.; Dakhil, S.R.; Garon, E.B.; Gerber, D.E.; Choksi, J.K.; Govindan, R.; Peng, G.; Koustenis, A.; Treat, J.; et al. Phase 2 study of pemetrexed plus carboplatin, or pemetrexed plus cisplatin with concurrent radiation therapy followed by pemetrexed consolidation in patients with favorable-prognosis inoperable stage IIIA/B non-small-cell lung cancer. J. Thorac. Oncol. 2013, 8, 1308–1316. [Google Scholar] [CrossRef]
- Faivre-Finn, C.; Vicente, D.; Kurata, T.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Spigel, D.R.; Garassino, M.C.; Reck, M.; Senan, S.; et al. Four-Year Survival with Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update from the PACIFIC Trial. J. Thorac. Oncol. 2021, 16, 860–867. [Google Scholar] [CrossRef]
- Fenwick, J.D.; Landau, D.B.; Baker, A.T.; Bates, A.T.; Eswar, C.; Garcia-Alonso, A.; Harden, S.V.; Illsley, M.C.; Laurence, V.; Malik, Z.; et al. Long-Term Results from the IDEAL-CRT Phase 1/2 Trial of Isotoxically Dose-Escalated Radiation Therapy and Concurrent Chemotherapy for Stage II/III Non-small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 733–742. [Google Scholar] [CrossRef]
- Flentje, M.; Huber, R.M.; Engel-Riedel, W.; Andreas, S.; Kollmeier, J.; Staar, S.; Dickgreber, N.; Vaissiere, N.; De Almeida, C.; Edlich, B.; et al. GILT—A randomised phase III study of oral vinorelbine and cisplatin with concomitant radiotherapy followed by either consolidation therapy with oral vinorelbine and cisplatin or best supportive care alone in stage III non-small cell lung cancer. Strahlenther. Onkol. 2016, 192, 216–222. [Google Scholar] [CrossRef]
- Fournel, P.; Vergnenegre, A.; Robinet, G.; Lena, H.; Gervais, R.; Le Caer, H.; Souquet, P.J.; Chavaillon, J.M.; Bozonnat, M.C.; Daures, J.P.; et al. Induction or consolidation chemotherapy for unresectable stage III non-small-cell lung cancer patients treated with concurrent chemoradiation: A randomised phase II trial GFPC-IFCT 02-01. Eur. J. Cancer 2016, 52, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Garrido, P.; Engel-Riedel, W.; Serke, M.; Giraud, P.; Ricardi, U.; Vallejo, C.; Visseren-Grul, C.; Ameryckx, S.; Soldatenkova, V.; Chouaki, N.; et al. Final results from a Phase II study of pemetrexed and cisplatin with concurrent thoracic radiation after Pem-Cis induction in patients with unresectable locally advanced non-squamous non-small cell lung cancer (NSCLC). Lung Cancer 2015, 88, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Govindan, R.; Bogart, J.; Stinchcombe, T.; Wang, X.; Hodgson, L.; Kratzke, R.; Garst, J.; Brotherton, T.; Vokes, E.E. Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small-cell lung cancer: Cancer and Leukemia Group B trial 30407. J. Clin. Oncol. 2011, 29, 3120–3125. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.; Dahlberg, S.E.; Schiller, J.H.; Mehta, M.P.; Fitzgerald, T.J.; Belinsky, S.A.; Johnson, D.H. Randomized phase III study of thoracic radiation in combination with paclitaxel and carboplatin with or without thalidomide in patients with stage III non-small-cell lung cancer: The ECOG 3598 study. J Clin Oncol 2012, 30, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, H.; Sekine, I.; Sumi, M.; Noda, K.; Goto, K.; Mori, K.; Tamura, T. Long-term results of concurrent chemoradiotherapy using cisplatin and vinorelbine for stage III non-small-cell lung cancer. Cancer Sci. 2013, 104, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; Konishi, K.; Uchida, J.; Nishino, K.; Okuyama, T.; Kumagai, T.; Kawaguchi, Y.; Nishiyama, K. Novel chemoradiotherapy with concomitant boost thoracic radiation and concurrent cisplatin and vinorelbine for stage IIIA and IIIB non-small-cell lung cancer. Clin. Lung Cancer 2014, 15, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Isla, D.; De Las Penas, R.; Insa, A.; Marse, R.; Martinez-Banaclocha, N.; Mut, P.; Moran, T.; Sala, M.A.; Massuti, B.; Ortega, A.L.; et al. Oral vinorelbine versus etoposide with cisplatin and chemo-radiation as treatment in patients with stage III non-small cell lung cancer: A randomized phase II (RENO study). Lung Cancer 2019, 135, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Takada, M.; Ando, M.; Okishio, K.; Atagi, S.; Fujita, Y.; Tomizawa, Y.; Hayashihara, K.; Okano, Y.; Takahashi, F.; et al. A multi-institutional phase II trial of consolidation S-1 after concurrent chemoradiotherapy with cisplatin and vinorelbine for locally advanced non-small cell lung cancer. Eur. J. Cancer 2012, 48, 672–677. [Google Scholar] [CrossRef]
- Kerner, G.S.; van Dullemen, L.F.; Wiegman, E.M.; Widder, J.; Blokzijl, E.; Driever, E.M.; van Putten, J.W.; Liesker, J.J.; Renkema, T.E.; Pieterman, R.M.; et al. Concurrent gemcitabine and 3D radiotherapy in patients with stage III unresectable non-small cell lung cancer. Radiat. Oncol. 2014, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, Y.R.; Paulus, R.; Langer, C.; Werner-Wasik, M.; Buyyounouski, M.K.; Komaki, R.; Machtay, M.; Smith, C.; Axelrod, R.S.; Wasserman, T.; et al. The addition of amifostine to carboplatin and paclitaxel based chemoradiation in locally advanced non-small cell lung cancer: Long-term follow-up of Radiation Therapy Oncology Group (RTOG) randomized trial 9801. Lung Cancer 2013, 80, 298–305. [Google Scholar] [CrossRef]
- Lerouge, D.; Riviere, A.; Dansin, E.; Chouaid, C.; Dujon, C.; Schott, R.; Lavole, A.; Le Pennec, V.; Fabre, E.; Crequit, J.; et al. A phase II study of cisplatin with intravenous and oral vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy with oral vinorelbine and cisplatin for locally advanced non-small cell lung cancer. BMC Cancer 2014, 14, 231. [Google Scholar] [CrossRef]
- Liang, J.; Bi, N.; Wu, S.; Chen, M.; Lv, C.; Zhao, L.; Shi, A.; Jiang, W.; Xu, Y.; Zhou, Z.; et al. Etoposide and cisplatin versus paclitaxel and carboplatin with concurrent thoracic radiotherapy in unresectable stage III non-small cell lung cancer: A multicenter randomized phase III trial. Ann. Oncol. 2017, 28, 777–783. [Google Scholar] [CrossRef]
- Lu, C.; Lee, J.J.; Komaki, R.; Herbst, R.S.; Feng, L.; Evans, W.K.; Choy, H.; Desjardins, P.; Esparaz, B.T.; Truong, M.T.; et al. Chemoradiotherapy with or without AE-941 in stage III non-small cell lung cancer: A randomized phase III trial. J. Natl. Cancer Inst. 2010, 102, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Niho, S.; Yoshida, T.; Akimoto, T.; Sakamaki, K.; Ono, A.; Seto, T.; Nishio, M.; Yamamoto, N.; Hida, T.; Okamoto, H.; et al. Randomized phase II study of chemoradiotherapy with cisplatin + S-1 versus cisplatin + pemetrexed for locally advanced non-squamous non-small cell lung cancer: SPECTRA study. Lung Cancer 2020, 141, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Noh, J.M.; Kim, Y.J.; Lee, H.S.; Cho, J.H.; Lim, S.W.; Ahn, Y.C.; Pyo, H.; Choi, Y.L.; Han, J.; et al. EGFR Mutation Is Associated with Short Progression-Free Survival in Patients with Stage III Non-squamous Cell Lung Cancer Treated with Concurrent Chemoradiotherapy. Cancer Res. Treat. 2019, 51, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Price, A.; Yellowlees, A.; Keerie, C.; Russell, S.; Faivre-Finn, C.; Gilligan, D.; Snee, M.; Skailes, G.; Hatton, M.; Erridge, S.; et al. Radical radiotherapy with or without gemcitabine in patients with early stage medically inoperable non-small cell lung cancer. Lung Cancer 2012, 77, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Provencio, M.; Majem, M.; Guirado, M.; Massuti, B.; de Las Penas, R.; Ortega, A.L.; Domine, M.; Marse, R.; Sala, M.A.; Paredes, A.; et al. Phase II clinical trial with metronomic oral vinorelbine and tri-weekly cisplatin as induction therapy, subsequently concomitant with radiotherapy (RT) in patients with locally advanced, unresectable, non-small cell lung cancer (NSCLC). Analysis of survival and value of ctDNA for patient selection. Lung Cancer 2021, 153, 25–34. [Google Scholar] [PubMed]
- Sasaki, T.; Seto, T.; Yamanaka, T.; Kunitake, N.; Shimizu, J.; Kodaira, T.; Nishio, M.; Kozuka, T.; Takahashi, T.; Harada, H.; et al. A randomised phase II trial of S-1 plus cisplatin versus vinorelbine plus cisplatin with concurrent thoracic radiotherapy for unresectable, locally advanced non-small cell lung cancer: WJOG5008L. Br. J. Cancer 2018, 119, 675–682. [Google Scholar] [CrossRef]
- Senan, S.; Brade, A.; Wang, L.H.; Vansteenkiste, J.; Dakhil, S.; Biesma, B.; Martinez Aguillo, M.; Aerts, J.; Govindan, R.; Rubio-Viqueira, B.; et al. PROCLAIM: Randomized Phase III Trial of Pemetrexed-Cisplatin or Etoposide-Cisplatin Plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, T.; Yamada, K.; Tanaka, H.; Kubota, K.; Takiguchi, Y.; Kishi, K.; Saito, H.; Hosomi, Y.; Kato, T.; Harada, D.; et al. Randomized phase II trial of S-1 plus cisplatin or docetaxel plus cisplatin with concurrent thoracic radiotherapy for inoperable stage III non-small cell lung cancer. Cancer Med. 2021, 10, 626–633. [Google Scholar] [CrossRef] [PubMed]
- van Baardwijk, A.; Reymen, B.; Wanders, S.; Borger, J.; Ollers, M.; Dingemans, A.M.; Bootsma, G.; Geraedts, W.; Pitz, C.; Lunde, R.; et al. Mature results of a phase II trial on individualised accelerated radiotherapy based on normal tissue constraints in concurrent chemo-radiation for stage III non-small cell lung cancer. Eur. J. Cancer 2012, 48, 2339–2346. [Google Scholar] [CrossRef]
- Vera, P.; Mihailescu, S.D.; Lequesne, J.; Modzelewski, R.; Bohn, P.; Hapdey, S.; Pepin, L.F.; Dubray, B.; Chaumet-Riffaud, P.; Decazes, P.; et al. Radiotherapy boost in patients with hypoxic lesions identified by (18)F-FMISO PET/CT in non-small-cell lung carcinoma: Can we expect a better survival outcome without toxicity? [RTEP5 long-term follow-up]. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1448–1456. [Google Scholar] [CrossRef]
- Wada, K.; Kishi, N.; Kanayama, N.; Hirata, T.; Morimoto, M.; Konishi, K.; Imamura, F.; Teshima, T.; Ogawa, K. Radiation Dose Escalation in Accelerated Hyperfractionated Radiotherapy for Stage III Non-small-cell Lung Cancer. Anticancer. Res. 2018, 38, 5951–5958. [Google Scholar] [CrossRef]
- Yamamoto, N.; Nakagawa, K.; Nishimura, Y.; Tsujino, K.; Satouchi, M.; Kudo, S.; Hida, T.; Kawahara, M.; Takeda, K.; Katakami, N.; et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J. Clin. Oncol. 2010, 28, 3739–3745. [Google Scholar] [CrossRef]
- Glinski, K.; Socha, J.; Wasilewska-Tesluk, E.; Komosinska, K.; Kepka, L. Accelerated hypofractionated radiotherapy with concurrent full dose chemotherapy for locally advanced non-small cell lung cancer: A phase I/II study. Radiother. Oncol. 2020, 148, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, R.; Le Chevalier, T.; Ruffie, P.; Chomy, P.; de Cremoux, H. Alternating radiotherapy and chemotherapy in limited small cell lung cancer: The IGR protocols. French FNCLCC Lung Cancer Study Group. Lung Cancer 1994, 10 (Suppl. S1), S289–S298. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, R.; le Chevalier, T.; Ruffie, P.; Baldeyrou, P.; De Cremoux, H.; Martin, M.; Chomy, P.; Cerrina, M.L.; Pellae-Cosset, B.; Tarayre, M.; et al. Alternating radiotherapy and chemotherapy in 173 consecutive patients with limited small cell lung carcinoma. GROP and the French Cancer Center’s Lung Group. Int. J. Radiat. Oncol. Biol. Phys. 1990, 19, 1135–1138. [Google Scholar] [CrossRef]
- Beith, J.M.; Clarke, S.J.; Woods, R.L.; Bell, D.R.; Levi, J.A. Long-term follow-up of a randomised trial of combined chemoradiotherapy induction treatment, with and without maintenance chemotherapy in patients with small cell carcinoma of the lung. Eur. J. Cancer 1996, 32A, 438–443. [Google Scholar] [CrossRef]
- Blackstock, A.W.; Bogart, J.A.; Matthews, C.; Lovato, J.F.; McCoy, T.; Livengood, K.; Ho, C.; White, D.; Atkins, J.N.; Miller, A.A. Split-course versus continuous thoracic radiation therapy for limited-stage small-cell lung cancer: Final report of a randomized phase III trial. Clin. Lung Cancer 2005, 6, 287–292. [Google Scholar] [CrossRef]
- Bogart, J.A.; Herndon, J.E.; Lyss, A.P., 2nd; Watson, D.; Miller, A.A.; Lee, M.E.; Turrisi, A.T.; Green, M.R. 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: Analysis of Cancer and Leukemia Group B study 39808. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 460–468. [Google Scholar] [CrossRef]
- Bonner, J.A.; Sloan, J.A.; Shanahan, T.G.; Brooks, B.J.; Marks, R.S.; Krook, J.E.; Gerstner, J.B.; Maksymiuk, A.; Levitt, R.; Mailliard, J.A.; et al. Phase III comparison of twice-daily split-course irradiation versus once-daily irradiation for patients with limited stage small-cell lung carcinoma. J. Clin. Oncol. 1999, 17, 2681–2691. [Google Scholar] [CrossRef] [PubMed]
- Edelman, M.J.; Chansky, K.; Gaspar, L.E.; Leigh, B.; Weiss, G.R.; Taylor, S.A.; Crowley, J.; Livingston, R.; Gandara, D.R. Phase II trial of cisplatin/etoposide and concurrent radiotherapy followed by paclitaxel/carboplatin consolidation for limited small-cell lung cancer: Southwest Oncology Group 9713. J. Clin. Oncol. 2004, 22, 127–132. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Berkey, B.A.; Abrams, R.A.; Fontanesi, J.; Machtay, M.; Duncan, P.J.; Curran, W.J., Jr.; Movsas, B.; Byhardt, R.W.; Radiation Therapy Oncology Group 9609. Study of paclitaxel, etoposide, and cisplatin chemotherapy combined with twice-daily thoracic radiotherapy for patients with limited-stage small-cell lung cancer: A Radiation Therapy Oncology Group 9609 phase II study. J. Clin. Oncol. 2005, 23, 4991–4998. [Google Scholar] [CrossRef]
- Glisson, B.; Scott, C.; Komaki, R.; Movsas, B.; Wagner, H. Cisplatin, ifosfamide, oral etoposide, and concurrent accelerated hyperfractionated thoracic radiation for patients with limited small-cell lung carcinoma: Results of radiation therapy oncology group trial 93-12. J. Clin. Oncol. 2000, 18, 2990–2995. [Google Scholar] [CrossRef]
- Goodman, G.E.; Crowley, J.J.; Blasko, J.C.; Livingston, R.B.; Beck, T.M.; Demattia, M.D.; Bukowski, R.M. Treatment of limited small-cell lung cancer with etoposide and cisplatin alternating with vincristine, doxorubicin, and cyclophosphamide versus concurrent etoposide, vincristine, doxorubicin, and cyclophosphamide and chest radiotherapy: A Southwest Oncology Group Study. J. Clin. Oncol. 1990, 8, 39–47. [Google Scholar] [PubMed]
- Gregor, A.; Drings, P.; Burghouts, J.; Postmus, P.E.; Morgan, D.; Sahmoud, T.; Kirkpatrick, A.; Dalesio, O.; Giaccone, G. Randomized trial of alternating versus sequential radiotherapy/chemotherapy in limited-disease patients with small-cell lung cancer: A European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group Study. J. Clin. Oncol. 1997, 15, 2840–2849. [Google Scholar] [CrossRef] [PubMed]
- Gronberg, B.H.; Killingberg, K.T.; Flotten, O.; Brustugun, O.T.; Hornslien, K.; Madebo, T.; Langer, S.W.; Schytte, T.; Nyman, J.; Risum, S.; et al. High-dose versus standard-dose twice-daily thoracic radiotherapy for patients with limited stage small-cell lung cancer: An open-label, randomised, phase 2 trial. Lancet Oncol. 2021, 22, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, T.O.; Sundstrom, S.; Flotten, O.; Brustugun, O.T.; Brunsvig, P.; Aasebo, U.; Bremnes, R.M.; Kaasa, S.; Gronberg, B.H. Comorbidity and outcomes of concurrent chemo- and radiotherapy in limited disease small cell lung cancer. Acta Oncol. 2016, 55, 1349–1354. [Google Scholar] [CrossRef]
- Horn, L.; Bernardo, P.; Sandler, A.; Wagner, H.; Levitan, N.; Levitt, M.L.; Johnson, D.H. A phase II study of paclitaxel + etoposide + cisplatin + concurrent radiation therapy for previously untreated limited stage small cell lung cancer (E2596): A trial of the Eastern Cooperative Oncology Group. J. Thorac. Oncol. 2009, 4, 527–533. [Google Scholar] [CrossRef]
- Hugli, A.; Moro, D.; Mermillod, B.; Bolla, M.; Alberto, P.; Bonnefoi, H.; Miralbell, R. Phase II trial of up-front accelerated thoracic radiotherapy combined with chemotherapy and optional up-front prophylactic cranial irradiation in limited small-cell lung cancer. Groupe d’Oncologie Thoracique des Regions Alpines. J. Clin. Oncol. 2000, 18, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Jett, J.R.; Everson, L.; Therneau, T.M.; Krook, J.E.; Dalton, R.J.; Marschke, R.F., Jr.; Veeder, M.H.; Brunk, S.F.; Mailliard, J.A.; Twito, D.I.; et al. Treatment of limited-stage small-cell lung cancer with cyclophosphamide, doxorubicin, and vincristine with or without etoposide: A randomized trial of the North Central Cancer Treatment Group. J. Clin. Oncol. 1990, 8, 33–38. [Google Scholar] [CrossRef]
- Kelley, M.J.; Bogart, J.A.; Hodgson, L.D.; Ansari, R.H.; Atkins, J.N.; Pang, H.; Green, M.R.; Vokes, E.E. Phase II study of induction cisplatin and irinotecan followed by concurrent carboplatin, etoposide, and thoracic radiotherapy for limited-stage small-cell lung cancer, CALGB 30206. J. Thorac. Oncol. 2013, 8, 102–108. [Google Scholar] [CrossRef]
- Komaki, R.; Paulus, R.; Ettinger, D.S.; Videtic, G.M.; Bradley, J.D.; Glisson, B.S.; Langer, C.J.; Sause, W.T.; Curran, W.J., Jr.; Choy, H. Phase II study of accelerated high-dose radiotherapy with concurrent chemotherapy for patients with limited small-cell lung cancer: Radiation Therapy Oncology Group protocol 0239. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e531–e536. [Google Scholar] [CrossRef]
- Kubota, K.; Hida, T.; Ishikura, S.; Mizusawa, J.; Nishio, M.; Kawahara, M.; Yokoyama, A.; Imamura, F.; Takeda, K.; Negoro, S.; et al. Etoposide and cisplatin versus irinotecan and cisplatin in patients with limited-stage small-cell lung cancer treated with etoposide and cisplatin plus concurrent accelerated hyperfractionated thoracic radiotherapy (JCOG0202): A randomised phase 3 study. Lancet Oncol. 2014, 15, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.T.; Moon, J.; Redman, M.; Williamson, S.K.; Lara, P.N., Jr.; Goldberg, Z.; Gaspar, L.E.; Crowley, J.J.; Moore, D.F., Jr.; Gandara, D.R. Phase II study of tirapazamine, cisplatin, and etoposide and concurrent thoracic radiotherapy for limited-stage small-cell lung cancer: SWOG 0222. J. Clin. Oncol. 2009, 27, 3014–3019. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laack, E.; Thom, I.; Krull, A.; Engel-Riedel, W.; Muller, T.; Meissner, C.; Durk, H.; Fischer, J.; Gutz, S.; Kortsik, C.; et al. A phase II study of irinotecan (CPT-11) and carboplatin in patients with limited disease small cell lung cancer (SCLC). Lung Cancer 2007, 57, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, P.; Salem, A.; Mistry, H.; Gornall, M.; Harden, S.; Julyan, P.; Locke, I.; McAleese, J.; McMenemin, R.; Mohammed, N.; et al. (18)F-Fludeoxyglucose PET/CT in SCLC: Analysis of the CONVERT Randomized Controlled Trial. J. Thorac. Oncol. 2019, 14, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Maurer, L.H.; Herndon, J.E., 2nd; Hollis, D.R.; Aisner, J.; Carey, R.W.; Skarin, A.T.; Perry, M.C.; Eaton, W.L.; Zacharski, L.L.; Hammond, S.; et al. Randomized trial of chemotherapy and radiation therapy with or without warfarin for limited-stage small-cell lung cancer: A Cancer and Leukemia Group B study. J. Clin. Oncol. 1997, 15, 3378–3387. [Google Scholar] [CrossRef]
- McClay, E.F.; Bogart, J.; Herndon, J.E., 2nd; Watson, D.; Evans, L.; Seagren, S.L.; Green, M.R.; Cancer and Leukemia Group B Study (9235). A phase III trial evaluating the combination of cisplatin, etoposide, and radiation therapy with or without tamoxifen in patients with limited-stage small cell lung cancer: Cancer and Leukemia Group B Study (9235). Am. J. Clin. Oncol. 2005, 28, 81–90. [Google Scholar] [CrossRef]
- Miller, A.A.; Wang, X.F.; Bogart, J.A.; Hodgson, L.D.; Rocha Lima, C.M.; Radford, J.E.; Vokes, E.E.; Green, M.R.; Cancer and Leukemia Group B (CALGB). Phase II trial of paclitaxel-topotecan-etoposide followed by consolidation chemoradiotherapy for limited-stage small cell lung cancer: CALGB 30002. J. Thorac. Oncol. 2007, 2, 645–651. [Google Scholar] [CrossRef]
- Murray, N.; Coy, P.; Pater, J.L.; Hodson, I.; Arnold, A.; Zee, B.C.; Payne, D.; Kostashuk, E.C.; Evans, W.K.; Dixon, P.; et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 1993, 11, 336–344. [Google Scholar] [CrossRef]
- Perry, M.C.; Herndon, J.E., 3rd; Eaton, W.L.; Green, M.R. Thoracic radiation therapy added to chemotherapy for small-cell lung cancer: An update of Cancer and Leukemia Group B Study 8083. J. Clin. Oncol. 1998, 16, 2466–2467. [Google Scholar] [CrossRef]
- Qiu, B.; Li, Q.; Liu, J.; Huang, Y.; Pang, Q.; Zhu, Z.; Yang, X.; Wang, B.; Chen, L.; Fang, J.; et al. Moderately Hypofractionated Once-Daily Compared with Twice-Daily Thoracic Radiation Therapy Concurrently with Etoposide and Cisplatin in Limited-Stage Small Cell Lung Cancer: A Multicenter, Phase II, Randomized Trial. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 424–435. [Google Scholar] [CrossRef]
- Salama, J.K.; Hodgson, L.; Pang, H.; Urbanic, J.J.; Blackstock, A.W.; Schild, S.E.; Crawford, J.; Bogart, J.A.; Vokes, E.E.; Cancer and Leukemia Group, B. A pooled analysis of limited-stage small-cell lung cancer patients treated with induction chemotherapy followed by concurrent platinum-based chemotherapy and 70 Gy daily radiotherapy: CALGB 30904. J. Thorac. Oncol. 2013, 8, 1043–1049. [Google Scholar] [CrossRef]
- Schild, S.E.; Bonner, J.A.; Shanahan, T.G.; Brooks, B.J.; Marks, R.S.; Geyer, S.M.; Hillman, S.L.; Farr, G.H., Jr.; Tazelaar, H.D.; Krook, J.E.; et al. Long-term results of a phase III trial comparing once-daily radiotherapy with twice-daily radiotherapy in limited-stage small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 943–951. [Google Scholar] [CrossRef]
- Spiro, S.G.; James, L.E.; Rudd, R.M.; Trask, C.W.; Tobias, J.S.; Snee, M.; Gilligan, D.; Murray, P.A.; Ruiz de Elvira, M.C.; O’Donnell, K.M.; et al. Early compared with late radiotherapy in combined modality treatment for limited disease small-cell lung cancer: A London Lung Cancer Group multicenter randomized clinical trial and meta-analysis. J. Clin. Oncol. 2006, 24, 3823–3830. [Google Scholar] [CrossRef]
- Takada, M.; Fukuoka, M.; Kawahara, M.; Sugiura, T.; Yokoyama, A.; Yokota, S.; Nishiwaki, Y.; Watanabe, K.; Noda, K.; Tamura, T.; et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: Results of the Japan Clinical Oncology Group Study 9104. J. Clin. Oncol. 2002, 20, 3054–3060. [Google Scholar] [CrossRef]
- Thomas, C.R.; Jr Giroux, D.J.; Stelzer, K.J.; Craig, J.B.; Laufman, L.R.; Taylor, S.A.; Goodwin, J.W.; Crowley, J.J.; Livingston, R.B. Concurrent cisplatin, prolonged oral etoposide, and vincristine plus chest and brain irradiation for limited small cell lung cancer: A phase II study of the Southwest Oncology Group (SWOG-9229). Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.R., Jr.; Giroux, D.J.; Janaki, L.M.; Turrisi, A.T., 3rd; Crowley, J.J.; Taylor, S.A.; McCracken, J.D.; Shankir Giri, P.G.; Gordon, W., Jr.; Livingston, R.B.; et al. Ten-year follow-up of Southwest Oncology Group 8269: A phase II trial of concomitant cisplatin-etoposide and daily thoracic radiotherapy in limited small-cell lung cancer. Lung Cancer 2001, 33, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Hong, L.Z.; Cai, X.W.; Zhu, Z.F.; Liu, Q.; Zhao, K.L.; Fan, M.; Mao, J.F.; Yang, H.J.; Wu, K.L.; et al. Phase 2 study of accelerated hypofractionated thoracic radiation therapy and concurrent chemotherapy in patients with limited-stage small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 517–523. [Google Scholar] [CrossRef]
- Tsuchiya-Kawano, Y.; Sasaki, T.; Yamaguchi, H.; Hirano, K.; Horiike, A.; Satouchi, M.; Hosokawa, S.; Morinaga, R.; Komiya, K.; Inoue, K.; et al. Updated Survival Data for a Phase I/II Study of Carboplatin plus Nab-Paclitaxel and Concurrent Radiotherapy in Patients with Locally Advanced Non-Small Cell Lung Cancer. Oncologist 2020, 25, 475–e891. [Google Scholar] [CrossRef]
- Puig, P.E.; Guilly, M.N.; Bouchot, A.; Droin, N.; Cathelin, D.; Bouyer, F.; Favier, L.; Ghiringhelli, F.; Kroemer, G.; Solary, E.; et al. Tumor cells can escape DNA-damaging cisplatin through DNA endoreduplication and reversible polyploidy. Cell Biol. Int. 2008, 32, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Liu, J.; Zhou, W.; Weng, K.; Long, B.; Wang, Y. The Role of Additional Chemotherapy in Combination with Concurrent Chemoradiotherapy for Locally Advanced Inoperable Non-Small Cell Lung Cancer, a Systematic Review and Meta-Analysis of 12 Randomized Trials. Cancer Investig. 2019, 37, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; He, Z.; Dang, J.; Li, G. Comparative efficacy and safety for different chemotherapy regimens used concurrently with thoracic radiation for locally advanced non-small cell lung cancer: A systematic review and network meta-analysis. Radiat. Oncol. 2019, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Sheiner, L.B.; Beal, S.L. Evaluation of methods for estimating population pharmacokinetic parameters, I.I. Biexponential model and experimental pharmacokinetic data. J. Pharmacokinet. Biopharm. 1981, 9, 635–651. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).