Personalized Radiation Therapy for Breast Cancer
Abstract
1. Introduction
2. Optimal Patient Selection
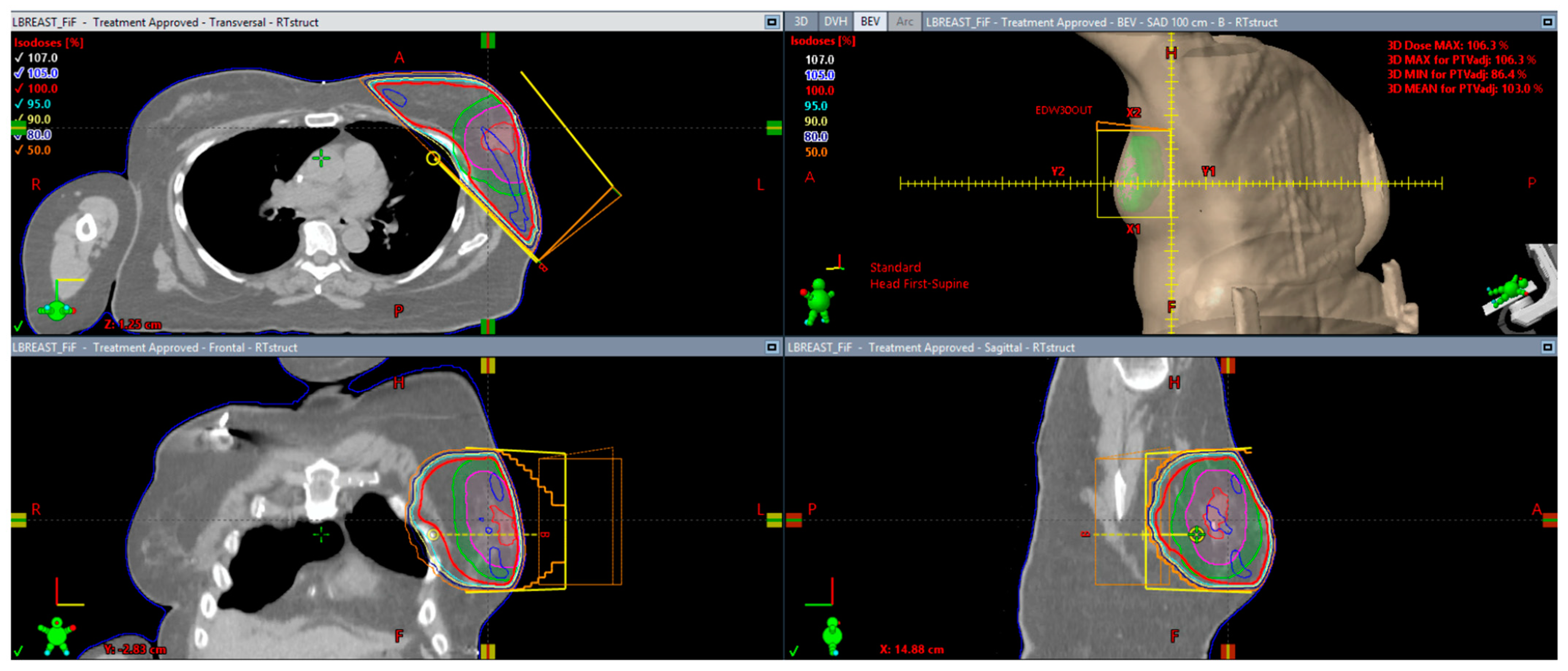
3. Radiation Therapy Technique
4. Fractionation
5. Genomic Personalization of Radiation Therapy
6. Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Breast Cancer. Version 4. 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 1 July 2023).
- Atkins, H.; Hayward, J.L.; Klugman, D.J.; Wayt, A.B. Treatment of early breast cancer: A report after ten years of a clinical trial. Br. Med. J. 1972, 2, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Montague, E.; Redmond, C.; Barton, B.; Borland, D.; Fisher, E.R.; Deutsch, M.; Schwarz, G.; Margolese, R.; Donegan, W.; et al. Comparison of radical mastectomy with alternative treatments for primary breast cancer: A first report of results from a prospective randomized clinical trial. Cancer 1977, 30, S2827–S2839. [Google Scholar] [CrossRef]
- Fisher, B.; Jeong, J.H.; Anderson, S.; Bryant, J.; Fisher, E.R.; Wolmark, N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N. Engl. J. Med. 2022, 347, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.H.; Wolmark, N. Twenty-year follow-up for a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.K.; Jagsi, R.; Woodward, W.A.; Ho, A. Breast cancer biology: Clinical implications for breast radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Buwenge, M.; Cammelli, S.; Ammendolia, I.; Tolento, G.; Zamagni, A.; Arcelli, A.; Macchia, G.; Deodato, F.; Cilla, S.; Morganti, A.G. Intensity modulated radiation therapy for breast cancer: Current perspectives. Breast Cancer Targets Ther. 2017, 9, 121–126. [Google Scholar] [CrossRef]
- Bergom, C.; Currey, A.; Desai, N.; Tai, A.; Strauss, J.B. Deep inspiration breath hold: Techniques and advantages for cardiac sparing during breast cancer irradiation. Front. Oncol. 2018, 8, 87. [Google Scholar] [CrossRef]
- McLellan, R.; Feig, S.A. Guidelines for mammography. In Breast Carcinoma: Current Diagnosis and Treatment; Feig, S.A., McLelland, R., Eds.; ACR & Mason: New York, NY, USA, 1983; pp. 365–369. [Google Scholar]
- Fisher, B.; Bryant, J.; Dignam, J.J.; Wickerham, D.L.; Mamounas, E.P.; Fisher, E.R.; Margolese, R.G.; Nesbitt, L.; Paik, S.; Pisansky, T.M.; et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J. Clin. Oncol. 2002, 20, 4141–4149. [Google Scholar] [CrossRef]
- Hughes, K.S.; Schnaper, L.A.; Bellon, J.R.; Cirrincione, C.T.; Berry, D.A.; McCormick, B.; Muss, H.B.; Smith, B.L.; Hudis, C.A.; Winer, E.P.; et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: Long-term follow-up of CALGB 9343. J. Clin. Oncol. 2013, 31, 2382–2387. [Google Scholar] [CrossRef]
- Kunkler, I.H.; Williams, L.J.; Jack, W.J.; Cameron, D.A.; Dixon, J.M. Breast-conserving surgery with or without irradiation in early breast cancer. N. Engl. J. Med. 2023, 388, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Whelan, T.J.; Smith, S.; Parpia, S.; Fyles, A.W.; Bane, A.; Liu, F.F.; Rakovitch, E.; Chang, L.; Stevens, C.; Bowen, J.; et al. LUMINA: Omitting radiotherapy after breast-conserving surgery in Luminal A breast cancer. N. Engl. J. Med. 2023, 389, 612–619. [Google Scholar] [CrossRef]
- Haque, W.; Yuan, D.M.K.; Verma, V.; Butler, E.B.; Teh, B.S.; Wiederhold, L.; Hatch, S. Radiation therapy utilization and outcomes for older women with breast cancer: Impact of molecular subtype and tumor grade. Breast 2017, 35, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Chlebowki, R.T.; Kim, J.; Haque, R. Adherence to endocrine therapy in breast cancer adjuvant and prevent settings. Cancer Prev. Res. 2014, 7, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Haussmann, J.; Budach, W.; Corradini, S.; Krug, D.; Bölke, E.; Tamaskovics, B.; Jazmati, D.; Haussmann, A.; Matuschek, C. Whole breast irradiation in comparison to endocrine therapy in early stage breast cancer—A direct and network meta-analysis of pubished randomized trials. Cancers 2023, 15, 4343. [Google Scholar] [CrossRef] [PubMed]
- Polgár, C.; Fodor, J.; Major, T.; Németh, G.; Lövey, K.; Orosz, Z.; Sulyok, Z.; Takácsi-Nagy, Z.; Kásler, M. Breast-conserving treatment with partial or whole breast irradiation for low-risk invasive breast carcinoma-5-year results of a randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Chao, K.K.; Vicini, F.A.; Wallace, M.; Mitchell, C.; Chen, P.; Ghilezan, M.; Gilbert, S.; Kunzman, J.; Benitez, P.; Martinez, A. Analysis of treatment efficacy, cosmesis, and toxicity using the MammoSite breast brachytherapy catheter to deliver accelerated partial-breast irradiation: The William Beaumont hospital experience. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 32–40. [Google Scholar] [CrossRef]
- Baglan, K.L.; Sharpe, M.B.; Jaffray, D.; Frazier, R.C.; Fayad, J.; Kestin, L.L.; Remouchamps, V.; A Martinez, A.; Wong, J.; A Vicini, F. Accelerated partial breast irradiation using 3D conformal radiation therapy (3D-CRT). Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 302–311. [Google Scholar] [CrossRef]
- Meattini, I.; Marrazzo, L.; Saieva, C.; Desideri, I.; Scotti, V.; Simontacchi, G.; Bonomo, P.; Greto, D.; Mangoni, M.; Scoccianti, S.; et al. Accelerated partial-breast irradiation compared with whole-breast irradiation for early breast cancer: Long-term results of the randomized phase III APBI-IMRT-Florence Trial. J. Clin. Oncol. 2020, 38, 41755–41783. [Google Scholar] [CrossRef]
- Coles, C.E.; Griffin, C.L.; Kirby, A.M.; Titley, J.; Agrawal, R.K.; Alhasso, A.; Bhattacharya, I.S.; Brunt, A.M.; Ciurlionis, L.; Chan, C.; et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomized, controlled, phase 3, non-inferiority trial. Lancet 2017, 390, 1048–1060. [Google Scholar] [CrossRef]
- Vicini, F.A.; Cecchini, R.S.; White, J.R.; Arthur, D.W.; Julian, T.B.; Rabinovitch, R.A.; Kuske, R.R.; Ganz, P.A.; Parda, D.S.; Scheier, M.F.; et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: A randomised phase 3, equivalence trial. Lancet 2019, 394, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
- Correa, C.; Harris, E.E.; Leonardi, M.C.; Smith, B.D.; Taghian, A.G.; Thompson, A.M.; White, J.; Harris, J.R. Accelerated Partial Breast Irradiation: Executive summary for the update of an ASTRO Evidence-Based Consensus Statement. Pr. Radiat Oncol. 2017, 7, 7309. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Conroy, L.; Quirk, S.; Watt, E.; Ecclestone, G.; Conway, J.L.; Olivotto, I.A.; Phan, T.; Smith, W.L. Deep inspiration breath hold level variability and deformation in locoregional breast irradiation. Pr. Radiat Oncol. 2018, 8, e109–e116. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Kim, S.S.; Ahn, S.D.; Kwak, J.; Jeong, C.; Ahn, S.-H.; Son, B.-H.; Lee, J.W. Cardiac dose reduction during tangential breast irradiation using deep inspiration breath hold: A dose comparison study based on deformable image registration. Radiat. Oncol. 2015, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.M.; Zhong, F.M.; Deng, J.M.; Hu, S.B.; Shen, R.B.; Luo, H.; Luo, Y.B. Prone position versus supine position in postoperative radiotherapy for cancer. Medicine 2021, 100, e26000. [Google Scholar] [CrossRef]
- Thilmann, C.; Sroka-Perez, G.; Krempien, R.; Hoess, A.; Wannenmacher, M.; Debus, J. Inversely planned intensity modulated radiotherapy of the breast including the internal mammary chain: A plan comparison study. Technol. Cancer Res. Treat. 2004, 3, 69–75. [Google Scholar] [CrossRef]
- Hou, P.Y.; Hsieh, C.H.; Wu, L.J.; Hsu, C.S.; Kuo, D.Y.; Lu, Y.F.; Wu, Y.W.; Tien, H.J.; Hsu, S.M.; Shueng, P.W. Cardiac function after modern radiation therapy with volumetric or helical tomotherapy for advanced left-breast cancer receiving irradiation. Bioengineering 2022, 9, 213. [Google Scholar] [CrossRef]
- Ares, C.; Khan, S.; MacArtain, A.M.; Heuberger, J.; Goitein, G.; Gruber, G.; Lutters, G.; Hug, E.B.; Bodis, S.; Lomax, A.J. Postoperative proton radiotherapy for localized and locoregional breast cancer: Potential for clinically relevant improvements? Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 685–697. [Google Scholar] [CrossRef]
- Mutter, R.W.; Choi, J.I.; Jimenez, R.B.; Kirova, Y.M.; Fagundes, M.; Haffty, B.G.; Amos, R.A.; Bradley, J.A.; Chen, P.Y.; Ding, X.; et al. Proton Therapy for Breast Cancer: A consensus statement from the Particle Therapy Cooperative Group (PTCOG) breast cancer subcommittee. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 337–359. [Google Scholar] [CrossRef]
- Ritter, M. Rationale, conduct, and outcome using hypofractionated radiotherapy in prostate cancer. Semin. Radiat. Oncol. 2008, 18, 249–256. [Google Scholar] [CrossRef]
- Ray, K.J.; Sibson, N.R.; Kiltie, A.E. Treatment of breast and prostate cancer by hypofractionated radiotherapy: Potential risks and benefits. Clin. Oncol. 2015, 27, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Whelan, T.; MacKenzie, R.; Julian, J.; Levine, M.; Shelley, W.; Grimard, L.; Lada, B.; Lukka, H.; Perera, F.; Fyles, A.; et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J. Natl. Cancer Inst. 2002, 94, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- START Trialists’ Group. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early stage breast cancer: A Randomized trial. Lancet Oncol. 2008, 9, 331–341. [Google Scholar] [CrossRef] [PubMed]
- START Trialists’ Group. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: A Randomizsd trial. Lancet 2008, 371, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Brunt, A.M.; Haviland, J.S.; Sydenham, M.; Agrawal, R.K.; Algurafi, H.; Alhasso, A.; Barrett-Lee, P.; Bliss, P.; Bloomfield, D.; Bowen, J.; et al. Ten-Year Results of FAST: A Randomized Controlled Trial of 5-Fraction Whole-Breast Radiotherapy for Early Breast Cancer. J. Clin. Oncol. 2020, 38, 3261–3272. [Google Scholar] [CrossRef] [PubMed]
- Brunt, A.M.; Haviland, J.S.; Wheatley, D.A.; Sydenham, M.A.; Alhasso, A.; Bloomfield, D.J.; Chan, C.; Churn, M.; Cleator, S.; Coles, C.E.; et al. Hypofractioanted breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiorly, randomized, phase 3 trial. Lancet 2020, 395, 1613–1626. [Google Scholar] [CrossRef] [PubMed]
- Van Hulle, H.; Desaunois, E.; Vakaet, V.; Paelinck, L.; Schoepen, M.; Post, G.; Van Greveling, A.; Speleers, B.; Mareel, M.; De Neve, W.; et al. Two-year toxicity of simultaneous integrated boost in hypofractionated prone breast cancer irradiation: Comparision with sequential boost in randomized trial. Radiother. Oncol. 2021, 158, 62–66. [Google Scholar] [CrossRef]
- Wang, S.L.; Fang, H.; Song, Y.W.; Wang, W.H.; Hu, C.; Liu, Y.P.; Jin, J.; Liu, X.F.; Yu, Z.H.; Ren, H.; et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer; a randomized, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 352–360. [Google Scholar] [CrossRef]
- Poppe, M.M.; Yehia, Z.A.; Baker, C.; Goyal, S.; Toppmeyer, D.; Kirstein, L.; Chen, C.; Moore, D.; Haffty, B.G.; Khan, A.J. 5-year update of a multi-institution, prospective phase 2 hypofractionated postmastectomy radiation therapy trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 694–700. [Google Scholar] [CrossRef]
- Paik, S.; Shak, S.; Tang, G.; Kim, C.; Baker, J.; Cronin, M.; Baehner, F.L.; Walker, M.G.; Watson, D.; Park, T.; et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 2004, 351, 2817–2826. [Google Scholar] [CrossRef] [PubMed]
- Mamounas, E.P.; Tang, G.; Fisher, B.; Paik, S.; Shak, S.; Costantino, J.P.; Watson, D.; Geyer, C.E., Jr.; Wickerham, D.L.; Wolmark, N. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: Results from NSABP B-14 and NSABP B-20. J. Clin. Oncol. 2010, 28, 1677–1683. [Google Scholar] [CrossRef]
- Mamounas, E.P.; Liu, Q.; Paik, S.; Baehner, F.L.; Tang, G.; Jeong, J.H.; Kim, S.R.; Butler, S.M.; Jamshidian, F.; Cherbavaz, D.B.; et al. 21-gene recurrence score and locoregional recurrence in node-positive/ER-positive breast cancer treated with chemo-endocrine therapy. J. Natl. Caner Inst. 2017, 109, djw259. [Google Scholar] [CrossRef] [PubMed]
- Chevli, N.; Haque, W.; Tran, K.T.; Farach, A.M.; Schwartz, M.R.; Hatch, S.S.; Butler, E.B.; Teh, B.S. Role of 21-gene recurrence score in predicting prognostic benefit of radiation therapy after breast-conserving surgery for T1N0 breast cancer. Pr. Radiat Oncol. 2023, 13, e230–e238. [Google Scholar] [CrossRef] [PubMed]
- Chevli, N.; Haque, W.; Tran, K.T.; Farach, A.M.; Schwartz, M.R.; Hatch, S.S.; Butler, E.B.; Teh, B.S. 21-gene recurrence score predictive for prognostic benefit of radiotherapy in patients age ≥ 70 with T1N0 ER/PR + HER2-breast cancer treated with breast conserving surgery and endocrine therapy. Radiother. Oncol. 2022, 174, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Fitzal, F.; Filipits, M.; Fesl, C.; Rudas, M.; Greil, R.; Balic, M.; Moinfar, F.; Herz, W.; Dubsky, P.; Bartsch, R.; et al. PAM-50 predicts local recurrence after breast cancer surgery in postmenopausal patients with ER+/HER2-disease: Results from 1204 patients in the randomized ABCSG-8 trial. Br. J. Surg. 2021, 108, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, M.; Fyles, A.; Liu, F.-F.; McCready, D.; Shi, W.; Rey-McIntyre, K.; Chang, S.L.; Feng, F.Y.; Speers, C.W.; Pierce, L.J.; et al. Development and validation of a genomic profile for the omission of local adjuvant radiation in breast cancer. J. Clin. Oncol. 2023, 41, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Eschrich, S.A.; Pramana, J.; Zhang, H.; Zhao, H.; Boulware, D.; Lee, J.-H.; Bloom, G.; Rocha-Lima, C.; Kelley, S.; Calvin, D.P.; et al. A gene expression model of intrinsic tumor radiosensitivity: Prediction of response and prognosis after chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 489–496. [Google Scholar] [CrossRef]
- Speers, C.; Zhao, S.; Liu, M.; Bartelink, H.; Pierce, L.J.; Feng, F.Y. Development and validation of a novel radiosensitivity signature in human breast cancer. Clin. Cancer Res. 2015, 21, 3667–3677. [Google Scholar] [CrossRef]
- Speers, C.; Zhao, S.; Liu, M.; Bartelink, H.; Pierce, L.J.; Feng, F.Y. Integrating radiosensitivity and immune gene signatures for predicting benefit of radiotherapy in breast cancer. Clin. Cancer Res. 2018, 24, 4754–4762. [Google Scholar]
- The IDEA Study (Individualized Decisions for Endocrine Therapy Alone); Clinicaltrials.Gov. Published: Washington, DC, USA, 2015. Available online: http://clinicaltrials.gov/show/NCT02400190 (accessed on 29 July 2023).
- PRECISION Trial (Profiling Early Breast Cancer for Radiotherapy Omission): A Phase II Study of Breast-Conserving Surgery without Adjuvant Radiotherapy for Favorable-Risk Breast Cancer; Clinicaltrials.Gov. Published: Washington, DC, USA, 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02653755 (accessed on 29 July 2023).
- Kirwan, C.; Coles, C.; Bliss, J.; Kilburn, L.; Fox, L.; Cheang, M.; Griffin, C.; Francis, A.; Kirby, A.; Ah-See, M.; et al. It’s PRIMETIME. Postoperative avoidance of radiotherapy: Biomarker selection of women at very low risk of local recurrence. Clin. Oncol. 2016, 28, 594–596. [Google Scholar] [CrossRef]
- De-Escalation of Breast Radiation Trial for Hormone Sensitive: HER-2 Negative, Oncotype Recurrence Score Less than or Equal to 18 Breast Cancer (DEBRA); Clinicialtrials.Gov. Published: Washington, DC, USA, 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04852887 (accessed on 29 July 2023).
- Examining Personalized Radiation Therapy for Low-Risk Early Breast Cancer (EXPERT); Clinicaltrials.Gov. Published: Washington, DC, USA, 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT0-2889874 (accessed on 29 July 2023).
- Overgaard, M.; Nielsen, H.M.; Tramm, T.; Højris, I.; Grantzau, T.L.; Alsner, J.; Offersen, B.V.; Overgaard, J. Postmastectomy radiotherapy in high-risk breast cancer patients given adjuvant systemic therapy. A 30-year long-term report from the Danish breast cancer cooperative group DBCG 82bc trial. Radiother. Oncol. 2022, 170, 4–13. [Google Scholar] [CrossRef]
- Ragaz, J.; Olivotto, I.A.; Spinelli, J.J.; Phillips, N.; Jackson, S.M.; Wilson, K.S.; Knowling, M.A.; Coppin, C.M.; Weir, L.; Gelmon, K.; et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy 20-year results of the British Columbia randomized trial. J. Natl. Cancer Inst. 2005, 97, 116–126. [Google Scholar] [CrossRef]
- Regional radiotherapy in Biomarker Low-Risk Positive and T3N0 Breast Cancer (TAILOR RT); Clinicaltrials.Gov. Published: Washington, DC, USA, 2018. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT0-03488693 (accessed on 26 August 2023).
- Mougalian, S.S.; Soulos, P.R.; Killelea, B.K.; Lannin, D.R.; Abu-Khalaf, M.M.; DiGiovanna, M.P.; Sanft, T.B.; Pusztai, L.; Gross, C.P.; Chagpar, A.B. Use of neoadjuvant chemotherapy for patients with Stage I to III breast cancer in the United States. Cancer 2015, 121, 2544–2552. [Google Scholar] [CrossRef]
- Haque, W.; Singh, A.; Verma, V.; Schwartz, M.R.; Chevli, N.; Hatch, S.; Desai, M.; Butler, E.B.; Arentz, C.; Farach, A.; et al. Postmastectomy radiation therapy following pathologic complete nodal response to neoadjuvant chemotherapy: A prelude to NSABP B-51. Radiother. Oncol. 2021, 162, 52–59. [Google Scholar] [CrossRef]
- Standard of Comprehensive Radiation Therapy in Treating Patients with Early-Stage Breast Cancer Previously Treated with Chemotherapy and Surgery; Clinicaltrials.Gov. Published: Washington, DC, USA, 2018. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT01872975 (accessed on 27 August 2023).
- Testing Radiation and HER2-Targeted Therapy versus HER2-Targeted Therapy Alone for Low-Risk HER2-Positive Breast Cancer (HERO); Clinicaltrials.Gov. Published: Washington, DC, USA, 2023. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05705401 (accessed on 3 September 2023).
- Hypofractionated Radiation Therapy after Mastectomy in Preventing Recurrence in Patients with Stage IIa-IIIa Breast Cancer; Clinicaltrials.Gov. Published: Washington, DC, USA, 2018. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03414970 (accessed on 3 September 2023).
| IDEA | PRECISION | PRIMETIME | DEBRA/NRG BR007 | EXPERT | HERO/NRG BR 008 | |
|---|---|---|---|---|---|---|
| Target patient accrual | 202 | 672 | 2400 | 1670 | 1167 | 1300 |
| Estimated date of completion | 2026 | 2026 | 2027 | 2026 | 2024 | 2034 |
| Age of patients | 50–69 | 50–75 | ≥60 | 50–70 | ≥50 | ≥40 |
| Inclusion | ER+HER2-, RS ≤ 18, T1N0 | ER+HER2-, T1N0, PAM 50 | ER+HER2-, T1N0, IHC4+C | ER+HER2-, T1N0, RS ≤ 18 | ER+HER2-, T1N0, PAM 50 | HER2+, T1N0 or (if neoadjuvant) T2N0 (<3 cm), HER2-directed therapy |
| Treatment arms | Single-arm, prospective, omission | Omission if low PAM 50; radiation if intermediate or high PAM 50 | Omission if very low IHC4+C; radiation if low, intermediate, or high IHC4+C | Observation or radiation | Observation or radiation | Observation or radiation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, W.; Butler, E.B.; Teh, B.S. Personalized Radiation Therapy for Breast Cancer. Curr. Oncol. 2024, 31, 1588-1599. https://doi.org/10.3390/curroncol31030121
Haque W, Butler EB, Teh BS. Personalized Radiation Therapy for Breast Cancer. Current Oncology. 2024; 31(3):1588-1599. https://doi.org/10.3390/curroncol31030121
Chicago/Turabian StyleHaque, Waqar, Edward Brian Butler, and Bin S. Teh. 2024. "Personalized Radiation Therapy for Breast Cancer" Current Oncology 31, no. 3: 1588-1599. https://doi.org/10.3390/curroncol31030121
APA StyleHaque, W., Butler, E. B., & Teh, B. S. (2024). Personalized Radiation Therapy for Breast Cancer. Current Oncology, 31(3), 1588-1599. https://doi.org/10.3390/curroncol31030121




