Vitiligo-like Lesions as a Predictor of Response to Immunotherapy in Non-Small Cell Lung Cancer: Comprehensive Review and Case Series from a University Center
Abstract
1. Introduction
2. Methods
3. Results
3.1. Case Reports—Unidade Local de Saúde de Santo António’s Experience
3.1.1. Case 1
3.1.2. Case 2
3.1.3. Case 3
3.2. Previously Reported Cases
4. Discussion
4.1. Vitiligo and Lung Cancer Risk
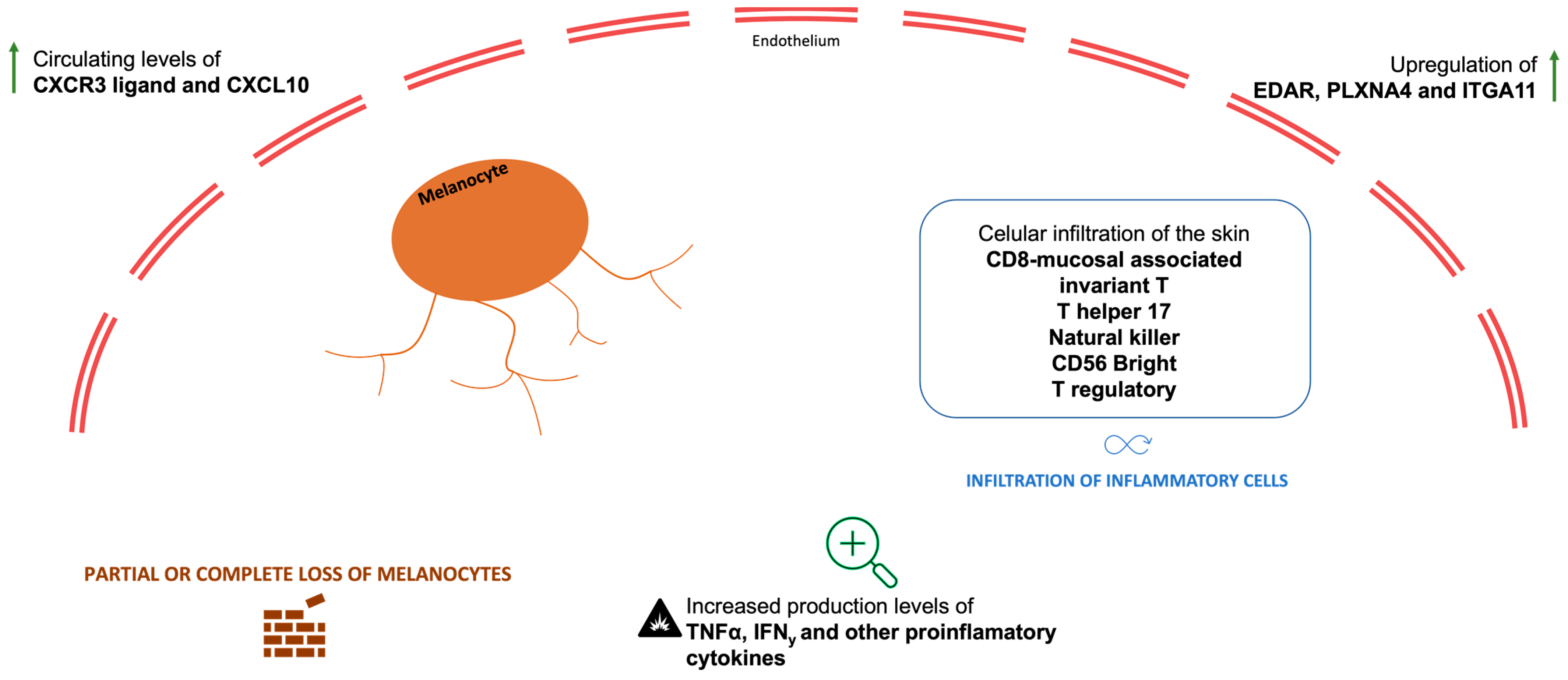
4.2. ICI-Induced VLLs
4.3. ICI-Induced VLLs and NSCLC
4.4. Reflection on Reported Experience
4.5. Possible Clinical Practice Implications
4.6. Predictive Biomarkers of ICI Response in NSCLC
| Biomarker | Distinctive Characteristic | Prognostic Value |
|---|---|---|
| PD-L1 expression in tumor sample [52,53,59,60,61,62,63,64] | Positive expression | Select patients who may benefit from ICI treatment |
| Higher values | Predictor of greater benefit from ICI treatment | |
| Soluble or Exosomal PD-L1 [67] | High pre-treatment levels | Unfavorable prognostic factors to patients undergoing ICI treatment |
| Exosomal PD-L1 [67] | Dynamic post-treatment upregulation | Favorable prognosis to patients undergoing ICI treatment |
| TMB [3,70,71] | High-TMB | Select patients who may benefit from ICI treatment |
| mTBI [72] | On-treatment mTBI decreasing levels | Predictor of greater benefit from ICI treatment |
| TILs [68] | High levels of tumor-reactive TILs | Select patients who may benefit from ICI treatment |
| NLR [69] | Dynamic evolution (changed to low) | Favorable prognosis to patients undergoing ICI treatment |
| Specific Mutations [73,74,75,76,77,78] | EGFR mutations ALK rearrangements MET exon 14 skipping HER2 amplification RET rearrangement KRAS mutations * | Unfavorable prognostic factors to patients undergoing ICI treatment |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garassino, M.C.; Gadgeel, S.; Speranza, G.; Felip, E.; Esteban, E.; Dómine, M.; Hochmair, M.J.; Powell, S.F.; Bischoff, H.G.; Peled, N.; et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes from the Phase 3 KEYNOTE-189 Study. J. Clin. Oncol. 2023, 41, 1992–1998. [Google Scholar] [CrossRef]
- Pacheco, J.M. KEYNOTE-407: Changing the Way We Treat Stage IV Squamous Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2020, 9, 148. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; de Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes from the PACIFIC Trial: Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef]
- Shao, L.; Lou, G. Neoadjuvant Immunotherapy in Non-Small Cell Lung Cancer: A Narrative Review on Mechanisms, Efficacy and Safety. J. Thorac. Dis. 2022, 14, 3565–3574. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, R.; Cheema, A.; Khan, T.; Amirpour, A.; Paul, A.; Chaughtai, S.; Patel, S.; Patel, T.; Bramson, J.; Gupta, V.; et al. Adverse Effects of Immune Checkpoint Inhibitors (Programmed Death-1 Inhibitors and Cytotoxic T-Lymphocyte-Associated Protein-4 Inhibitors): Results of a Retrospective Study. J. Clin. Med. Res. 2019, 11, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Austin, M.; Kluger, H.M.; Trunova, N.; Mann, H.; Shire, N.; Morgan, C.; Zhou, D.; Mugundu, G.M. Association between Immune-Mediated Adverse Events and Efficacy in Metastatic Non-Small-Cell Lung Cancer Patients Treated with Durvalumab and Tremelimumab. Front. Immunol. 2022, 13, 1026964. [Google Scholar] [CrossRef] [PubMed]
- Teulings, H.E.; Limpens, J.; Jansen, S.N.; Zwinderman, A.H.; Reitsma, J.B.; Spuls, P.I.; Luiten, R.M. Vitiligo-like Depigmentation in Patients with Stage III–IV Melanoma Receiving Immunotherapy and Its Association with Survival: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2015, 33, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Boussemart, L.; Mateus, C.; Routier, E.; Boutros, C.; Cazenave, H.; Viollet, R.; Thomas, M.; Roy, S.; Benannoune, N.; et al. Association of Vitiligo with Tumor Response in Patients with Metastatic Melanoma Treated with Pembrolizumab. JAMA Dermatol. 2016, 152, 45–51. [Google Scholar] [CrossRef]
- Nardin, C.; Jeand’heur, A.; Bouiller, K.; Valnet-Rabier, M.B.; Dresco, F.; Castagna, J.; Mareschal, A.; Carlet, C.; Nerich, V.; Limat, S.; et al. Vitiligo under Anti-Programmed Cell Death-1 Therapy Is Associated with Increased Survival in Melanoma Patients. J. Am. Acad. Dermatol. 2020, 82, 770–772. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Hwang, S.J.E.; Byth, K.; Kyaw, M.; Carlino, M.S.; Chou, S.; Fernandez-Penas, P. Survival and Prognosis of Individuals Receiving Programmed Cell Death 1 Inhibitor with and without Immunologic Cutaneous Adverse Events. J. Am. Acad. Dermatol. 2020, 82, 311–316. [Google Scholar] [CrossRef]
- Sanlorenzo, M.; Vujic, I.; Daud, A.; Algazi, A.; Gubens, M.; Luna, S.A.; Lin, K.; Quaglino, P.; Rappersberger, K.; Ortiz-Urda, S. Pembrolizumab Cutaneous Adverse Events and Their Association with Disease Progression. JAMA Dermatol. 2015, 151, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Tanaka, R.; Asami, Y.; Teramoto, Y.; Imamura, T.; Sato, S.; Maruyama, H.; Fujisawa, Y.; Matsuya, T.; Fujimoto, M.; et al. Correlation between Vitiligo Occurrence and Clinical Benefit in Advanced Melanoma Patients Treated with Nivolumab: A Multi-Institutional Retrospective Study. J. Dermatol. 2017, 44, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Le Gal, F.A.; Avril, M.F.; Bosq, J.; Lefebvre, P.; Deschemin, J.C.; Andrieu, M.; Dore, M.X.; Guillet, J.G. Direct Evidence to Support the Role of Antigen-Specific CD8+ T Cells in Melanoma-Associated Vitiligo. J. Investig. Dermatol. 2001, 117, 1464–1470. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, K.; Tian, J.; Xia, X.; Ma, J.; Tang, X.; Xu, H.; Wang, S. Granulocytic Myeloid-Derived Suppressor Cells Promote the Stemness of Colorectal Cancer Cells through Exosomal S100A9. Adv. Sci. 2019, 6, 1901278. [Google Scholar] [CrossRef]
- Mandelcorn-Monson, R.L.; Shear, N.H.; Yau, E.; Sambhara, S.; Barber, B.H.; Spaner, D.; DeBenedette, M.A. Cytotoxic T Lymphocyte Reactivity to Gp100, MelanA/MART-1, and Tyrosinase, in HLA-A2-Positive Vitiligo Patients. J. Investig. Dermatol. 2003, 121, 550–556. [Google Scholar] [CrossRef]
- Geisler, A.N.; Phillips, G.S.; Barrios, D.M.; Wu, J.; Leung, D.Y.M.; Moy, A.P.; Kern, J.A.; Lacouture, M.E. Immune Checkpoint Inhibitor—Related Dermatologic Adverse Events. J. Am. Acad. Dermatol. 2020, 83, 1255. [Google Scholar] [CrossRef]
- Nikolaou, V.A.; Apalla, Z.; Carrera, C.; Fattore, D.; Sollena, P.; Riganti, J.; Segura, S.; Freites-Martinez, A.; Lallas, K.; Romano, M.C.; et al. Clinical Associations and Classification of Immune Checkpoint Inhibitor-Induced Cutaneous Toxicities: A Multicentre Study from the European Academy of Dermatology and Venereology Task Force of Dermatology for Cancer Patients. Br. J. Dermatol. 2022, 187, 962–969. [Google Scholar] [CrossRef]
- Yun, S.J.; Oh, I.J.; Park, C.K.; Kim, Y.C.; Kim, H.B.; Kim, H.K.; Ram Hong, A.; Kim, I.Y.; Ahn, S.J.; Na, K.J.; et al. Vitiligo-like Depigmentation after Pembrolizumab Treatment in Patients with Non-Small Cell Lung Cancer: A Case Report. Transl. Lung Cancer Res. 2020, 9, 1585. [Google Scholar] [CrossRef]
- Kosche, C.; Mohindra, N.; Choi, J.N. Vitiligo in a Patient Undergoing Nivolumab Treatment for Non–Small Cell Lung Cancer. JAAD Case Rep. 2018, 4, 1042. [Google Scholar] [CrossRef][Green Version]
- Nishino, K.; Ohe, S.; Kitamura, M.; Kunimasa, K.; Kimura, M.; Inoue, T.; Tamiya, M.; Kumagai, T.; Nakatsuka, S.; Isei, T.; et al. Nivolumab Induced Vitiligo-like Lesions in a Patient with Metastatic Squamous Cell Carcinoma of the Lung. J. Thorac. Dis. 2018, 10, E481–E484. [Google Scholar] [CrossRef]
- Bulat, V.; Likic, R.; Bradic, L.; Speeckaert, R.; Azdajic, M.D. Pembrolizumab-Induced Vitiligo in a Patient with Lung Adenocarcinoma: A Case Report. Br. J. Clin. Pharmacol. 2021, 87, 2614–2618. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.C.; Consuegra, G.; Chou, S.; Fernandez Peñas, P. Vitiligo-like Depigmentation in Oncology Patients Treated with Immunotherapies for Nonmelanoma Metastatic Cancers. Clin. Exp. Dermatol. 2019, 44, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Perreault, C.; Finn, O.J.; Kroemer, G. Beneficial Autoimmunity Improves Cancer Prognosis. Nat. Rev. Clin. Oncol. 2021, 18, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.M.; Chung, K.Y.; Yun, S.J.; Kim, H.; Park, B.C.; Kim, J.S.; Seo, S.H.; Ahn, H.H.; Lee, D.Y.; Kim, Y.C.; et al. Markedly Reduced Risk of Internal Malignancies in Patients with Vitiligo: A Nationwide Population-Based Cohort Study. J. Clin. Oncol. 2019, 37, 903–911. [Google Scholar] [CrossRef]
- Paradisi, A.; Tabolli, S.; Didona, B.; Sobrino, L.; Russo, N.; Abeni, D. Markedly Reduced Incidence of Melanoma and Nonmelanoma Skin Cancer in a Nonconcurrent Cohort of 10,040 Patients with Vitiligo. J. Am. Acad. Dermatol. 2014, 71, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wu, X.; Peng, H.; Li, C.; Jiang, Y.; Liang, H.; Zhong, R.; Liu, J.; He, J.; Liang, W. Cancer Risks in Patients with Vitiligo: A Mendelian Randomization Study. J. Cancer Res. Clin. Oncol. 2020, 146, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Seo, J.; Tiu, B.C.; Le, T.K.; Pahalyants, V.; Raval, N.S.; Ugwu-Dike, P.O.; Zubiri, L.; Naranbhai, V.; Carrington, M.; et al. Association of Cutaneous Immune-Related Adverse Events with Increased Survival in Patients Treated with Anti–Programmed Cell Death 1 and Anti–Programmed Cell Death Ligand 1 Therapy. JAMA Dermatol. 2022, 158, 1. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, R.; van Geel, N. Vitiligo: An Update on Pathophysiology and Treatment Options. Am. J. Clin. Dermatol. 2017, 18, 733–744. [Google Scholar] [CrossRef]
- Luiten, R.M.; Van Den Boorn, J.G.; Konijnenberg, D.; Dellemijn, T.A.M.; Van Der Veen, J.P.W.; Bos, J.D.; Melief, C.J.M.; Vyth-Dreese, F.A. Autoimmune Destruction of Skin Melanocytes by Perilesional T Cells from Vitiligo Patients. J. Investig. Dermatol. 2009, 129, 2220–2232. [Google Scholar] [CrossRef]
- Klarquist, J.; Denman, C.J.; Hernandez, C.; Wainwright, D.J.; Strickland, F.M.; Overbeck, A.; Mehrotra, S.; Nishimura, M.I.; Le Poole, I.C. Reduced Skin Homing by Functional Treg in Vitiligo. Pigment Cell Melanoma Res. 2010, 23, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.A.; Fisher, D.E.; Flaherty, K.T. Prognostic Significance of Cutaneous Adverse Events Associated with Pembrolizumab Therapy. JAMA Oncol. 2015, 1, 1340. [Google Scholar] [CrossRef] [PubMed]
- Hermann, N.; Maul, L.V.; Ameri, M.; Traidl, S.; Ziadlou, R.; Papageorgiou, K.; Kolm, I.; Levesque, M.; Maul, J.T.; Brüggen, M.C. Clinical Presentation and Prognostic Features in Patients with Immunotherapy-Induced Vitiligo-like Depigmentation: A Monocentric Prospective Observational Study. Cancers 2022, 14, 4576. [Google Scholar] [CrossRef] [PubMed]
- Dousset, L.; Pacaud, A.; Barnetche, T.; Kostine, M.; Dutriaux, C.; Pham-Ledard, A.; Beylot-Barry, M.; Gérard, E.; Prey, S.; Andreu, N.; et al. Analysis of Tumor Response and Clinical Factors Associated with Vitiligo in Patients Receiving Anti-Programmed Cell Death-1 Therapies for Melanoma: A Cross-Sectional Study. JAAD Int. 2021, 5, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Babai, S.; Voisin, A.L.; Bertin, C.; Gouverneur, A.; Le-Louet, H. Occurrences and Outcomes of Immune Checkpoint Inhibitors-Induced Vitiligo in Cancer Patients: A Retrospective Cohort Study. Drug Saf. 2020, 43, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.; Guo, Z.; Wen, X.; Zeng, X.; Wu, L.; Huang, L. Case Report: Immune Checkpoint Inhibitor-Related Vitiligo-like Depigmentation in Non-Melanoma Advanced Cancer: A Report of Three Cases and a Pooled Analysis of Individual Patient Data. Front. Oncol. 2023, 12, 1099108. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Grizzi, G.; Ghidini, M.; Ghidini, A.; Ratti, M.; Panni, S.; Cabiddu, M.; Ghilardi, M.; Borgonovo, K.; Parati, M.C.; et al. Immune-Related Adverse Events and Survival in Solid Tumors Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. J. Immunother. 2020, 43, 1–7. [Google Scholar] [CrossRef]
- Romão, R.; Mendes, A.S.; Ranchor, R.; Ramos, M.J.; Coelho, J.; Pichel, R.C.; Azevedo, S.X.; Fidalgo, P.; Araújo, A. Impact of Immune-Related Adverse Events on Immune Checkpoint Inhibitors Treated Cancer Patients’ Survival: Single Center Experience and Literature Review. Cancers 2023, 15, 888. [Google Scholar] [CrossRef]
- Zhou, X.; Yao, Z.; Yang, H.; Liang, N.; Zhang, X.; Zhang, F. Are Immune-Related Adverse Events Associated with the Efficacy of Immune Checkpoint Inhibitors in Patients with Cancer? A Systematic Review and Meta-Analysis. BMC Med. 2020, 18, 87. [Google Scholar] [CrossRef]
- Ramondetta, A.; Ribero, S.; Conti, L.; Fava, P.; Marra, E.; Broganelli, P.; Caliendo, V.; Picciotto, F.; Guida, M.; Ierro, M.T.; et al. Clinical and Pathological Relevance of Drug-Induced Vitiligo in Patients Treated for Metastatic Melanoma with Anti-PD1 or BRAF/MEK Inhibitors. Acta Derm. Venereol. 2020, 100, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Malik, B.T.; Byrne, K.T.; Vella, J.L.; Zhang, P.; Shabaneh, T.B.; Steinberg, S.M.; Molodtsov, A.K.; Bowers, J.S.; Angeles, C.V.; Paulos, C.M.; et al. Resident Memory T Cells in the Skin Mediate Durable Immunity to Melanoma. Sci. Immunol. 2017, 2, eaam6346. [Google Scholar] [CrossRef]
- Overwijk, W.W.; Lee, D.S.; Surman, D.R.; Irvine, K.R.; Touloukian, C.E.; Chan, C.C.; Carroll, M.W.; Moss, B.; Rosenberg, S.A.; Restifo, N.P. Vaccination with a Recombinant Vaccinia Virus Encoding a “Self” Antigen Induces Autoimmune Vitiligo and Tumor Cell Destruction in Mice: Requirement for CD4+ T Lymphocytes. Proc. Natl. Acad. Sci. USA 1999, 96, 2982. [Google Scholar] [CrossRef] [PubMed]
- Van Geel, N.A.C.; Mollet, I.G.; De Schepper, S.; Tjin, E.P.M.; Vermaelen, K.; Clark, R.A.; Kupper, T.S.; Luiten, R.M.; Lambert, J. First Histopathological and Immunophenotypic Analysis of Early Dynamic Events in a Patient with Segmental Vitiligo Associated with Halo Nevi. Pigment Cell Melanoma Res. 2010, 23, 375–384. [Google Scholar] [CrossRef]
- Riding, R.L.; Harris, J.E. The Role of Memory CD8+ T Cells in Vitiligo. J. Immunol. 2019, 203, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.E.; Harris, T.H.; Weninger, W.; Wherry, E.J.; Hunter, C.A.; Turka, L.A. A Mouse Model of Vitiligo with Focused Epidermal Depigmentation Requires IFN-γ for Autoreactive CD8+ T-Cell Accumulation in the Skin. J. Investig. Dermatol. 2012, 132, 1869–1876. [Google Scholar] [CrossRef]
- Williams, K.C.; Gault, A.; Anderson, A.E.; Stewart, C.J.; Lamb, C.A.; Speight, R.A.; Rajan, N.; Plummer, R.; Pratt, A.G. Immune-Related Adverse Events in Checkpoint Blockade: Observations from Human Tissue and Therapeutic Considerations. Front. Immunol. 2023, 14, 1122430. [Google Scholar] [CrossRef] [PubMed]
- Larsabal, M.; Marti, A.; Jacquemin, C.; Rambert, J.; Thiolat, D.; Dousset, L.; Taieb, A.; Dutriaux, C.; Prey, S.; Boniface, K.; et al. Vitiligo-like Lesions Occurring in Patients Receiving Anti-Programmed Cell Death-1 Therapies Are Clinically and Biologically Distinct from Vitiligo. J. Am. Acad. Dermatol. 2017, 76, 863–870. [Google Scholar] [CrossRef]
- Carbone, M.L.; Capone, A.; Guercio, M.; Reddel, S.; Silvestris, D.A.; Lulli, D.; Ramondino, C.; Peluso, D.; Quintarelli, C.; Volpe, E.; et al. Insight into Immune Profile Associated with Vitiligo Onset and Anti-Tumoral Response in Melanoma Patients Receiving Anti-PD-1 Immunotherapy. Front. Immunol. 2023, 14, 1197630. [Google Scholar] [CrossRef]
- Hasan Ali, O.; Diem, S.; Markert, E.; Jochum, W.; Kerl, K.; French, L.E.; Speiser, D.E.; Früh, M.; Flatz, L. Characterization of Nivolumab-Associated Skin Reactions in Patients with Metastatic Non-Small Cell Lung Cancer. Oncoimmunology 2016, 5, e1231292. [Google Scholar] [CrossRef]
- Lo, J.; Hanania, H.L.; Keiser, M.F.; Patel, A.B. Immune Checkpoint Inhibitor-Induced Vitiligo in Cancer Patients: Characterization and Management. Arch. Dermatol. Res. 2023, 315, 1697–1703. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus Chemotherapy for Previously Untreated, PD-L1-Expressing, Locally Advanced or Metastatic Non-Small-Cell Lung Cancer (KEYNOTE-042): A Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef] [PubMed]
- Goulding, R.E.; Chenoweth, M.; Carter, G.C.; Boye, M.E.; Sheffield, K.M.; John, W.J.; Leusch, M.S.; Muehlenbein, C.E.; Li, L.; Jen, M.H.; et al. KRAS Mutation as a Prognostic Factor and Predictive Factor in Advanced/Metastatic Non-Small Cell Lung Cancer: A Systematic Literature Review and Meta-Analysis. Cancer Treat. Res. Commun. 2020, 24, 100200. [Google Scholar] [CrossRef]
- Gu, M.; Xu, T.; Chang, P. KRAS/LKB1 and KRAS/TP53 Co-Mutations Create Divergent Immune Signatures in Lung Adenocarcinomas. Ther. Adv. Med. Oncol. 2021, 13, 17588359211006950. [Google Scholar] [CrossRef]
- Ricciuti, B.; Arbour, K.C.; Lin, J.J.; Vajdi, A.; Vokes, N.; Hong, L.; Zhang, J.; Tolstorukov, M.Y.; Li, Y.Y.; Spurr, L.F.; et al. Diminished Efficacy of Programmed Death-(Ligand)1 Inhibition in STK11- and KEAP1-Mutant Lung Adenocarcinoma Is Affected by KRAS Mutation Status. J. Thorac. Oncol. 2022, 17, 399–410. [Google Scholar] [CrossRef]
- Casagrande, G.M.S.; Silva, M.d.O.; Reis, R.M.; Leal, L.F. Liquid Biopsy for Lung Cancer: Up-to-Date and Perspectives for Screening Programs. Int. J. Mol. Sci. 2023, 24, 2505. [Google Scholar] [CrossRef]
- Mack, P.C.; Klein, M.I.; Ayers, K.L.; Zhou, X.; Guin, S.; Fink, M.; Rossi, M.; AI-Kateb, H.; O’Connell, T.; Hantash, F.M.; et al. Targeted Next-Generation Sequencing Reveals Exceptionally High Rates of Molecular Driver Mutations in Never-Smokers with Lung Adenocarcinoma. Oncologist 2022, 27, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Barlesi, F.; West, H.; Ball, S.; Bordoni, R.; Cobo, M.; Longeras, P.D.; Goldschmidt, J.; Novello, S.; Orlandi, F.; et al. Atezolizumab Plus Chemotherapy for First-Line Treatment of Nonsquamous NSCLC: Results from the Randomized Phase 3 IMpower132 Trial. J. Thorac. Oncol. 2021, 16, 653–664. [Google Scholar] [CrossRef]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant Atezolizumab after Adjuvant Chemotherapy in Resected Stage IB–IIIA Non-Small-Cell Lung Cancer (IMpower010): A Randomised, Multicentre, Open-Label, Phase 3 Trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1–Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.-E.; Badin, F.; et al. First-Line Nivolumab in Stage IV or Recurrent Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.; Kilickap, S.; Gümüş, M.; Bondarenko, I.; Özgüroğlu, M.; Gogishvili, M.; Turk, H.M.; Cicin, I.; Bentsion, D.; Gladkov, O.; et al. Cemiplimab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer with PD-L1 of at Least 50%: A Multicentre, Open-Label, Global, Phase 3, Randomised, Controlled Trial. Lancet 2021, 397, 592–604. [Google Scholar] [CrossRef]
- Pabst, L.; Lopes, S.; Bertrand, B.; Creusot, Q.; Kotovskaya, M.; Pencreach, E.; Beau-Faller, M.; Mascaux, C. Prognostic and Predictive Biomarkers in the Era of Immunotherapy for Lung Cancer. Int. J. Mol. Sci. 2023, 24, 7577. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chau, Y.F.; Bai, H.; Wu, X.; Duan, J. Biomarkers for Immunotherapy in Driver-Gene-Negative Advanced NSCLC. Int. J. Mol. Sci. 2023, 24, 14521. [Google Scholar] [CrossRef]
- Cui, Q.; Li, W.; Wang, D.; Wang, S.; Yu, J. Prognostic Significance of Blood-Based PD-L1 Analysis in Patients with Non-Small Cell Lung Cancer Undergoing Immune Checkpoint Inhibitor Therapy: A Systematic Review and Meta-Analysis. World J. Surg. Oncol. 2023, 21, 318. [Google Scholar] [CrossRef]
- Hummelink, K.; van der Noort, V.; Muller, M.; Schouten, R.D.; Lalezari, F.; Peters, D.; Theelen, W.S.M.E.; Koelzer, V.H.; Mertz, K.D.; Zippelius, A.; et al. PD-1T TILs as a Predictive Biomarker for Clinical Benefit to PD-1 Blockade in Patients with Advanced NSCLC. Clin. Cancer Res. 2022, 28, 4893–4906. [Google Scholar] [CrossRef]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the Lung Immune Prognostic Index with Immune Checkpoint Inhibitor Outcomes in Patients with Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Vryza, P.; Fischer, T.; Mistakidi, E.; Zaravinos, A. Tumor Mutation Burden in the Prognosis and Response of Lung Cancer Patients to Immune-Checkpoint Inhibition Therapies. Transl. Oncol. 2023, 38, 101788. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Tang, M.; Cui, L.; Bai, J.; Yu, J.; Gao, J.; Nie, X.; Li, X.; Xia, X.; Yi, X.; et al. Prognostic and Predictive Impact of Molecular Tumor Burden Index in Non-Small Cell Lung Cancer Patients. Thorac. Cancer 2023, 14, 3097–3107. [Google Scholar] [CrossRef] [PubMed]
- Biton, J.; Mansuet-Lupo, A.; Pécuchet, N.; Alifano, M.; Ouakrim, H.; Arrondeau, J.; Boudou-Rouquette, P.; Goldwasser, F.; Leroy, K.; Goc, J.; et al. TP53, STK11, and EGFR Mutations Predict Tumor Immune Profile and the Response to Anti–PD-1 in Lung Adenocarcinoma. Clin. Cancer Res. 2018, 24, 5710–5723. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.B.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune Checkpoint Inhibitors for Patients with Advanced Lung Cancer and Oncogenic Driver Alterations: Results from the IMMUNOTARGET Registry. Ann. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.Y.; Zhang, J.T.; Liu, S.Y.; Su, J.; Zhang, C.; Xie, Z.; Zhou, Q.; Tu, H.Y.; Xu, C.R.; Yan, L.X.; et al. EGFR Mutation Correlates with Uninflamed Phenotype and Weak Immunogenicity, Causing Impaired Response to PD-1 Blockade in Non-Small Cell Lung Cancer. Oncoimmunology 2017, 6, e1356145. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, C.G.; Andelkovic, V.; Ladwa, R.; Pavlakis, N.; Zhou, C.; Hirsch, F.; Richard, D.; O’Byrne, K. Targeting BRAF Mutations in Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2019, 8, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Papillon-Cavanagh, S.; Doshi, P.; Dobrin, R.; Szustakowski, J.; Walsh, A.M. STK11 and KEAP1 Mutations as Prognostic Biomarkers in an Observational Real-World Lung Adenocarcinoma Cohort. ESMO Open 2020, 5, e000706. [Google Scholar] [CrossRef]
- Ying, K.; Zou, L.; Wang, D.; Wang, R.; Qian, J. Co-Mutation of TP53 and TTN Is Correlated with the Efficacy of Immunotherapy in Lung Squamous Cell Carcinoma. Comb. Chem. High Throughput Screen. 2023, 26. [Google Scholar] [CrossRef]
- De Marchi, P.; Leal, L.F.; da Silva, L.S.; Cavagna, R.d.O.; da Silva, F.A.F.; da Silva, V.D.; da Silva, E.C.; Saito, A.O.; de Lima, V.C.C.; Reis, R.M. Gene Expression Profiles (GEPs) of Immuno-Oncologic Pathways as Predictors of Response to Checkpoint Inhibitors in Advanced NSCLC. Transl. Oncol. 2023, 39, 101818. [Google Scholar] [CrossRef]
- Mouritzen, M.T.; Ladekarl, M.; Hager, H.; Mattesen, T.B.; Lippert, J.B.; Frank, M.S.; Nøhr, A.K.; Egendal, I.B.; Carus, A. Gene Expressions and High Lymphocyte Count May Predict Durable Clinical Benefits in Patients with Advanced Non-Small-Cell Lung Cancer Treated with Immune Checkpoint Inhibitors. Cancers 2023, 15, 4480. [Google Scholar] [CrossRef]
- Huai, Q.; Luo, C.; Song, P.; Bie, F.; Bai, G.; Li, Y.; Liu, Y.; Chen, X.; Zhou, B.; Sun, X.; et al. Peripheral Blood Inflammatory Biomarkers Dynamics Reflect Treatment Response and Predict Prognosis in Non-Small Cell Lung Cancer Patients with Neoadjuvant Immunotherapy. Cancer Sci. 2023, 114, 4484–4498. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Dai, S.; Li, Y.; Tian, P.; Li, Q.; Cai, X. Peripheral Blood Laboratory Test Results Combined with TCF1+CD8+ T Lymphocytes Ratio to Predict the Response and Prognosis of Immunotherapy to Advanced Lung Cancer. Zhongguo Fei Ai Za Zhi 2023, 26, 605–614. [Google Scholar] [CrossRef] [PubMed]
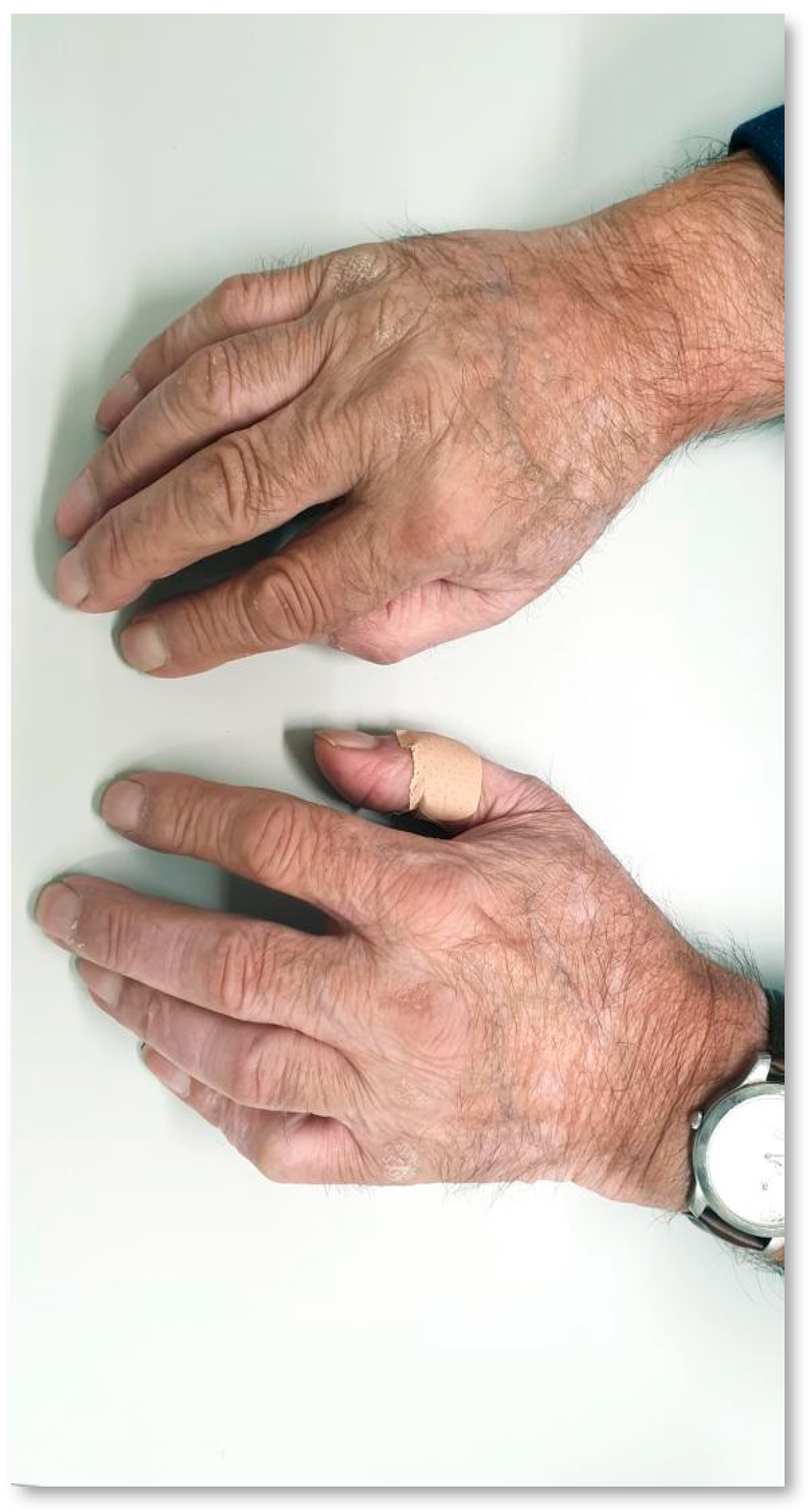
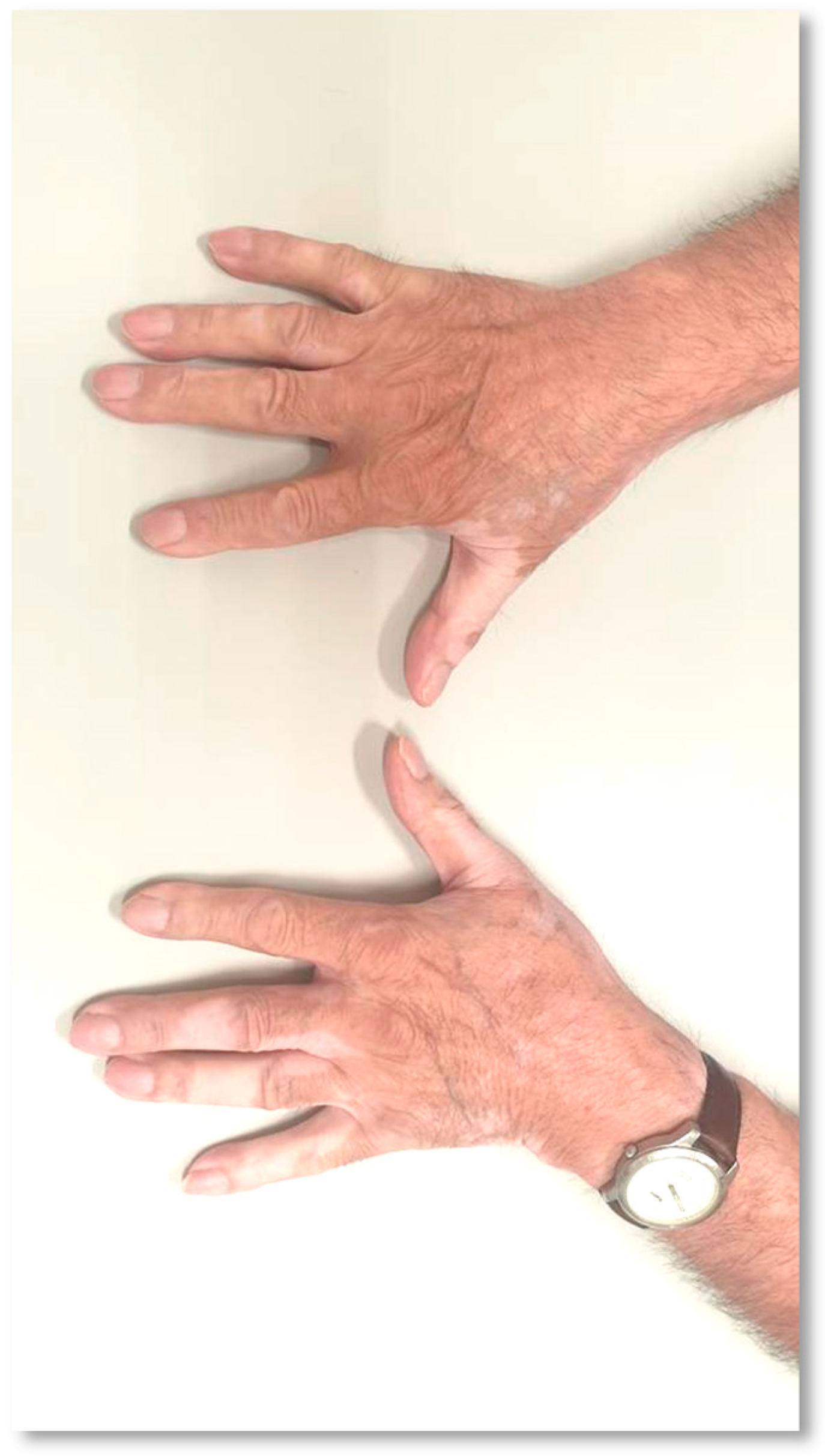
| Case Report | TX Line | Histologic Subtype | ICI | PD-L1 (TPS) | Overall Response (RECIST v1.1) | Time to VLL Onset since ICI Initiation | Vitiligo (CTCAE v5.0) | Other irAE | Time to PD since ICI Initiation | Clinical State |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2nd | SCC | Pembrolizumab | 1–5% | PR | 5 months | G1 | G2 hypothyroidism | 24 months | Alive (43 months of f-up) |
| 2 | 1st | AC | Atezolizumab | UNK | PR | 33 months | G1 | G1 asthenia G1 arthralgias G1 diarrhea G1 SCE | 54 months | Alive (70 months of f-up) |
| 3 | 1st | AC | Pembrolizumab | 0% | PD | 2 months | G1 | None | 3 months | Death (6 months of f-up) |
| Case Reviewed | TX Line | Histologic Subtype | ICI | PD-L1 (TPS) | Overall Response (RECIST v1.1) | Time to VLL Onset since ICI Initiation | Vitiligo (CTCAE v5.0) | Other irAE | Time to PD since ICI Initiation | Clinical State |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 4th | SCC | Nivolumab | UNK | PR/CR | 15 months | UNK | None | Still responding | Alive (34 months of f-up) |
| B | 1st | SCC | Pembrolizumab | UNK | PR/CR/SD | 2 months | UNK | G2 dermatitis | 10 months | Death (13 months of f-up) |
| C | 2nd | AC | Pembrolizumab | 70% | SD | 5 months | UNK | G2 hypothyroidism | 14 months | Alive (25 months of f-up) |
| D | 1st | AC | Pembrolizumab | 100% | CR | 3 months | UNK | None | Still responding | Alive (18 months of f-up) |
| E | 2nd | UNK | Nivolumab | 1–24% | PR | 20 months | UNK | G1 pneumonitis G1 arthralgias G1 hypothyroidism | Still responding | Alive (22 months of f-up) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, J.Q.; Romão, R.; Sousa, M.J.; Azevedo, S.X.; Fidalgo, P.; Araújo, A. Vitiligo-like Lesions as a Predictor of Response to Immunotherapy in Non-Small Cell Lung Cancer: Comprehensive Review and Case Series from a University Center. Curr. Oncol. 2024, 31, 1113-1128. https://doi.org/10.3390/curroncol31020083
Coelho JQ, Romão R, Sousa MJ, Azevedo SX, Fidalgo P, Araújo A. Vitiligo-like Lesions as a Predictor of Response to Immunotherapy in Non-Small Cell Lung Cancer: Comprehensive Review and Case Series from a University Center. Current Oncology. 2024; 31(2):1113-1128. https://doi.org/10.3390/curroncol31020083
Chicago/Turabian StyleCoelho, João Queirós, Raquel Romão, Maria João Sousa, Sérgio Xavier Azevedo, Paula Fidalgo, and António Araújo. 2024. "Vitiligo-like Lesions as a Predictor of Response to Immunotherapy in Non-Small Cell Lung Cancer: Comprehensive Review and Case Series from a University Center" Current Oncology 31, no. 2: 1113-1128. https://doi.org/10.3390/curroncol31020083
APA StyleCoelho, J. Q., Romão, R., Sousa, M. J., Azevedo, S. X., Fidalgo, P., & Araújo, A. (2024). Vitiligo-like Lesions as a Predictor of Response to Immunotherapy in Non-Small Cell Lung Cancer: Comprehensive Review and Case Series from a University Center. Current Oncology, 31(2), 1113-1128. https://doi.org/10.3390/curroncol31020083





