The Influence of Nordic Walking Training on the Serum Levels of Sirtuins, FOXO3a, and Vitamin D Metabolites in Patients with Multiple Myeloma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Study Protocol
- Medical interview and examination (including questionnaires, body composition analysis, anthropometric measurements, and aerobic capacity assessment);
- Venous blood collection for biochemical analyses;
- Randomized assignment to one of the following two groups, training group (TG) or control group (CG).
2.3. Methods
2.3.1. Interview—Questionnaires
2.3.2. Body Composition Analysis, Anthropometric Measurements, and Aerobic Capacity Assessment
2.3.3. Venous Blood Collection
2.4. Nordic Walking Training
2.5. Statistical Analysis
3. Results
3.1. Study Group Characteristics
3.2. Anthropometric Measurements, Body Composition Analysis, and Physical Activity Level
3.3. Serum Biochemical Parameters
4. Discussion
Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wojciechowska, U.; Barańska, K.; Miklewska, M.; Didkowska, J.A. Cancer Incidence and Mortality in Poland in 2020. Nowotw. J. Oncol. 2023, 73, 129–145. [Google Scholar] [CrossRef]
- Giannopoulos, K.; Jamroziak, K.; Usnarska-Zubkiewicz, L.; Dytfeld, D.; Jurczyszyn, A.; Walewski, J.; Lech-Marańda, E.; Walter-Croneck, A.; Pieńkowska-Grela, B.; Wróbel, T.; et al. Zalecenia Polskiej Grupy Szpiczakowej Dotyczące Rozpoznawania i Leczenia Szpiczaka Plazmocytowego Oraz Innych Dyskrazji Plazmocytowych Na Rok 2021; Polska Grupa Szpiczakowa: Poland, 2020. [Google Scholar]
- Mikhael, J.; Ismaila, N.; Cheung, M.C.; Costello, C.; Dhodapkar, M.V.; Kumar, S.; Lacy, M.; Lipe, B.; Little, R.F.; Nikonova, A.; et al. Treatment of Multiple Myeloma: ASCO and CCO Joint Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 1228–1263. [Google Scholar] [CrossRef] [PubMed]
- Witamina D—Od Dawnego Czynnika Przeciwkrzywiczego Do Nowej Substancji o Działaniu Plejotropowym Vitamin D—From the Past Antirachitic Factor to New Pleiotropic Substance. Available online: https://ppm.edu.pl/info/article/UMBe5f8bd0b9624473fbb6858d0c4b50d62?aq=mesh%3AMeSH-D014807&affil=&r=publication&ps=20&tab=&title=Publikacja%2B%25E2%2580%2593%2BWitamina%2BD%2B-%2Bod%2Bdawnego%2Bczynnika%2Bprzeciwkrzywiczego%2Bdo%2Bnowej%2Bsubstancji%2Bo%2Bdzia%25C5%2582aniu%2Bplejotropowym%2B%25E2%2580%2593%2BPolska%2BPlatforma%2BMedyczna&lang=pl (accessed on 1 November 2024).
- Mason, R.S.; Sequeira, V.B.; Gordon-Thomson, C. Vitamin D: The Light Side of Sunshine. Eur. J. Clin. Nutr. 2011, 65, 986–993. [Google Scholar] [CrossRef]
- An, B.-S.; Tavera-Mendoza, L.E.; Dimitrov, V.; Wang, X.; Calderon, M.R.; Wang, H.-J.; White, J.H. Stimulation of Sirt1-Regulated FoxO Protein Function by the Ligand-Bound Vitamin D Receptor. Mol. Cell. Biol. 2010, 30, 4890–4900. [Google Scholar] [CrossRef]
- Jeon, S.M.; Shin, E.A. Exploring Vitamin D Metabolism and Function in Cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Adamkiewicz, D.; Adamkiewicz, M.; Śniegocki, M.; Podhorecka, M.; Szychta, P.; Malinowski, B. Impact of Vitamin D on Physical Efficiency and Exercise Performance—A Review. Nutrients 2019, 11, 2826. [Google Scholar] [CrossRef] [PubMed]
- Pilch, W.; Tyka, A.; Cebula, A.; Śliwicka, E.; Pilaczyńska-Szcześniak, Ł.; Tyka, A. Effects of a 6-Week Nordic Walking Training on Changes in 25(OH)D Blood Concentration in Women Aged over 55. J. Sports Med. Phys. Fit. 2017, 57, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Czerwińska-Ledwig, O.; Vesole, D.H.; Piotrowska, A.; Gradek, J.; Pilch, W.; Jurczyszyn, A. Effect of a 6-Week Cycle of Nordic Walking Training on Vitamin 25(OH)D3, Calcium-Phosphate Metabolism and Muscle Damage in Multiple Myeloma Patients–Randomized Controlled Trial. J. Clin. Med. 2022, 11, 6534. [Google Scholar] [CrossRef]
- Liu, Y.; Ao, X.; Ding, W.; Ponnusamy, M.; Wu, W.; Hao, X.; Yu, W.; Wang, Y.; Li, P.; Wang, J. Critical Role of FOXO3a in Carcinogenesis. Mol. Cancer 2018, 17, 104. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Bidere, N.; Lenardo, M. Another Fork in the Road: Foxo3a Regulates NF-ΚB Activation. Immunity 2004, 21, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Skórka, K.; Giannopoulos, K. Budowa i Funkcje Jądrowego Czynnika Transkrypcyjnego NF Kappa B (NF-ΚB) Oraz Jego Znaczenie w Przewlekłej Białaczce Limfocytowej. Acta Haematol. Pol. 2012, 43, 54–62. [Google Scholar] [CrossRef]
- Thépot, S.; Lainey, E.; Cluzeau, T.; Sébert, M.; Leroy, C.; Adès, L.; Tailler, M.; Galluzzi, L.; Baran-Marszak, F.; Roudot, H.; et al. Hypomethylating Agents Reactivate FOXO3A in Acute Myeloid Leukemia. Cell Cycle 2011, 10, 2323–2330. [Google Scholar] [CrossRef]
- Frazzi, R.; Valli, R.; Tamagnini, I.; Casali, B.; Latruffe, N.; Merli, F. Resveratrol-Mediated Apoptosis of Hodgkin Lymphoma Cells Involves SIRT1 Inhibition and FOXO3a Hyperacetylation. Int. J. Cancer 2013, 132, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, J.; Jiang, H.; Wang, J.; Li, X.; Wang, J.; Zhu, S.; Guo, J.; Li, T.; Zhong, Y.; et al. Proteasome Inhibitor Induced SIRT1 Deacetylates GLI2 to Enhance Hedgehog Signaling Activity and Drug Resistance in Multiple Myeloma. Oncogene 2019, 39, 922–934. [Google Scholar] [CrossRef]
- Chalkiadaki, A.; Guarente, L. The Multifaceted Functions of Sirtuins in Cancer. Nat. Rev. Cancer 2015, 15, 608–624. [Google Scholar] [CrossRef]
- Allegra, A.; Innao, V.; Polito, F.; Oteri, R.; Alibrandi, A.; Allegra, A.G.; Oteri, G.; Di Giorgio, R.M.; Musolino, C.; Aguennouz, M. SIRT2 and SIRT3 Expression Correlates with Redox Imbalance and Advanced Clinical Stage in Patients with Multiple Myeloma. Clin. Biochem. 2021, 93, 42–49. [Google Scholar] [CrossRef]
- Juan, C.G.; Matchett, K.B.; Davison, G.W. A Systematic Review and Meta-Analysis of the SIRT1 Response to Exercise. Sci. Rep. 2023, 13, 14752. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Pinho, R.; Gu, Y.; Radak, Z. The Role of SIRT3 in Exercise and Aging. Cells 2022, 11, 2596. [Google Scholar] [CrossRef]
- Takeshima, N.; Islam, M.M.; Rogers, M.E.; Rogers, N.L.; Sengoku, N.; Koizumi, D.; Kitabayashi, Y.; Imai, A.; Naruse, A. Effects of Nordic Walking Compared to Conventional Walking and Band-Based Resistance Exercise on Fitness in Older Adults. J. Sports Sci. Med. 2013, 12, 422. [Google Scholar] [PubMed]
- Peyré-Tartaruga, L.A.; Boccia, G.; Feijó Martins, V.; Zoppirolli, C.; Bortolan, L.; Pellegrini, B. Margins of Stability and Trunk Coordination during Nordic Walking. J. Biomech. 2022, 134, 111001. [Google Scholar] [CrossRef]
- Sánchez-Lastra, M.A.; Torres, J.; Martínez-Lemos, I.; Ayán, C. Nordic Walking for Women with Breast Cancer: A Systematic Review. Eur. J. Cancer Care 2019, 28, e13130. [Google Scholar] [CrossRef] [PubMed]
- Stupnicki, R. International Physical Activity Questionnaire (IPAQ)—Polish Version Mi ę Dzynarodowy Kwestionariusz Aktywno ś Ci Fizycznej (IPAQ)—Wersja Polska. Phys. Educ. Sport 2007, 51, 47–54. [Google Scholar]
- Szponar, L.; Wolnicka, K.; Rychlik, E. Album Fotografii Produktów i Potraw; Prace IŻŻ: Warszawa, Poland, 2000; ISBN 83-86060-51-4.
- Jones, J.; Rikli, R. Fitness of Older Adults. J. Act. Aging 2002, 56, 24–30. [Google Scholar] [CrossRef]
- Nes, B.M.; Janszky, I.; Wisløff, U.; Støylen, A.; Karlsen, T. Age-Predicted Maximal Heart Rate in Healthy Subjects: The HUNT Fitness Study. Scand. J. Med. Sci. Sports 2013, 23, 697–704. [Google Scholar] [CrossRef]
- Paul, K.L. Rehabilitation and Exercise Considerations in Hematologic Malignancies. Am. J. Phys. Med. Rehabil. 2011, 90, S88–S94. [Google Scholar] [CrossRef]
- Czerwińska-Ledwig, O.; Żychowska, M.; Jurczyszyn, A.; Kryst, J.; Deląg, J.; Borkowska, A.; Reczkowicz, J.; Pałka, T.; Bujas, P.; Piotrowska, A. The Impact of a 6-Week Nordic Walking Training Cycle and a 14-Hour Intermittent Fasting on Disease Activity Markers and Serum Levels of Wnt Pathway-Associated Proteins in Patients with Multiple Myeloma. J. Clin. Med. 2024, 13, 2771. [Google Scholar] [CrossRef]
- Czerwińska-Ledwig, O.; Jurczyszyn, A.; Piotrowska, A.; Pilch, W.; Antosiewicz, J.; Żychowska, M. The Effect of a Six-Week Nordic Walking Training Cycle on Oxidative Damage of Macromolecules and Iron Metabolism in Older Patients with Multiple Myeloma in Remission—Randomized Clinical Trial. Int. J. Mol. Sci. 2023, 24, 15358. [Google Scholar] [CrossRef]
- Jarden, M.; Tscherning Lindholm, S.; Kaldan, G.; Grønset, C.; Faebo Larsen, R.; Larsen, A.T.S.; Schaufuss Engedal, M.; Kramer Mikkelsen, M.; Nielsen, D.; Vinther, A.; et al. Limited Evidence for the Benefits of Exercise in Older Adults with Hematological Malignancies: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 2962. [Google Scholar] [CrossRef]
- Moore, M.; Northey, J.M.; Crispin, P.; Semple, S.; Toohey, K. Effects of Exercise Rehabilitation on Physical Function in Adults With Hematological Cancer Receiving Active Treatment: A Systematic Review and Meta-Analysis. Semin Oncol. Nurs. 2023, 39, 151504. [Google Scholar] [CrossRef]
- Coleman, E.A.; Goodwin, J.A.; Kennedy, R.; Coon, S.K.; Richards, K.; Enderlin, C.; Stewart, C.B.; McNatt, P.; Lockhart, K.; Anaissie, E. Effects of Exercise on Fatigue, Sleep, and Performance: A Randomized Trial. Physiol. Behav. 2012, 39, 468–477. [Google Scholar] [CrossRef]
- Hacker, E.D.; Richards, R.L.; Abu Zaid, M.; Chung, S.Y.; Perkins, S.; Farag, S.S. STEPS to Enhance Physical Activity After Hematopoietic Cell Transplantation for Multiple Myeloma. Cancer Nurs. 2022, 45, 211–223. [Google Scholar] [CrossRef]
- Marques-Mourlet, C.; Di Iorio, R.; Fairfield, H.; Reagan, M.R. Obesity and Myeloma: Clinical and Mechanistic Contributions to Disease Progression. Front. Endocrinol. 2023, 14, 1118691. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Wang, Z.; Chen, W.Y. Sirtuins in Hematological Aging and Malignancy. Crit. Rev. Oncog. 2013, 18, 531. [Google Scholar] [CrossRef]
- Kumar, R.; Mohan, N.; Upadhyay, A.D.; Singh, A.P.; Sahu, V.; Dwivedi, S.; Dey, A.B.; Dey, S. Identification of Serum Sirtuins as Novel Noninvasive Protein Markers for Frailty. Aging Cell 2014, 13, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, C.; Yuan, F.; Li, F. Clinical Significance of SIRT3 and Inflammatory Factors in Multiple Myeloma Patients with Bortezomib-Induced Peripheral Neuropathy: A Cohort Study. Scand. J. Clin. Lab. Investig. 2021, 81, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; MacK, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643. [Google Scholar] [CrossRef]
- Carrelli, A.; Bucovsky, M.; Horst, R.; Cremers, S.; Zhang, C.; Bessler, M.; Schrope, B.; Evanko, J.; Blanco, J.; Silverberg, S.J.; et al. Vitamin D Storage in Adipose Tissue of Obese and Normal Weight Women. J. Bone Miner. Res. 2016, 32, 237. [Google Scholar] [CrossRef]
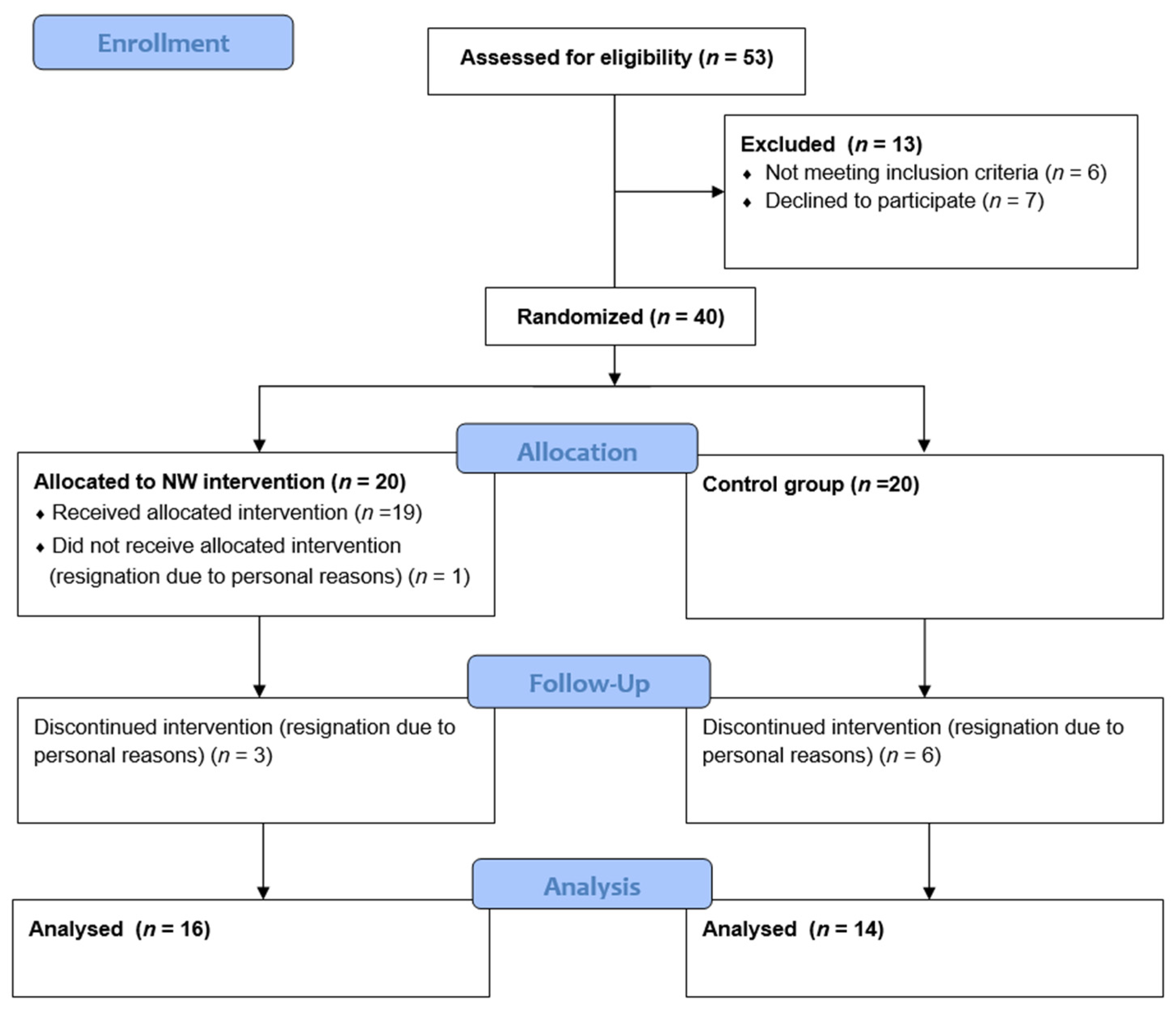
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Multiple myeloma in plateau stage without cytostatic treatment | Significant liver and kidney damage |
| Permissible bisphosphonate therapy | Acute respiratory tract infection or other infectious disease |
| Overall good condition of the patient | Another malignancy |
| Vitamin D and calcium supplementation in accordance with standards | Recent fall from one’s own height resulting in injury |
| TG (n = 16) | CG (n = 14) | |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| Energy [kcal] | 2373.6 ± 305.6 | 2450.9 ± 310.5 |
| Water [g] | 2857.6 ± 730.7 | 3184.1 ± 777.8 |
| Protein [g] | 110.4 ± 23.0 | 97.1 ± 20.2 |
| Fat [g] | 90.8 ± 18.9 | 93.9 ± 31.0 |
| Carbohydrates [g] | 293.0 ± 68.6 | 328.8 ± 76.1 |
| Vitamin A [μg] | 1465.3 ± 876.0 | 1406.4 ± 768.1 |
| Vitamin E [μg] | 9.5 ± 4.1 | 9.1 ± 4.6 |
| Vitamin C [mg] | 100.6 ± 58.1 | 89.3 ± 46.9 |
| Folic acid [μg] | 343.9 ± 103.3 | 343.5 ± 54.8 |
| Vitamin D [μg] | 5.1 ± 3.6 | 5.4 ± 7.7 |
| Energy from protein [%] | 18.7 ± 3.0 | 16.0 ± 3.2 |
| Energy from fat [%] | 34.1 ± 6.7 | 34.0 ± 10.6 |
| Energy from carbohydrates [%] | 45.4 ± 6.4 | 50.1 ± 10.8 |
| TG (n = 16) | CG (n = 14) | |||||
|---|---|---|---|---|---|---|
| Baseline (I) Mean ± SD | After 6 Weeks (III) Mean ± SD | Δ | Baseline Mean ± SD | After 6 Weeks (III) Mean ± SD | Δ | |
| Body composition | ||||||
| Body mass [kg] | 78.72 ± 10.44 | 78.53 ± 10.57 | −0.19 | 79.44 ± 15.56 | 79.91 ± 15.61 | +0.47 |
| BMI [kg/m] | 29.29 ± 3.40 | 29.26 ± 3.40 | −0.04 | 28.34 ± 4.28 | 28.48 ± 4.34 | +0.14 |
| FAT% [%] | 34.40 ± 7.13 | 34.28 ± 7.32 | −0.12 | 33.12 ± 7.47 | 32.79 ± 6.22 | −0.33 |
| FFM [kg] | 51.52 ± 8.40 | 51.51 ± 8.43 | −0.02 | 52.61 ± 9.84 | 55.44 ± 11.50 | +2.83 |
| MM [kg] | 48.91 ± 8.02 | 48.89 ± 8.04 | −0.02 | 49.95 ± 9.37 | 50.57 ± 9.65 | +0.62 |
| TBW [%] | 45.82 ± 4.80 | 45.80 ± 4.82 | −0.03 | 46.44 ± 4.66 | 47.05 ± 4.0 | +0.61 |
| Anthropometrics | ||||||
| Body height [cm] | 164.0 ± 10.2 | - | - | 166.9 ± 6.1 | - | |
| Waist [cm] | 94.32 ± 8.84 | 95.36 ± 9.22 | +1.04 | 97.57 ± 15.66 | 97.87 ± 16.11 | +0.30 |
| Hips [cm] | 104.23 ± 8.52 | 103.24 ± 7.76 | −0.99 | 103.57 ± 10.87 | 103.40 ± 10.74 | −0.17 |
| Aerobic capacity | ||||||
| 2 min step test [repetitions] | 96.4 ± 19.7 * | 108.8 ± 14.0 *,$ | +10.81 | 91.0 ± 18.3 | 92.5 ± 15.70 $ | +1.36 |
| TG (n = 16) | CG (n = 14) | TG (n = 16) | CG (n = 14) | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||||
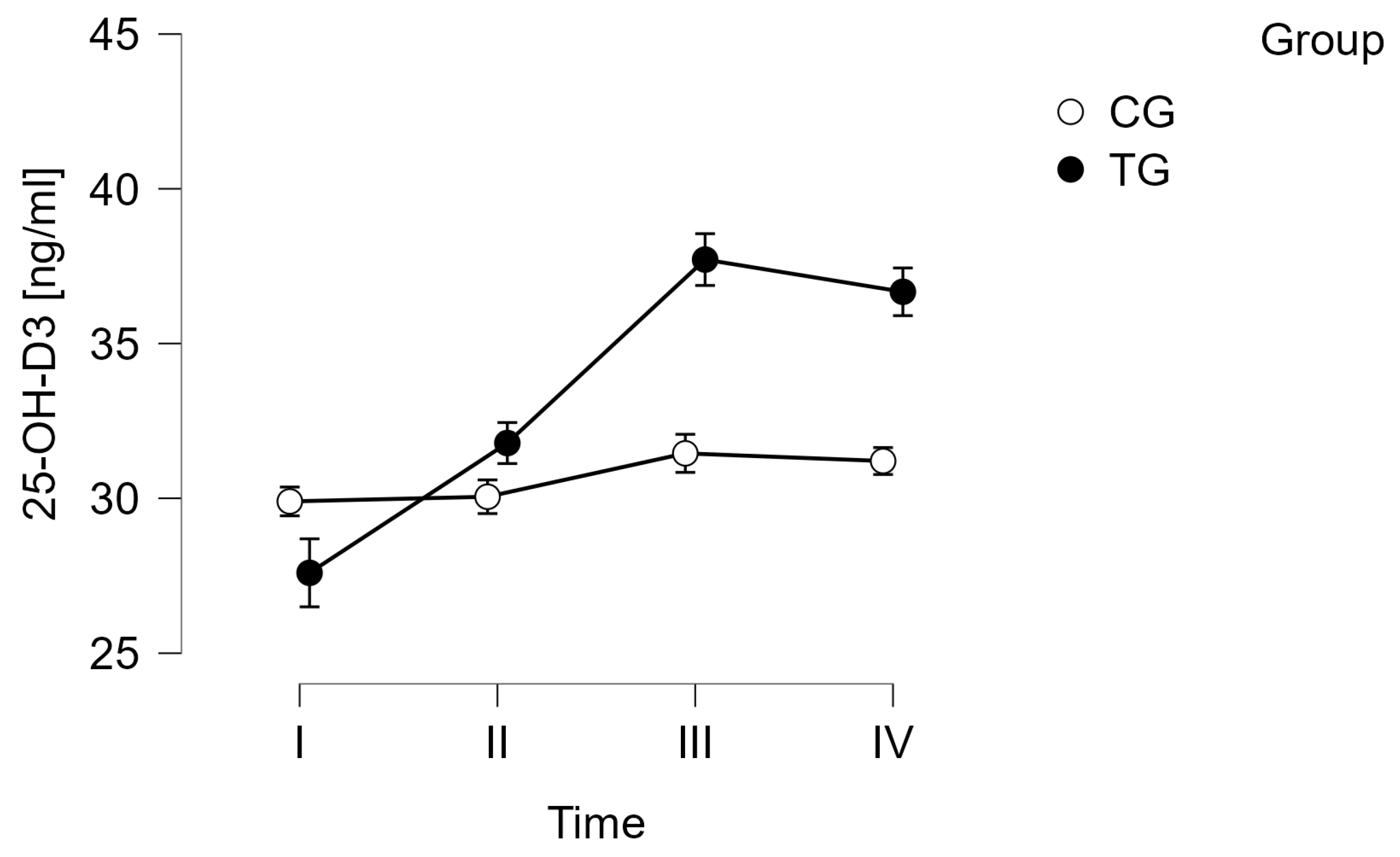
| Sirt1 [ng/mL] | I | 4.12 ± 0.16 | 4.13 ± 0.19 | 25-(OH)-D3 [ng/mL] | I | 27.60 ± 12.00 * | 29.90 ± 7.46 |
| II | 4.21 ± 0.09 | 4.19 ± 0.17 | II | 31.78 ± 10.56 *,#,$ | 30.05 ± 6.53 | ||
| III | 4.19 ± 0.07 | 4.30 ± 0.17 | III | 37.71 ± 11.28 *,# | 31.45 ± 7.77 | ||
| IV | 4.19 ± 0.12 | 4.28 ± 0.16 | IV | 36.67 ± 10.60 *,$ | 31.20 ± 6.79 | ||
| Sirt3 [ng/mL] | I | 4.97 ± 0.39 | 5.18 ± 0.35 | 1,25-(OH)-D3 [ng/mL] | I | 205.42 ± 17.72 | 207.19 ± 20.28 |
| II | 5.16 ± 0.21 | 5.21 ± 0.21 | II | 210.82 ± 8.01 | 211.39 ± 6.41 | ||
| III | 5.06 ± 0.17 | 5.08 ± 0.65 | III | 210.65 ± 4.24 | 209.34 ± 16.41 | ||
| IV | 5.16 ± 0.18 | 5.37 ± 0.28 | IV | 213.85 ± 6.15 | 208.76 ± 5.89 | ||
| Foxo3 [ng/mL] | I | 2.16 ± 0.16 | 2.16 ± 0.22 | VDR [pmol/l] | I | 23.88 ± 0.91 | 24.44 ± 0.66 |
| II | 2.24 ± 0.07 | 2.26 ± 0.11 | II | 24.17 ± 0.66 | 24.07 ± 0.71 | ||
| III | 2.23 ± 0.06 | 2.19 ± 0.35 | III | 23.98 ± 0.48 | 23.90 ± 1.69 | ||
| IV | 2.23 ± 0.05 | 2.30 ± 0.10 | IV | 24.30 ± 0.69 | 23.78 ± 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerwińska-Ledwig, O.; Żychowska, M.; Jurczyszyn, A.; Kryst, J.; Dzidek, A.; Zuziak, R.; Jurczyszyn, A.; Piotrowska, A. The Influence of Nordic Walking Training on the Serum Levels of Sirtuins, FOXO3a, and Vitamin D Metabolites in Patients with Multiple Myeloma. Curr. Oncol. 2024, 31, 7960-7970. https://doi.org/10.3390/curroncol31120587
Czerwińska-Ledwig O, Żychowska M, Jurczyszyn A, Kryst J, Dzidek A, Zuziak R, Jurczyszyn A, Piotrowska A. The Influence of Nordic Walking Training on the Serum Levels of Sirtuins, FOXO3a, and Vitamin D Metabolites in Patients with Multiple Myeloma. Current Oncology. 2024; 31(12):7960-7970. https://doi.org/10.3390/curroncol31120587
Chicago/Turabian StyleCzerwińska-Ledwig, Olga, Małgorzata Żychowska, Artur Jurczyszyn, Joanna Kryst, Adrianna Dzidek, Roxana Zuziak, Anna Jurczyszyn, and Anna Piotrowska. 2024. "The Influence of Nordic Walking Training on the Serum Levels of Sirtuins, FOXO3a, and Vitamin D Metabolites in Patients with Multiple Myeloma" Current Oncology 31, no. 12: 7960-7970. https://doi.org/10.3390/curroncol31120587
APA StyleCzerwińska-Ledwig, O., Żychowska, M., Jurczyszyn, A., Kryst, J., Dzidek, A., Zuziak, R., Jurczyszyn, A., & Piotrowska, A. (2024). The Influence of Nordic Walking Training on the Serum Levels of Sirtuins, FOXO3a, and Vitamin D Metabolites in Patients with Multiple Myeloma. Current Oncology, 31(12), 7960-7970. https://doi.org/10.3390/curroncol31120587







