Real-World, Observational, Retrospective Study to Evaluate the Effectiveness and Safety of Treatment with Sorafenib in Patients with Advanced Hepatocellular Carcinoma
Abstract
1. Introduction
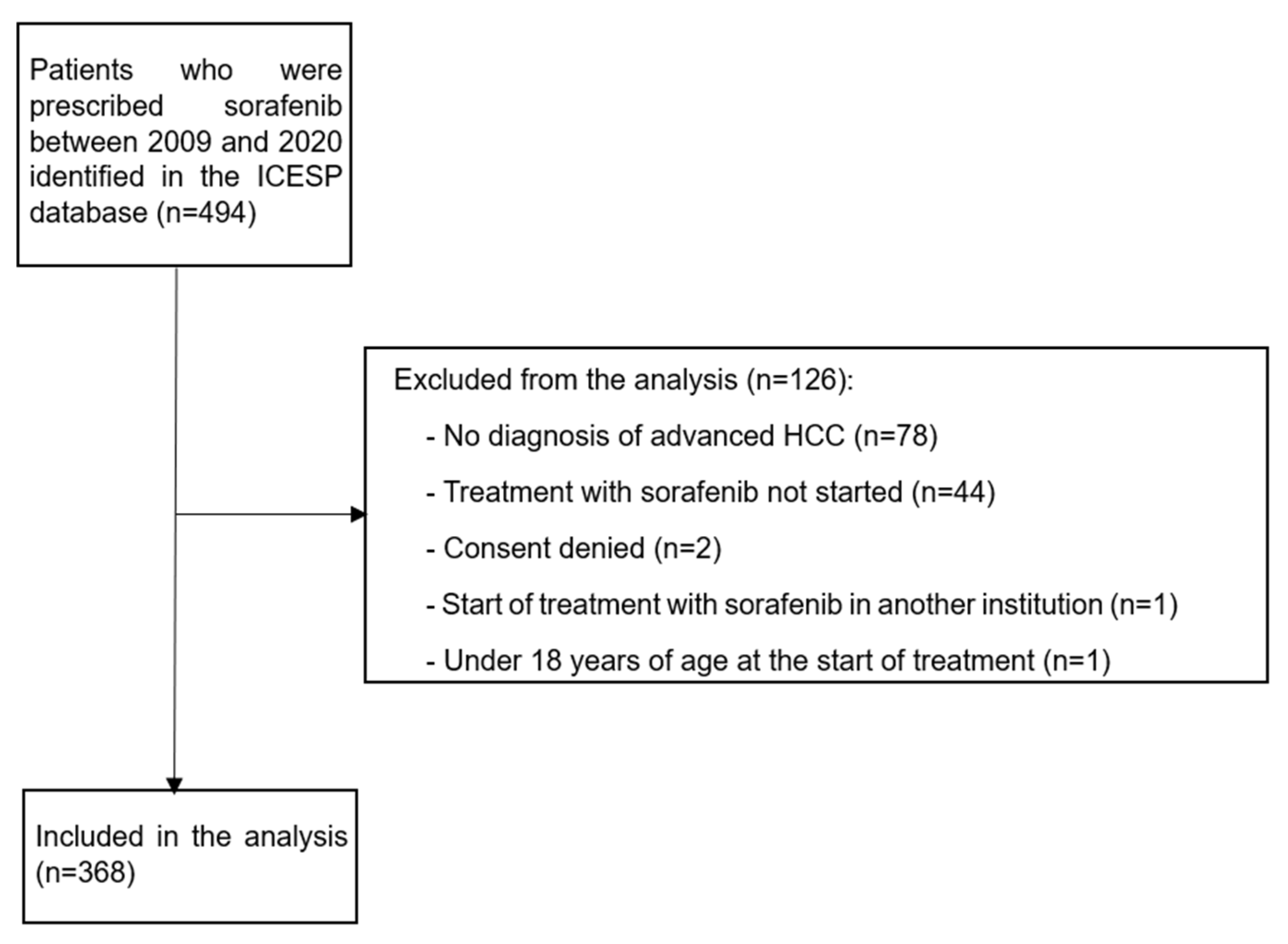
2. Patients and Methods
3. Results
3.1. Patient Characteristics
3.2. Duration of Treatment
3.3. Progression Data
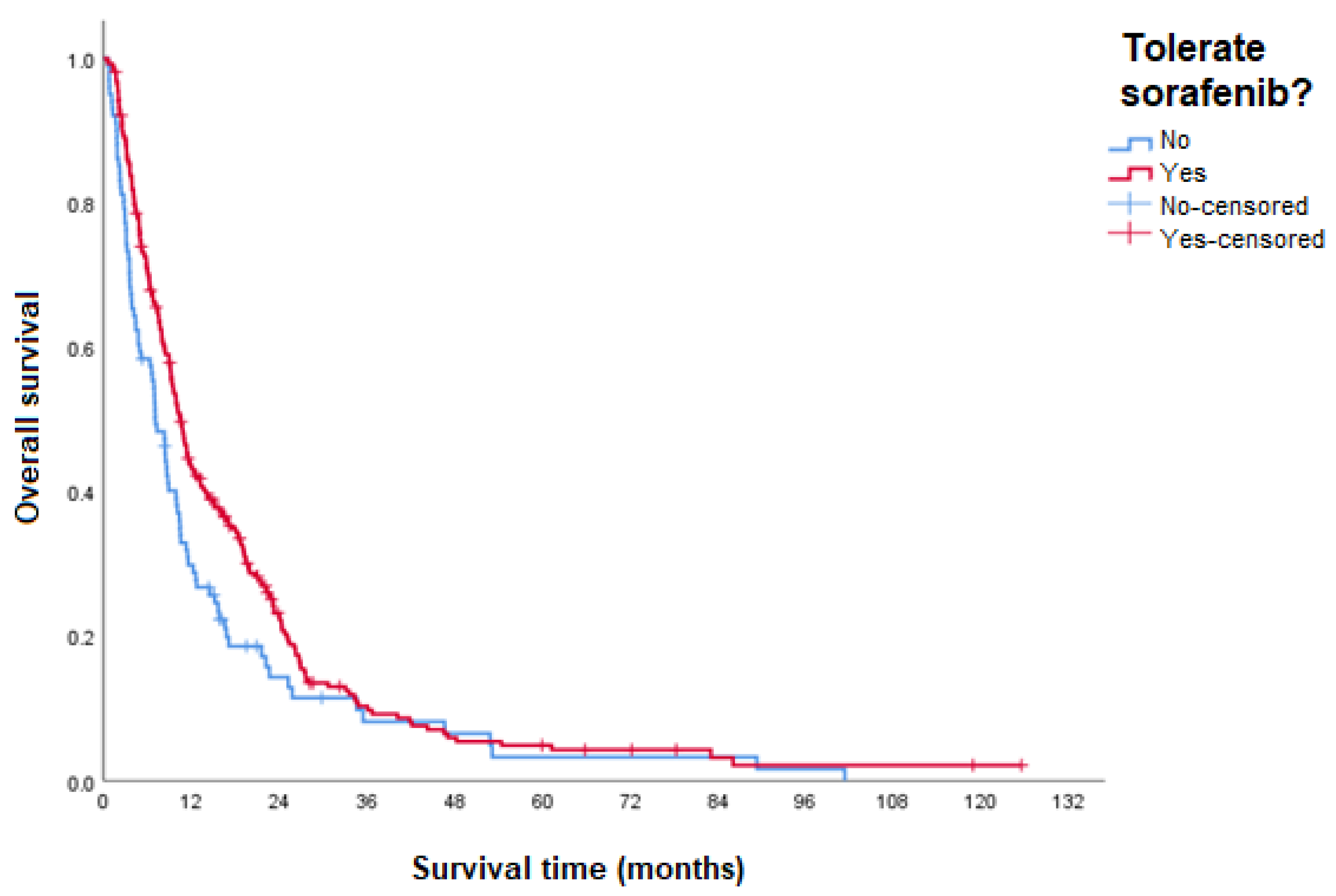
3.4. Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IARC. Cancer Fact Sheets: Stomach Cancer. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf (accessed on 6 September 2023).
- IARC. Cancer Today. Available online: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=population&mode_population=countries&population=900&populations=900&key=asr&sex=0&cancer=11&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=0&include_nmsc_other=1 (accessed on 6 September 2023).
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global epidemiology of hepatocellular carcinoma: An emphasis on demographic and regional variability. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular carcinoma. N. Engl. J. Med. 2019, 381, 1450–1462. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman- Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef]
- Moriguchi, M.; Umemura, A.; Itoh, Y. Current status and future prospects of chemotherapy for advanced hepatocellular carcinoma. Clin. J. Gastroenterol. 2016, 9, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2021, 76, 681–693. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, H.; Zhang, L.; Zhu, A.X.; Bernards, R.; Qin, W.; Wang, C. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 203–222. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Galle, P.R.; Dufour, J.F.; Peck-Radosavljevic, M.; Trojan, J.; Vogel, A. Systemic therapy of advanced hepatocellular carcinoma. Future Oncol. 2021, 17, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.K.; Dao, T.V.; De Toni, E.N.; et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.H.; Park, J.W.; et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef]
- Marisi, G.; Cucchetti, A.; Ulivi, P.; Canale, M.; Cabibbo, G.; Solaini, L.; Foschi, F.G.; De Matteis, S.; Ercolani, G.; Valgiusti, M.; et al. Ten years of sorafenib in hepatocellular carcinoma: Are there any predictive and/or prognostic markers? World J. Gastroenterol. 2018, 24, 4152. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Schirmacher, P.; Vilgrain, V. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Blonde, L.; Khunti, K.; Harris, S.B.; Meizinger, C.; Skolnik, N.S. Interpretation and impact of real-world clinical data for the practicing clinician. Adv. Ther. 2018, 35, 1763–1774. [Google Scholar] [CrossRef]
- FDA. Real-World Evidence. Available online: https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence (accessed on 5 October 2023).
- Camargo-Pinheiro-Alves, R.; Viera-Alves, D.E.; Malzyner, A.; Gampel, O.; de F Almeida-Costa, T.; Guz, B.; Poletti, P. Experience with sorafenib in 3 hospitals in Sao Paulo. Ann. Hepatol. 2019, 18, 172–176. [Google Scholar] [CrossRef]
- Longo, L.; de Freitas, L.B.R.; Santos, D.; Grivicich, I.; Álvares-da-Silva, M.R. Sorafenib for advanced hepatocellular carcinoma: A real-life experience. Dig. Dis. 2018, 36, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.; Marsh, P.; Gill, R.; Rodov, M.; Mohsen, W.; Varma, P.; Hong, T.; Strasser, S.I.; Bell, S.; Ryan, M.; et al. Sorafenib in the treatment of hepatocellular carcinoma: A multi-centre real-world study. Scand. J. Gastroenterol. 2016, 51, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Leathers, J.S.; Balderramo, D.; Prieto, J.; Diehl, F.; Gonzalez-Ballerga, E.; Ferreiro, M.R.; Carrera, E.; Barreyro, F.; Diaz-Ferrer, J.; Singh, D.; et al. Sorafenib for treatment of hepatocellular carcinoma: A survival analysis from the South American liver research network. J. Clin. Gastroenterol. 2019, 53, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Branco, F.; Alencar, R.S.M.; Volt, F.; Sartori, G.; Dode, A.; Kikuchi, L.; Tani, C.M.; Chagas, A.L.; Pfiffer, T.; Hoff, P.; et al. The impact of early dermatologic events in the survival of patients with hepatocellular carcinoma treated with sorafenib. Ann. Hepatol. 2017, 16, 263–268. [Google Scholar] [CrossRef]
- Dang, A. Real-world evidence: A primer. Pharm. Med. 2023, 37, 25–36. [Google Scholar] [CrossRef]


| Characteristic (n = 368) | n | % |
|---|---|---|
| Sex | ||
| Male | 279 | 75.8 |
| Female | 89 | 24.2 |
| Age (years) | ||
| Mean (SD) | 61.5 (10.4) | |
| Median (minimum-maximum) | 62.3 (19–86.3) | |
| Etiology | ||
| HCV | 188 | 51.1 |
| HBV | 52 | 14.1 |
| Alcohol only | 52 | 14.1 |
| NASH | 36 | 9.8 |
| Cryptogenic cirrhosis | 14 | 3.8 |
| Other * | 16 | 4.3 |
| Unknown/Uninformed | 10 | 2.7 |
| ECOG | ||
| 0 | 212 | 57.6 |
| 1 | 154 | 41.8 |
| 2 | 1 | 0.3 |
| 3 | 1 | 0.3 |
| Child–Pugh class | ||
| A (5) | 218 | 59.2 |
| A (6) | 88 | 23.9 |
| B (7) | 43 | 11.7 |
| B (8) | 16 | 4.3 |
| B (9) | 3 | 0.8 |
| BCLC stage | ||
| A | 3 | 0.8 |
| B | 88 | 23.9 |
| C | 277 | 75.3 |
| Time to Progression (TTP) | Median (95% CI) (Months) |
|---|---|
| Time to radiological progression | 5.3 (4.7–6.0) |
| Time to symptomatic/clinical progression | 2.3 (1.3–3.4) |
| Overall Survival (OS) | Median (95% CI) (Months) | p Value | HR (95% CI) |
|---|---|---|---|
| General study population | |||
| OS | 9.6 (8.5–10.7) | ||
| OS according to the type of progression at the time of treatment discontinuation | |||
| Radiological progression | 11.5 (9.7–13.2) | <0.001 | 1 |
| Symptomatic/clinical progression | 3.6 (2.6–4.7) | 3.1 (2.3–4.1) | |
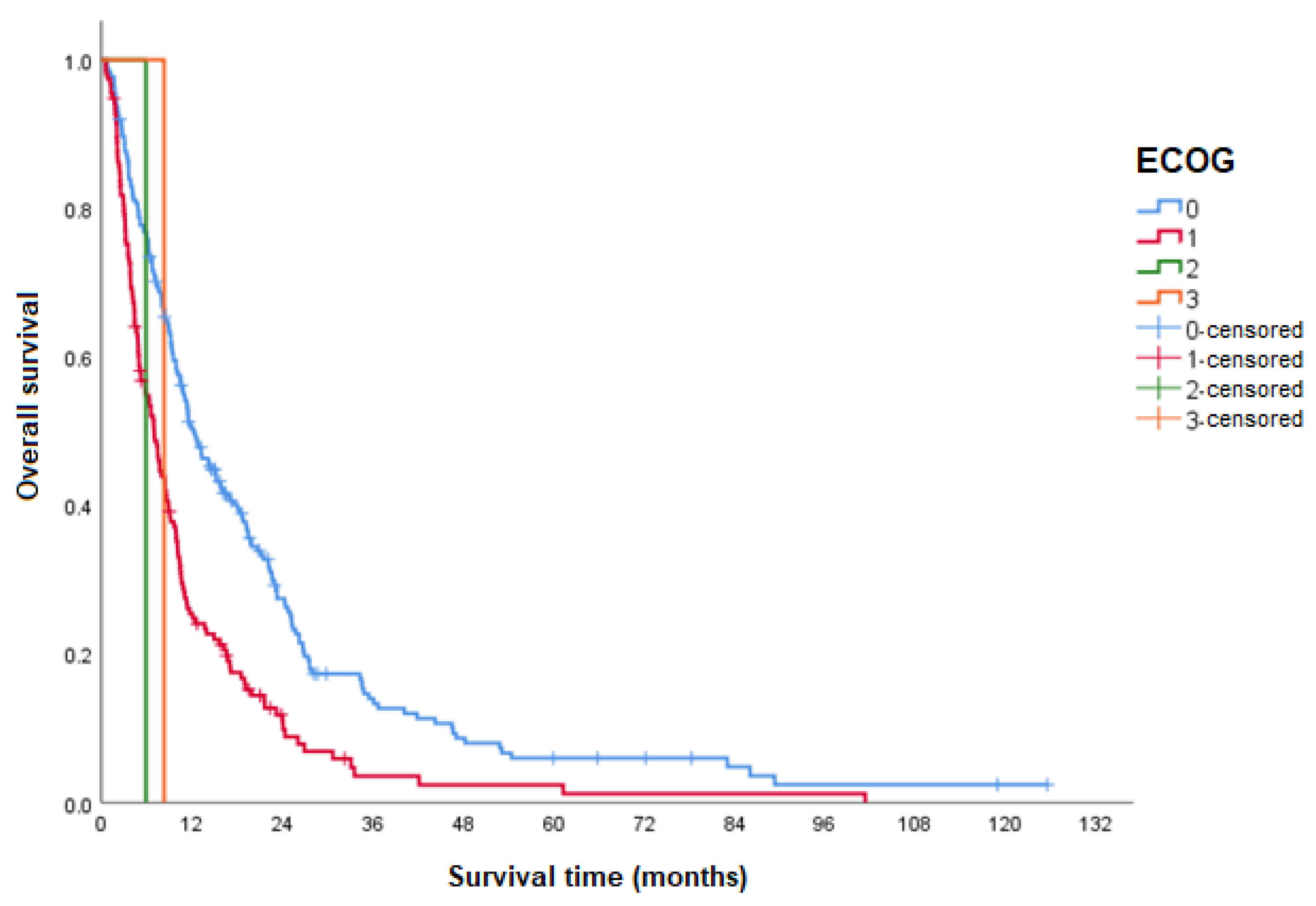
| OS according to the ECOG performance status | |||
| ECOG 0 | 12.3 (9.9–14.6) | <0.001 | 1 |
| ECOG 1 | 7.0 (5.6–8.4) | 1.8 (1.4–2.3) | |
| ECOG 2 | 5.9 | 3.4 (0.5–24.1) | |
| ECOG 3 | 8.3 | 2.2 (0.3–16.3) | |
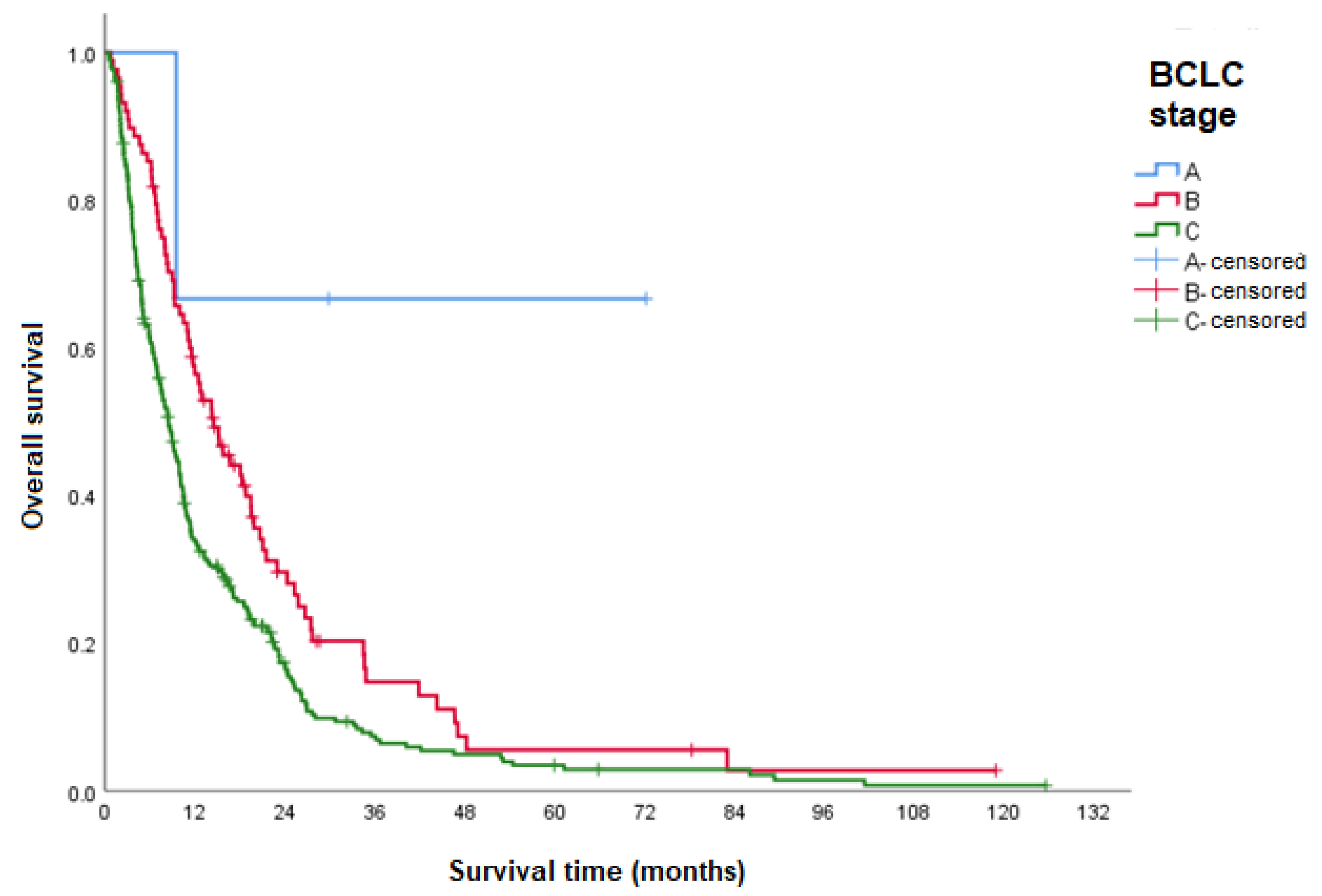
| OS according to BCLC stage | |||
| BCLC B (intermediate) | 14.5 (10.5–18.5) | <0.001 | 1 |
| BCLC C (advanced) | 8.5 (7.2–9.8) | 1.5 (1.2–2.0) | |
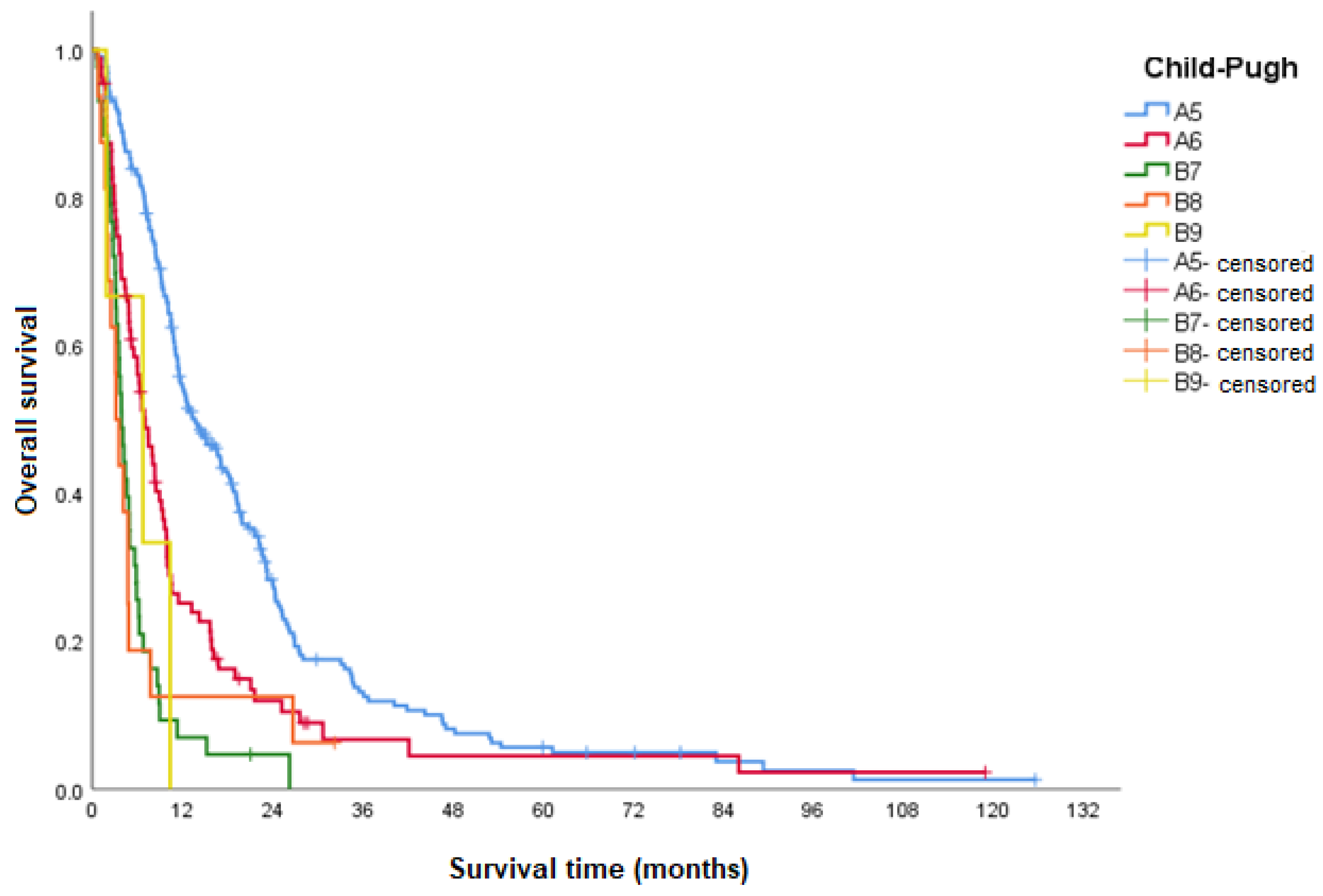
| OS according to Child–Pugh class | |||
| Child–Pugh A(5) | 13.7 (10.4–17.0) | <0.001 | 1 |
| Child–Pugh A(6) | 7.0 (5.3–8.8) | <0.001 | 1.8 (1.4–2.4) |
| Child–Pugh B(7) | 3.9 (3.2–4.7) | <0.001 | 4.0 (2.8–5.7) |
| Child–Pugh B(8) | 3.2 (2.3–4.0) | <0.001 | 3.1 (1.8–5.3) |
| Child–Pugh B(9) | 6.7 (0–14.5) | 0.037 | 3.3 (1.1–10.7) |
| OS according to etiology | |||
| HCV | 10.5 (8.4–12.7) | 0.023 | 1 |
| HBV | 7.1 (5.7–8.4) | 0.019 | 1.5 (1.1–2.0) |
| Alcohol only | 9.9 (6.9–12.9) | 0.202 | 1.2 (0.9–1.7) |
| NASH | 8.0 (7.3–8.7) | 0.049 | 1.5 (1.0–2.1) |
| Cryptogenic cirrhosis | 5.0 (0.0–11.4) | 0.040 | 1.8 (1.0–3.1) |
| Other | 10.3 (4.1–16.6) | 0.719 | 0.9 (0.5–1.6) |
| Unknown/Uninformed | 12.0 (3.0–20.9) | 0.171 | 0.6 (0.3–1.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csipak, A.R.; da Fonseca, L.G.; López, R.V.M.; Estevez-Diz, M.D.P. Real-World, Observational, Retrospective Study to Evaluate the Effectiveness and Safety of Treatment with Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Curr. Oncol. 2024, 31, 6778-6790. https://doi.org/10.3390/curroncol31110500
Csipak AR, da Fonseca LG, López RVM, Estevez-Diz MDP. Real-World, Observational, Retrospective Study to Evaluate the Effectiveness and Safety of Treatment with Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Current Oncology. 2024; 31(11):6778-6790. https://doi.org/10.3390/curroncol31110500
Chicago/Turabian StyleCsipak, Angélica Richart, Leonardo G. da Fonseca, Rossana Verónica Mendoza López, and Maria Del Pilar Estevez-Diz. 2024. "Real-World, Observational, Retrospective Study to Evaluate the Effectiveness and Safety of Treatment with Sorafenib in Patients with Advanced Hepatocellular Carcinoma" Current Oncology 31, no. 11: 6778-6790. https://doi.org/10.3390/curroncol31110500
APA StyleCsipak, A. R., da Fonseca, L. G., López, R. V. M., & Estevez-Diz, M. D. P. (2024). Real-World, Observational, Retrospective Study to Evaluate the Effectiveness and Safety of Treatment with Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Current Oncology, 31(11), 6778-6790. https://doi.org/10.3390/curroncol31110500





