Current Radiotherapy Management of Extensive-Stage Small-Cell Lung Cancer in the Immunotherapy Era: An Italian National Survey on Behalf of the Italian Association of Radiotherapy and Clinical Oncology (AIRO)
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographics and Expertise in ES-SCLC Treatment
3.2. Management of ES-SCLC
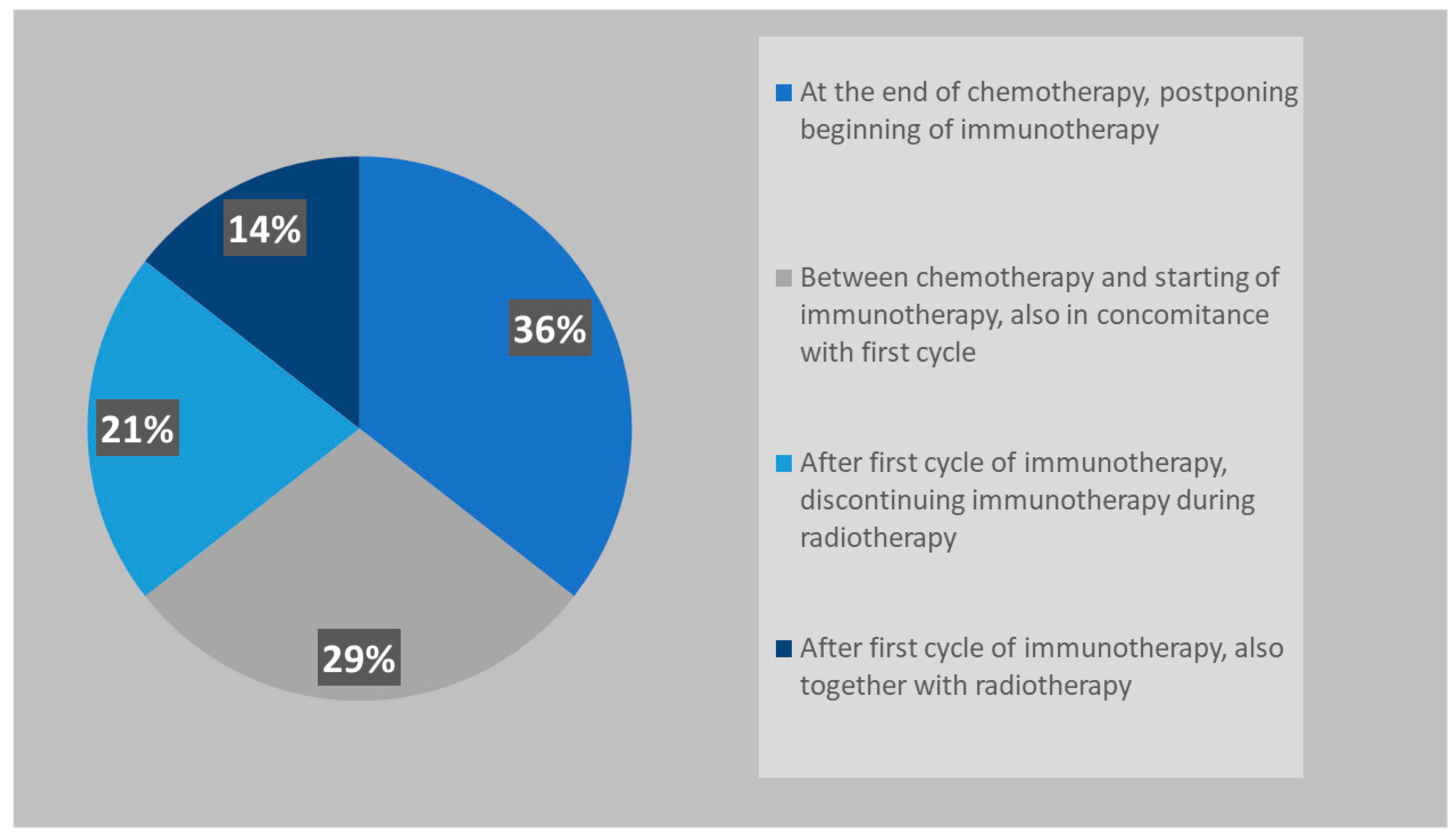
3.2.1. Role of Systemic Treatment
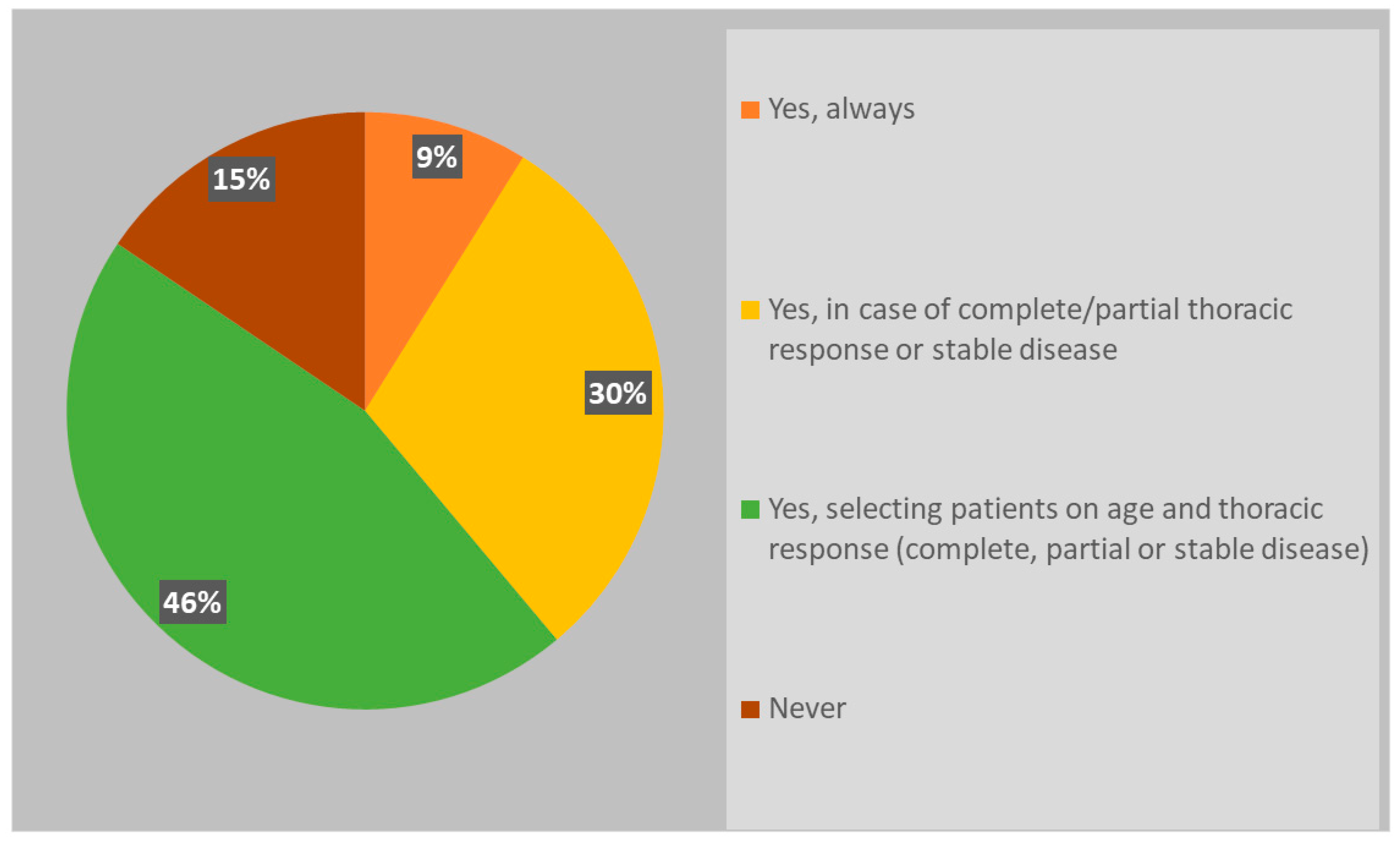
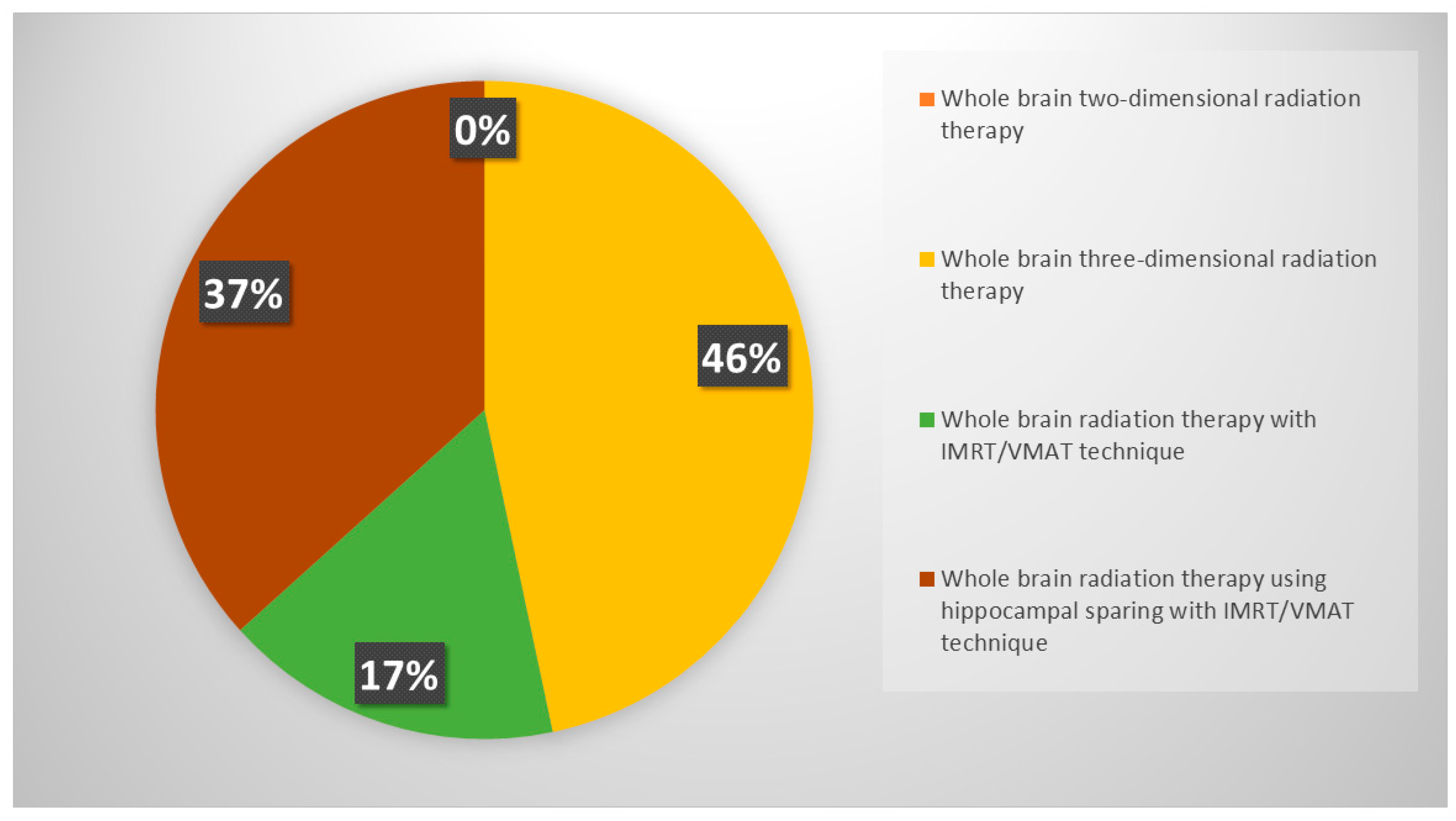
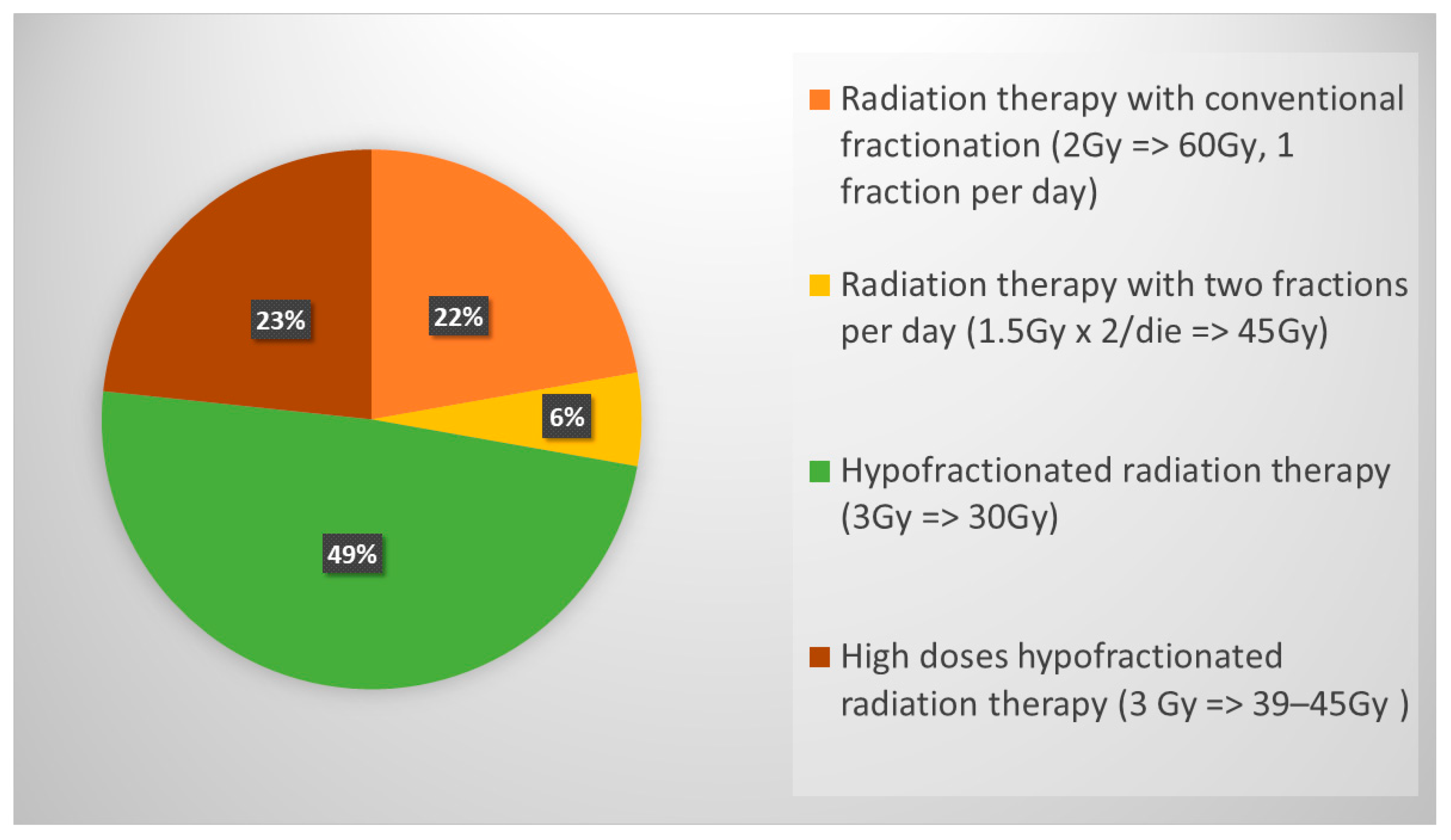
3.2.2. Role of PCI
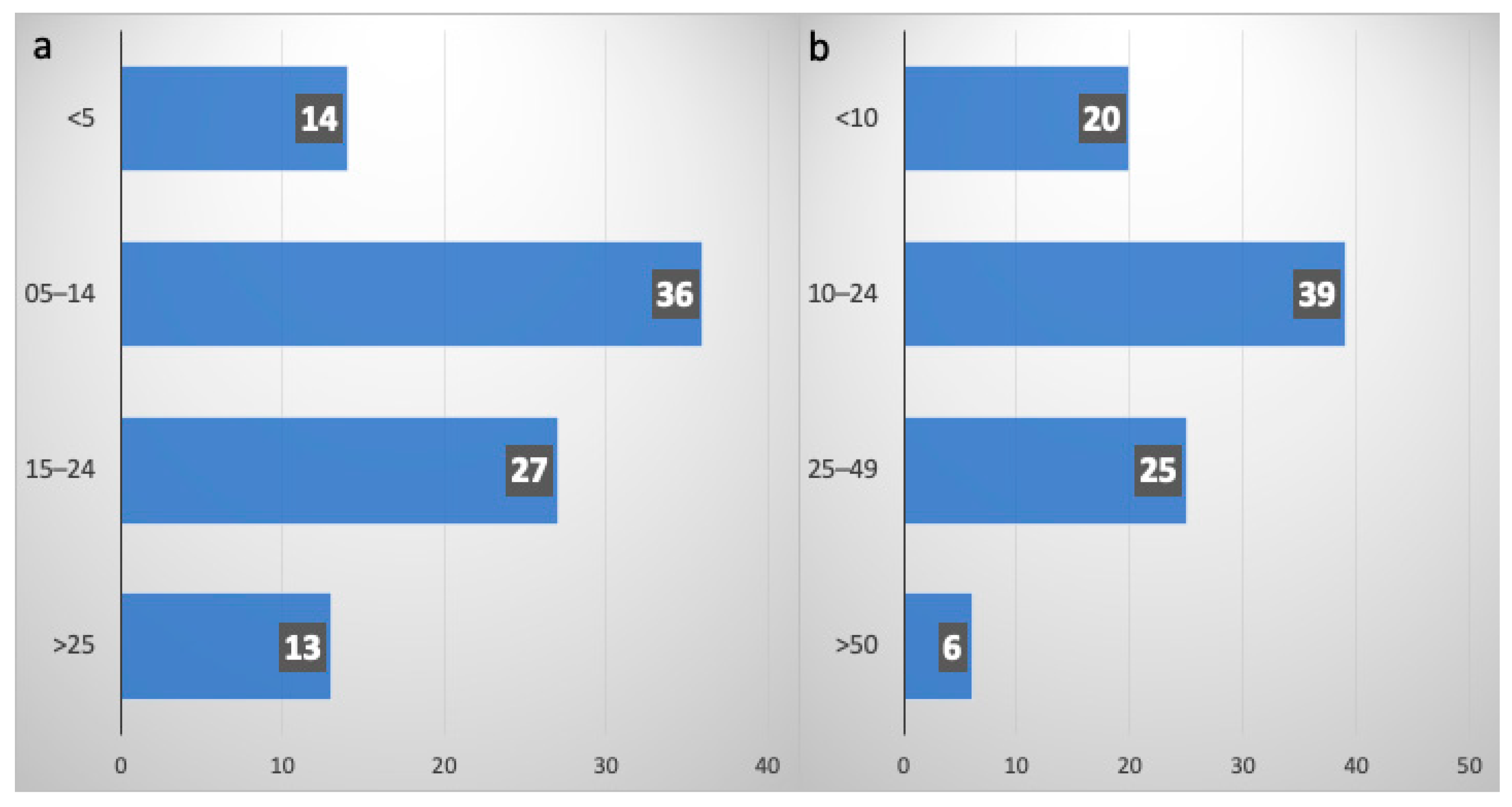
3.2.3. Role of Thoracic Consolidative RT
3.2.4. Management of Oligoprogression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daly, M.E.; Ismaila, N.; Decker, R.H.; Higgins, K.; Owen, D.; Saxena, A.; Franklin, G.E.; Donaldson, D.; Schneider, B.J. Radiation Therapy for Small-Cell Lung Cancer: ASCO Guideline Endorsement of an ASTRO Guideline. J. Clin. Oncol. 2021, 39, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Primers 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.G.; Chansky, K.; Crowley, J.; Beyruti, R.; Kubota, K.; Turrisi, A.; Eberhardt, W.E.; van Meerbeeck, J.; Rami-Porta, R.; Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions; et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the Clinical and Pathologic Staging of Small Cell Lung Cancer in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, H.; Ismaila, N.; Bian, J.; Dabney, R.; Das, M.; Ellis, P.; Feldman, J.; Hann, C.; Kulkarni, S.; Laskin, J.; et al. Systemic Therapy for Small-Cell Lung Cancer: ASCO-Ontario Health (Cancer Care Ontario) Guideline. J. Clin. Oncol. 2023, 41, 5448–5472. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Spigel, D.R.; Cho, B.C.; Laktionov, K.K.; Fang, J.; Chen, Y.; Zenke, Y.; Lee, K.H.; Wang, Q.; Navarro, A.; et al. Durvalumab after Chemoradiotherapy in Limited-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2024, 391, 1313–1327. [Google Scholar] [CrossRef] [PubMed]
- Lally, B.E.; Urbanic, J.J.; Blackstock, A.W.; Miller, A.A.; Perry, M.C. Small cell lung cancer: Have we made any progress over the last 25 years? Oncologist 2007, 12, 1096–1104. [Google Scholar] [CrossRef]
- Levy, A.; Botticella, A.; Le Péchoux, C.; Faivre-Finn, C. Thoracic radiotherapy in small cell lung cancer-a narrative review. Transl. Lung Cancer Res. 2021, 10, 2059–2070. [Google Scholar] [CrossRef]
- Goldman, J.W.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): Updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 51–65. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; Garassino, M.C.; et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open 2022, 7, 100408. [Google Scholar] [CrossRef]
- Liu, S.V.; Reck, M.; Mansfield, A.S.; Mok, T.; Scherpereel, A.; Reinmuth, N.; Garassino, M.C.; De Castro Carpeno, J.; Califano, R.; Nishio, M.; et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients with Extensive-Stage Small-Cell Lung Cancer Treated with Atezolizumab, Carboplatin, and Etoposide (IMpower133). J. Clin. Oncol. 2021, 39, 619–630. [Google Scholar] [CrossRef]
- Sun, A.; Abdulkarim, B.; Blais, N.; Greenland, J.; Louie, A.V.; Melosky, B.; Schellenberg, D.; Snow, S.; Liu, G. Use of radiation therapy among patients with Extensive-stage Small-cell lung cancer receiving Immunotherapy: Canadian consensus recommendations. Lung Cancer 2023, 179, 107166. [Google Scholar] [CrossRef] [PubMed]
- Chemo-Immunotherapy Plus Thoracic Radiotherapy in Extensive Stage Small-Cell Lung Cancer (TRIPLEX). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05223647 (accessed on 1 September 2024).
- Bozorgmehr, F.; Christopoulos, P.; Chung, I.; Cvetkovic, J.; Feißt, M.; Krisam, J.; Schneider, M.A.; Heußel, C.P.; Kreuter, M.; Müller, D.W.; et al. Protocol of the TREASURE study: Thoracic RadiothErapy with Atezolizumab in Small cell lUng canceR Extensive disease—A randomized, open-label, multicenter phase II trial. BMC Cancer 2022, 22, 1011. [Google Scholar] [CrossRef] [PubMed]
- NIH, Testing the Addition of Radiation Therapy to the Usual Immune Therapy Treatment (Atezolizumab) for Extensive Stage Small Cell Lung Cancer, The RAPTOR Trial. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04402788 (accessed on 1 September 2024).
- Takahashi, T.; Yamanaka, T.; Seto, T.; Harada, H.; Nokihara, H.; Saka, H.; Nishio, M.; Kaneda, H.; Takayama, K.; Ishimoto, O.; et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Ciammella, P.; Timon, G.; Bruni, A.; Franceschini, D.; Borghetti, P.; Giaj-Levra, N.; Greco, C.; Scotti, V.; Trovo, M.; On the Behalf of Associazione Italiana Radioterapia Oncologica (AIRO). Radiation therapy in small cell lung cancer: A national Italian survey. Radiol. Med. 2018, 123, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Liu, A.; Almquist, D.; Langlais, B.T.; Leventakos, K.; Yu, N.Y.; Manochakian, R.; Ernani, V. Chemoimmunotherapy in patients with extensive-stage small cell lung cancer and a poor performance status. Cancer 2023, 129, 3546–3553. [Google Scholar] [CrossRef]
- Agarwal, M.; Liu, A.; Langlais, B.T.; Leventakos, K.; Yu, N.Y.; Almquist, D.; Manochakian, R.; Ernani, V. Chemoimmunotherapy as the First-Line Treatment for Patients with Extensive-Stage Small-Cell Lung Cancer and an ECOG Performance Status 2 or 3. Clin. Lung Cancer 2023, 24, 591–597. [Google Scholar] [CrossRef]
- Longo, V.; Pizzutilo, P.; Catino, A.; Montrone, M.; Pesola, F.; Marerch, I.; Galetta, D. Prognostic factors for survival in extensive-stage small cell lung cancer: An Italian real-world retrospective analysis of 244 patients treated over the last decade. Thorac. Cancer 2022, 13, 3486–3495. [Google Scholar] [CrossRef]
- Slotman, B.; Faivre-Finn, C.; Kramer, G.; Rankin, E.; Snee, M.; Hatton, M.; Postmus, P.; Collette, L.; Musat, E.; Senan, S.; et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N. Engl. J. Med. 2007, 357, 664–672. [Google Scholar] [CrossRef]
- Gondi, V.; Paulus, R.; Bruner, D.W.; Meyers, C.A.; Gore, E.M.; Wolfson, A.; Werner-Wasik, M.; Sun, A.Y.; Choy, H.; Movsas, B. Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: Pooled secondary analysis of Radiation Therapy Oncology Group randomized trials 0212 and 0214. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 656–664. [Google Scholar] [CrossRef]
- Higgins, K.A.; Curran WJJr Liu, S.V.; Yu, W.; Brockman, M.; Johnson, A.; Bara, I.; Bradley, J.D. Patterns of Disease Progression after Carboplatin/Etoposide + Atezolizumab in Extensive-Stage Small-Cell Lung Cancer (ES-SCLC). Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 1398. [Google Scholar] [CrossRef]
- Chen, Y.; Paz-Ares, L.; Reinmuth, N.; Garassino, M.C.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Verderame, F.; Havel, L.; Losonczy, G.; et al. Impact of Brain Metastases on Treatment Patterns and Outcomes with First-Line Durvalumab Plus Platinum-Etoposide in Extensive-Stage SCLC (CASPIAN): A Brief Report. JTO Clin. Res. Rep. 2022, 3, 100330. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.J.; Sheikh, S.; Kharouta, M.; Chaung, K.; Choi, S.; Margevicius, S.; Fu, P.; Machtay, M.; Bruno, D.b.S.; Dowlati, A.; et al. The Impact of Prophylactic Cranial Irradiation and Consolidative Thoracic Radiation Therapy for Extensive Stage Small-Cell Lung Cancer in the Transition to the Chemo-Immunotherapy Era: A Single Institution Series. Clin. Lung Cancer 2023, 24, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Kong, W.; Shang, J.; Zhe, H.; Wang, Y.Y. Hippocampal-Sparing Whole-Brain Radiotherapy for Lung Cancer. Clin. Lung Cancer 2017, 18, 127–131. [Google Scholar] [CrossRef] [PubMed]
- S1827 (MAVERICK) Testing Whether the Use of Brain Scans Alone Instead of Brain Scans Plus Preventive Brain Radiation Affects Lifespan in Patients with Small Cell Lung Cancer. Available online: https://clinicaltrials.gov/study/NCT04155034 (accessed on 1 September 2024).
- PRophylactic Cerebral Irradiation or Active MAgnetic Resonance Imaging Surveillance in Small-Cell Lung Cancer Patients (PRIMALung Study) (PRIMALung). Available online: https://clinicaltrials.gov/study/NCT04790253 (accessed on 1 September 2024).
- Expert Panel Thoracic Malignancies; Higgins, K.A.; Simone, C.B., 2nd; Amini, A.; Chetty, I.J.; Donington, J.; Edelman, M.J.; Chun, S.G.; Kestin, L.L.; Movsas, B.; et al. American Radium Society Appropriate Use Criteria on Radiation Therapy for Extensive-Stage SCLC. J. Thorac. Oncol. 2021, 16, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, A.C.; Früh, M.; Ardizzoni, A.; Besse, B.; Faivre-Finn, C.; Hendriks, L.E.; Lantuejoul, S.; Peters, S.; Reguart, N.; Rudin, C.M.; et al. Electronic address: Clinicalguidelines@esmo.org. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann. Oncol. 2021, 32, 839–853. [Google Scholar] [CrossRef]
- Gore, E.M.; Hu, C.; Sun, A.Y.; Grimm, D.F.; Ramalingam, S.S.; Dunlap, N.E.; Higgins, K.A.; Werner-Wasik, M.; Allen, A.M.; Iyengar, P.; et al. Randomized Phase II Study Comparing Prophylactic Cranial Irradiation Alone to Prophylactic Cranial Irradiation and Consolidative Extracranial Irradiation for Extensive-Disease Small Cell Lung Cancer (ED SCLC): NRG Oncology RTOG 0937. J. Thorac. Oncol. 2017, 12, 1561–1570. [Google Scholar] [CrossRef]
- Slotman, B.J.; van Tinteren, H.; Praag, J.O.; Knegjens, J.L.; El Sharouni, S.Y.; Hatton, M.; Keijser, A.; Faivre-Finn, C.; Senan, S. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: A phase 3 randomised controlled trial. Lancet 2015, 385, 36–42. [Google Scholar] [CrossRef]
- Cozzi, S.; Bruni, A.; Ruggieri, M.P.; Borghetti, P.; Scotti, V.; Franceschini, D.; Fiore, M.; Taraborrelli, M.; Salvi, F.; Galaverni, M.; et al. Thoracic Radiotherapy in Extensive Disease Small Cell Lung Cancer: Multicenter Prospective Observational TRENDS Study. Cancers 2023, 15, 434. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Sun, H.; Sun, Y.; Ge, Y.; Cheng, Q.; Wang, D.; Wang, X.; Fu, X.; Li, J.; et al. 2000P Efficacy and safety of thoracic radiotherapy after first-line immunotherapy in extensive stage small cell lung cancer: A multi-center retrospective study. Ann. Oncol. 2023, 34, S1067. [Google Scholar] [CrossRef]
- Bozorgmehr, F.; Weykamp, F.; Overbeck, T.R.; Maguire, N.; Buchmeier, E.L.; Hammer-Hellmig, M.; Gauler, T.C.; Wermke, M.; Troost, E.G.C.; Ulmer, M.; et al. 1988MO Recruitment discontinuation in TREASURE trial (thoracic radiotherapy with atezolizumab in small cell lung cancer extensive disease) due to unexpected safety data. Ann. Oncol. 2023, 34 (Suppl. 2), S1060. [Google Scholar] [CrossRef]
- Rusthoven, C.G.; Yamamoto, M.; Bernhardt, D.; Smith, D.E.; Gao, D.; Serizawa, T.; Yomo, S.; Aiyama, H.; Higuchi, Y.; Shuto, T.; et al. Evaluation of First-line Radiosurgery vs Whole-Brain Radiotherapy for Small Cell Lung Cancer Brain Metastases: The FIRE-SCLC Cohort Study. JAMA Oncol. 2020, 6, 1028–1037, Erratum in: JAMA Oncol. 2020, 6, 1473. https://doi.org/10.1001/jamaoncol.2020.3404. [Google Scholar] [CrossRef] [PubMed]
- Gjyshi, O.; Lin, S.H.; Pezzi, T.A.; Ning, M.S.; Ma, J.; Liu, S.; Rusthoven, C.G. Care Patterns for Stereotactic Radiosurgery in Small Cell Lung Cancer Brain Metastases. Clin. Lung Cancer 2022, 23, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Borghetti, P.; Facheris, G.; Ciammella, P.; Galaverni, M.; Granello, L.; Scotti, V.; Franceschini, D.; Romei, A.; Giaj Levra, N.; Federico, M.; et al. Sterotactic Ablative Radiotherapy in a Multicentric Series of Oligometastatic SCLC: The SAMOS Cohort. Clin. Lung Cancer 2024, 25, 151–158. [Google Scholar] [CrossRef] [PubMed]
| Medical Specialty (%) | Radiation Oncologist | 89 | Pneumologists | 4 | Medical Oncologist | 6 | Other Specialties | 1 |
|---|---|---|---|---|---|---|---|---|
| Years of experience (%) | <5 years | 30 | 6–10 years | 19 | 11–15 years | 31 | >15 years | 20 |
| Geographical distribution (%) | North % | 51 | Centre | 21 | South | 28 | ||
| Institution of work (%) | Academic hospital | 24 | Non-academic hospital | 46 | IRCCS Cancer Care Center | 24 | Private hospital | 6 |
| Time spent working on lung cancer (%) | <50% | 31 | 50–70% | 44 | 70–90% | 15 | <90% | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruni, A.; Scotti, V.; Zerella, M.A.; Bertolini, F.; Imbrescia, J.; Olmetto, E.; Bennati, C.; Cuccia, F.; Miele, M.; Giaj-Levra, N.; et al. Current Radiotherapy Management of Extensive-Stage Small-Cell Lung Cancer in the Immunotherapy Era: An Italian National Survey on Behalf of the Italian Association of Radiotherapy and Clinical Oncology (AIRO). Curr. Oncol. 2024, 31, 6791-6802. https://doi.org/10.3390/curroncol31110501
Bruni A, Scotti V, Zerella MA, Bertolini F, Imbrescia J, Olmetto E, Bennati C, Cuccia F, Miele M, Giaj-Levra N, et al. Current Radiotherapy Management of Extensive-Stage Small-Cell Lung Cancer in the Immunotherapy Era: An Italian National Survey on Behalf of the Italian Association of Radiotherapy and Clinical Oncology (AIRO). Current Oncology. 2024; 31(11):6791-6802. https://doi.org/10.3390/curroncol31110501
Chicago/Turabian StyleBruni, Alessio, Vieri Scotti, Maria Alessia Zerella, Federica Bertolini, Jessica Imbrescia, Emanuela Olmetto, Chiara Bennati, Francesco Cuccia, Marianna Miele, Niccolò Giaj-Levra, and et al. 2024. "Current Radiotherapy Management of Extensive-Stage Small-Cell Lung Cancer in the Immunotherapy Era: An Italian National Survey on Behalf of the Italian Association of Radiotherapy and Clinical Oncology (AIRO)" Current Oncology 31, no. 11: 6791-6802. https://doi.org/10.3390/curroncol31110501
APA StyleBruni, A., Scotti, V., Zerella, M. A., Bertolini, F., Imbrescia, J., Olmetto, E., Bennati, C., Cuccia, F., Miele, M., Giaj-Levra, N., Tiseo, M., Ciammella, P., Vagge, S., Galaverni, M., Pontoriero, A., Badellino, S., Spoto, R., Alì, E., & Borghetti, P., on behalf of the Associazione Italiana Radioterapia Oncologica (AIRO). (2024). Current Radiotherapy Management of Extensive-Stage Small-Cell Lung Cancer in the Immunotherapy Era: An Italian National Survey on Behalf of the Italian Association of Radiotherapy and Clinical Oncology (AIRO). Current Oncology, 31(11), 6791-6802. https://doi.org/10.3390/curroncol31110501







