Health-Related Quality of Life and Treatment Satisfaction of Patients with Malignant IDH Wild-Type Gliomas and Their Caregivers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Sociodemographic, Clinical and Treatment Factors
2.4. Psycho-Oncological Need and Treatment
2.5. Health-Related Quality of Life and Patient and Caregiver Treatment Satisfaction
2.6. Statistics
2.7. Ethical and Regulatory Framework
3. Results
3.1. Patient Characteristics
3.2. Distribution of HR-QoL and Patient/Caregiver Treatment Satisfaction Questionnaires
3.3. Health-Related Quality of Life
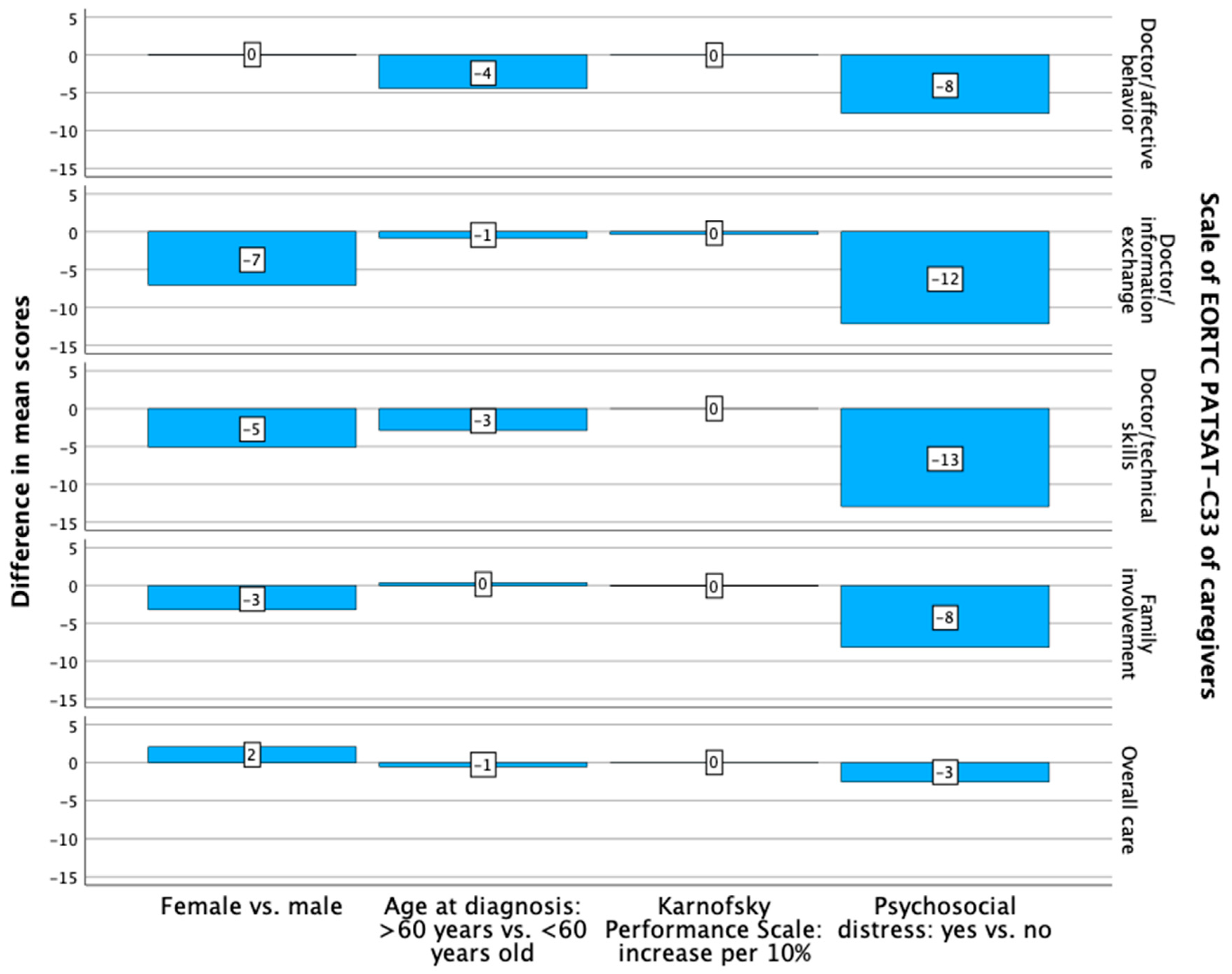
3.4. Patient and Caregiver Treatment Satisfaction
4. Discussion
4.1. Role of Sex in HR-QoL
4.2. Role of Age in HR-QoL
4.3. Role of Functional Status in HR-QoL
4.4. Role of Psychosocial Distress in HR-QoL
4.5. Treatment Satisfaction in the Patient Cohort
4.6. Treatment Satisfaction of Caregivers
4.7. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro Oncol. 2021, 23, iii1–iii105. [Google Scholar] [CrossRef] [PubMed]
- Coomans, M.B.; Dirven, L.; Aaronson, N.K.; Baumert, B.G.; Van Den Bent, M.; Bottomley, A.; Brandes, A.A.; Chinot, O.; Coens, C.; Gorlia, T.; et al. Symptom clusters in newly diagnosed glioma patients: Which symptom clusters are independently associated with functioning and global health status? Neuro Oncol. 2019, 21, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Wiewrodt, D. Psycho-oncological care of brain tumor patients. Der Onkol. 2018, 25, 67–72. [Google Scholar] [CrossRef]
- Coomans, M.; Dirven, L.; Aaronson, N.K.; Baumert, B.G.; van den Bent, M.; Bottomley, A.; Brandes, A.A.; Chinot, O.; Coens, C.; Gorlia, T.; et al. The added value of health-related quality of life as a prognostic indicator of overall survival and progression-free survival in glioma patients: A meta-analysis based on individual patient data from randomised controlled trials. Eur. J. Cancer 2019, 116, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.C.M.; Zwinkels, H.; Koekkoek, J.A.F.; Vos, M.J.; Dirven, L.; Taphoorn, M.J.B. The Impact of the Timing of Health-Related Quality of Life Assessments on the Actual Results in Glioma Patients: A Randomized Prospective Study. Cancers 2020, 12, 2172. [Google Scholar] [CrossRef]
- Aaronson, N.K. Quality of life: What is it? How should it be measured? Oncology 1988, 2, 64+69–76. [Google Scholar]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; Haes, J.C.D.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Taphoorn, M.J.; Claassens, L.; Aaronson, N.K.; Coens, C.; Mauer, M.; Osoba, D.; Stupp, R.; Mirimanoff, R.O.; van den Bent, M.J.; Bottomley, A.; et al. An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur. J. Cancer 2010, 46, 1033–1040. [Google Scholar] [CrossRef]
- Dirven, L.; Vos, M.E.; Walbert, T.; Armstrong, T.S.; Arons, D.; van den Bent, M.J.; Blakeley, J.; Brown, P.D.; Bulbeck, H.; Chang, S.M.; et al. Systematic review on the use of patient-reported outcome measures in brain tumor studies: Part of the Response Assessment in Neuro-Oncology Patient-Reported Outcome (RANO-PRO) initiative. Neurooncol. Pract. 2021, 8, 417–425. [Google Scholar] [CrossRef]
- Karnofsky, D.A.; Abelmann, W.H.; Craver, L.F.; Burchenal, J.H. The use of the nitrogen mustards in the palliative treatment of carcinoma: With particular reference to bronchogenic carcinoma. Cancer 1948, 1, 634–656. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Yang, S.; Wu, X.; Wang, S.; Li, X.; Zhang, F.; Wang, P.; Zhao, J. The quality of life in nasopharyngeal carcinoma radiotherapy: A longitudinal study. Asia Pac. J. Oncol. Nurs. 2023, 10, 100251. [Google Scholar] [CrossRef]
- Oertelt-Prigione, S.; de Rooij, B.H.; Mols, F.; Oerlemans, S.; Husson, O.; Schoormans, D.; Haanen, J.B.; van de Poll-Franse, L.V. Sex-differences in symptoms and functioning in >5000 cancer survivors: Results from the PROFILES registry. Eur. J. Cancer 2021, 156, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Efficace, F.; Al Essa, W.; Platzbecker, U.; Niscola, P.; Palumbo, G.A.; Caocci, G.; Cottone, F.; Breccia, M.; Luppi, M.; Stauder, R.; et al. Health-related Quality of Life Profile of Newly Diagnosed Patients with Myelodysplastic Syndromes by Age, Sex, and Risk Group: A Real-world Study by the GIMEMA. Hemasphere 2023, 7, e944. [Google Scholar] [CrossRef] [PubMed]
- Eichler, M.; Hentschel, L.; Singer, S.; Hornemann, B.; Richter, S.; Hofbauer, C.; Hohenberger, P.; Kasper, B.; Andreou, D.; Pink, D.; et al. Health related Quality of Life over time in German sarcoma patients. An analysis of associated factors—Results of the PROSa study. Front. Endocrinol. 2023, 14, 1166838. [Google Scholar] [CrossRef] [PubMed]
- Formica, V.; Nardecchia, A.; Morelli, C.; Lucchetti, J.; Giuliano, G.; Renzi, N.; Gallo, C.; Pellegrino, R.; Massimiliani, V.; Serci, C.; et al. Health-related quality of life in patients with advanced colorectal cancer: A predictive nomogram including BMI, sex and age. Ann. Palliat. Med. 2021, 10, 4252–4261. [Google Scholar] [CrossRef]
- Mainio, A.; Hakko, H.; Niemelä, A.; Koivukangas, J.; Räsänen, P. Gender difference in relation to depression and quality of life among patients with a primary brain tumor. Eur. Psychiatry 2006, 21, 194–199. [Google Scholar] [CrossRef]
- Coomans, M.B.; Dirven, L.; Aaronson, N.; Baumert, B.G.; van den Bent, M.; Bottomley, A.; Brandes, A.A.; Chinot, O.; Coens, C.; Gorlia, T.; et al. Factors associated with health-related quality of life (HRQoL) deterioration in glioma patients during the progression-free survival period. Neuro Oncol. 2022, 24, 2159–2169. [Google Scholar] [CrossRef]
- Milanetto, A.C.; Armellin, C.; Brigiari, G.; Lorenzoni, G.; Pasquali, C. Younger Age and Parenchyma-Sparing Surgery Positively Affected Long-Term Health-Related Quality of Life after Surgery for Pancreatic Neuroendocrine Neoplasms. J. Clin. Med. 2023, 12, 6529. [Google Scholar] [CrossRef]
- Schut, A.W.; Lidington, E.; Timbergen, M.J.M.; Younger, E.; van der Graaf, W.T.A.; van Houdt, W.J.; Bonenkamp, J.J.; Jones, R.L.; Grunhagen, D.J.; Sleijfer, S.; et al. Unraveling Desmoid-Type Fibromatosis-Specific Health-Related Quality of Life: Who Is at Risk for Poor Outcomes. Cancers 2022, 14, 2979. [Google Scholar] [CrossRef]
- Renovanz, M.; Hickmann, A.K.; Nadji-Ohl, M.; Keric, N.; Weimann, E.; Wirtz, C.R.; Singer, S.; Ringel, F.; Coburger, J. Health-related quality of life and distress in elderly vs. younger patients with high-grade glioma-results of a multicenter study. Support. Care Cancer 2020, 28, 5165–5175. [Google Scholar] [CrossRef]
- Strittmatter, G.; Tilkorn, M.; Mawick, R. How to Identify Patients in Need of Psychological Intervention. Cancer Res. 2002, 160, 353–361. [Google Scholar]
- Holmes, A.C.N.; Adams, S.J.; Hall, S.; Rosenthal, M.A.; Drummond, K.J. Liaison psychiatry in a central nervous system tumor service. Neurooncol. Pract. 2015, 2, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Schipmann, S.; Suero Molina, E.; Frasch, A.; Stummer, W.; Wiewrodt, D. Initial psycho-oncological counselling in neuro-oncology: Analysis of topics and needs of brain tumour patients. J. Neurooncol. 2018, 136, 505–514. [Google Scholar] [CrossRef]
- Hoffmann, K.; Kamp, M.; Steiger, H.J.; Sabel, M.; Rapp, M. Correlation of psychooncological distress- screening and quality of life assessment in neurosurgical patients. Oncotarget 2017, 8, 111396. [Google Scholar] [CrossRef] [PubMed]
- Boele, F.W.; Hoeben, W.; Hilverda, K.; Lenting, J.; Calis, A.L.; Sizoo, E.M.; Collette, E.H.; Heimans, J.J.; Taphoorn, M.J.; Reijneveld, J.C.; et al. Enhancing quality of life and mastery of informal caregivers of high-grade glioma patients: A randomized controlled trial. J. Neurooncol. 2013, 111, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Hannon, B.; Swami, N.; Krzyzanowska, M.K.; Leighl, N.; Rodin, G.; Le, L.W.; Zimmermann, C. Satisfaction with oncology care among patients with advanced cancer and their caregivers. Qual. Life Res. 2013, 22, 2341–2349. [Google Scholar] [CrossRef]
- Drury, A.; Payne, S.; Brady, A.M. Identifying associations between quality of life outcomes and healthcare-related variables among colorectal cancer survivors: A cross-sectional survey study. Int. J. Nurs. Stud. 2020, 101, 103434. [Google Scholar] [CrossRef]
- Litzelman, K.; Kent, E.E.; Mollica, M.; Rowland, J.H. How Does Caregiver Well-Being Relate to Perceived Quality of Care in Patients with Cancer? Exploring Associations and Pathways. J. Clin. Oncol. 2016, 34, 3554–3561. [Google Scholar] [CrossRef]
- Zamanipoor Najafabadi, A.H.; van der Meer, P.B.; Boele, F.W.; Taphoorn, M.J.B.; Klein, M.; Peerdeman, S.M.; van Furth, W.R.; Dirven, L.; Dutch Meningioma, C. The long-term caregiver burden in World Health Organization grade I and II meningioma: It is not just the patient. Neurooncol. Adv. 2021, 3, vdaa169. [Google Scholar] [CrossRef]
- Sauer, C.; Ihrig, A.; Hanslmeier, T.; Huber, J.; Hiller, K.; Friederich, H.C.; Maatouk, I. Health-related quality of life of advanced prostate cancer patients and spouses: Results from actor-partner interdependence models. Support. Care Cancer 2022, 30, 6985–6993. [Google Scholar] [CrossRef] [PubMed]
- Boele, F.W.; Butler, S.; Nicklin, E.; Bulbeck, H.; Pointon, L.; Short, S.C.; Murray, L. Communication in the context of glioblastoma treatment: A qualitative study of what matters most to patients, caregivers and health care professionals. Palliat. Med. 2023, 37, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Retzer, A.; Baddeley, E.; Sivell, S.; Seddon, K.; Bulbeck, H.; Nelson, A.; Calvert, M.; Grant, R.; Adams, R.; Watts, C.; et al. Core outcomes in Brain Tumour Trials—The COBra Study Review of Glioma Trial Registration Data. Neuro Oncol. 2022, 24 (Suppl. 4), iv5. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; DeAngelis, L.M.; Brandes, A.A.; Peereboom, D.M.; Galanis, E.; Lin, N.U.; Soffietti, R.; Macdonald, D.R.; Chamberlain, M.; Perry, J.; et al. The Neurologic Assessment in Neuro-Oncology (NANO) scale: A tool to assess neurologic function for integration into the Response Assessment in Neuro-Oncology (RANO) criteria. Neuro Oncol. 2017, 19, 625–635. [Google Scholar] [CrossRef]
- Fischl, A.; Gerken, M.; Roos, P.; Haedenkamp, T.; Hillberg, A.; Klinkhammer-Schalke, M.; Kölbl, O.; Linker, R.; Proescholdt, M.; Pukrop, T.; et al. Does the distance to the cancer center affect psycho-oncological care and emergency visits of patients with IDH wild-type gliomas?—A retrospective study. Neuro-Oncol. Pract. 2023, 10, 446–453. [Google Scholar] [CrossRef]
- Bredart, A.; Anota, A.; Young, T.; Tomaszewski, K.A.; Arraras, J.I.; Moura De Albuquerque Melo, H.; Schmidt, H.; Friend, E.; Bergenmar, M.; Costantini, A.; et al. Phase III study of the European Organisation for Research and Treatment of Cancer satisfaction with cancer care core questionnaire (EORTC PATSAT-C33) and specific complementary outpatient module (EORTC OUT-PATSAT7). Eur. J. Cancer Care 2018, 27, e12786. [Google Scholar] [CrossRef]
- Cull, A.; Sprangers, M.; Bjordal, K.; Aaronson, N.; West, K.; Bottomley, A.; on behalf of the EORTC Quality of Life Group. EORTC Quality of Life Group Translation Procedure, 2nd ed.; EORTC Data Center: Brussels, Belgium, 2002. [Google Scholar]
- Reijneveld, J.C.; Machingura, A.; Coens, C.; Taphoorn, M.J.B.; Taal, W.; Clement, P.M.; Idbaih, A.; de Vos, F.Y.F.; Klein, M.; Wick, W.; et al. Health-related quality-of-life results from the randomised phase II TAVAREC trial on temozolomide with or without bevacizumab in 1p/19q intact first-recurrence World Health Organization grade 2 and 3 glioma (European Organization for Research and Treatment of Cancer 26091). Eur. J. Cancer 2023, 190, 112946. [Google Scholar] [CrossRef]
- Reijneveld, J.C.; Taphoorn, M.J.B.; Coens, C.; Bromberg, J.E.C.; Mason, W.P.; Hoang-Xuan, K.; Ryan, G.; Hassel, M.B.; Enting, R.H.; Brandes, A.A.; et al. Health-related quality of life in patients with high-risk low-grade glioma (EORTC 22033-26033): A randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016, 17, 1533–1542. [Google Scholar] [CrossRef]
- Habets, E.J.; Taphoorn, M.J.; Nederend, S.; Klein, M.; Delgadillo, D.; Hoang-Xuan, K.; Bottomley, A.; Allgeier, A.; Seute, T.; Gijtenbeek, A.M.; et al. Health-related quality of life and cognitive functioning in long-term anaplastic oligodendroglioma and oligoastrocytoma survivors. J. Neurooncol. 2014, 116, 161–168. [Google Scholar] [CrossRef]
- Weller, J.; Tzaridis, T.; Mack, F.; Steinbach, J.P.; Schlegel, U.; Hau, P.; Krex, D.; Grauer, O.; Goldbrunner, R.; Bähr, O.; et al. Health-related quality of life and neurocognitive functioning with lomustine-temozolomide versus temozolomide in patients with newly diagnosed, MGMT-methylated glioblastoma (CeTeG/NOA-09): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Fayers, P.; Aaronson, N.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A.; EORTC Quality of Life Group. EORTC QLQ-C30 Scoring Manual; EORTC Data Center: Brussels, Belgium, 2001. [Google Scholar]
- Koch, M.; Grafenstein, L.; Karnosky, J.; Schulz, C.; Koller, M. Psychosocial Burden and Quality of Life of Lung Cancer Patients: Results of the EORTC QLQ-C30/QLQ-LC29 Questionnaire and Hornheide Screening Instrument. Cancer Manag. Res. 2021, 13, 6191–6197. [Google Scholar] [CrossRef] [PubMed]
- Brix, C.; Schleussner, C.; Fuller, J.; Roehrig, B.; Wendt, T.G.; Strauss, B. The need for psychosocial support and its determinants in a sample of patients undergoing radiooncological treatment of cancer. J. Psychosom. Res. 2008, 65, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, J.T.; Van Harn, M.; Haider, S.A.; Phillips, J.; Walbert, T. Caregiver perceptions of end-of-life care in patients with high-grade glioma. Neurooncol. Pract. 2021, 8, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.K.; Ketcher, D.; Heyman, R.E.; Vadaparampil, S.T.; Ellington, L.; Reblin, M. Communication between Advanced Cancer Patients and Their Family Caregivers: Relationship with Caregiver Burden and Preparedness for Caregiving. Health Commun. 2021, 36, 714–721. [Google Scholar] [CrossRef]
- Dhumal, T.; Siddiqui, Z.A.; Kelley, G.A.; Harper, F.; Kelly, K.M. Systematic review and meta-analysis of randomized controlled trials of interventions addressing caregiver distress and burden among cancer caregivers. PEC Innov. 2023, 2, 100145. [Google Scholar] [CrossRef]
- Tzelepis, F.; Rose, S.K.; Sanson-Fisher, R.W.; Clinton-McHarg, T.; Carey, M.L.; Paul, C.L. Are we missing the Institute of Medicine’s mark? A systematic review of patient-reported outcome measures assessing quality of patient-centred cancer care. BMC Cancer 2014, 14, 41. [Google Scholar] [CrossRef]



| Valid Number | Percent | ||
|---|---|---|---|
| Age groups at diagnosis | 20.0–59.9 | 33 | 54.1% |
| 60.0–99.9 | 28 | 45.9% | |
| Sex | Male | 30 | 49.2% |
| Female | 31 | 50.8% | |
| Tumor localization | Cerebrum | 1 | 1.6% |
| Frontal lobe | 13 | 21.3% | |
| Temporal lobe | 18 | 29.5% | |
| Parietal lobe | 14 | 23.0% | |
| Occipital lobe | 2 | 3.3% | |
| Brain, subareas overlapping | 8 | 13.1% | |
| Brain, not specified | 5 | 8.2% | |
| Karnofsky Performance Scale at last QLQ-C30 assessment | 100 | 1 | 1.6% |
| 90 | 22 | 36.1% | |
| 80 | 20 | 32.8% | |
| 70 | 12 | 19.7% | |
| 60 | 3 | 4.9% | |
| 50 | 3 | 4.9% | |
| Epilepsy at first visit | No | 28 | 45.9% |
| Yes | 33 | 54.1% | |
| Range: diagnosis to last QLQ-C30 assessment | <18 months | 33 | 54.1% |
| ≥18 months | 28 | 45.9% | |
| Psycho-oncological treatment | No | 32 | 52.5% |
| Yes | 29 | 47.5% | |
| Psychosocial distress at first visit * | No (<4) | 37 | 60.7% |
| Yes (≥4) | 24 | 39.3% | |
| Total | 61 | 100.0% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischl, A.; Gerken, M.; Lindberg-Scharf, P.; Haedenkamp, T.M.; Rosengarth, K.; Hillberg, A.; Vogelhuber, M.; Schön, I.; Proescholdt, M.; Araceli, T.; et al. Health-Related Quality of Life and Treatment Satisfaction of Patients with Malignant IDH Wild-Type Gliomas and Their Caregivers. Curr. Oncol. 2024, 31, 6155-6170. https://doi.org/10.3390/curroncol31100459
Fischl A, Gerken M, Lindberg-Scharf P, Haedenkamp TM, Rosengarth K, Hillberg A, Vogelhuber M, Schön I, Proescholdt M, Araceli T, et al. Health-Related Quality of Life and Treatment Satisfaction of Patients with Malignant IDH Wild-Type Gliomas and Their Caregivers. Current Oncology. 2024; 31(10):6155-6170. https://doi.org/10.3390/curroncol31100459
Chicago/Turabian StyleFischl, Anna, Michael Gerken, Patricia Lindberg-Scharf, Tareq M. Haedenkamp, Katharina Rosengarth, Andrea Hillberg, Martin Vogelhuber, Ingrid Schön, Martin Proescholdt, Tommaso Araceli, and et al. 2024. "Health-Related Quality of Life and Treatment Satisfaction of Patients with Malignant IDH Wild-Type Gliomas and Their Caregivers" Current Oncology 31, no. 10: 6155-6170. https://doi.org/10.3390/curroncol31100459
APA StyleFischl, A., Gerken, M., Lindberg-Scharf, P., Haedenkamp, T. M., Rosengarth, K., Hillberg, A., Vogelhuber, M., Schön, I., Proescholdt, M., Araceli, T., Koller, M., Herrmann, A., Kölbl, O., Pukrop, T., Riemenschneider, M. J., Schmidt, N. O., Klinkhammer-Schalke, M., Linker, R., Hau, P., & Bumes, E. (2024). Health-Related Quality of Life and Treatment Satisfaction of Patients with Malignant IDH Wild-Type Gliomas and Their Caregivers. Current Oncology, 31(10), 6155-6170. https://doi.org/10.3390/curroncol31100459







