Patient-Reported Outcomes after Surgical, Endoscopic, or Radiological Techniques for Nutritional Support in Esophageal Cancer Patients: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
2.6. Data Synthesis
3. Results
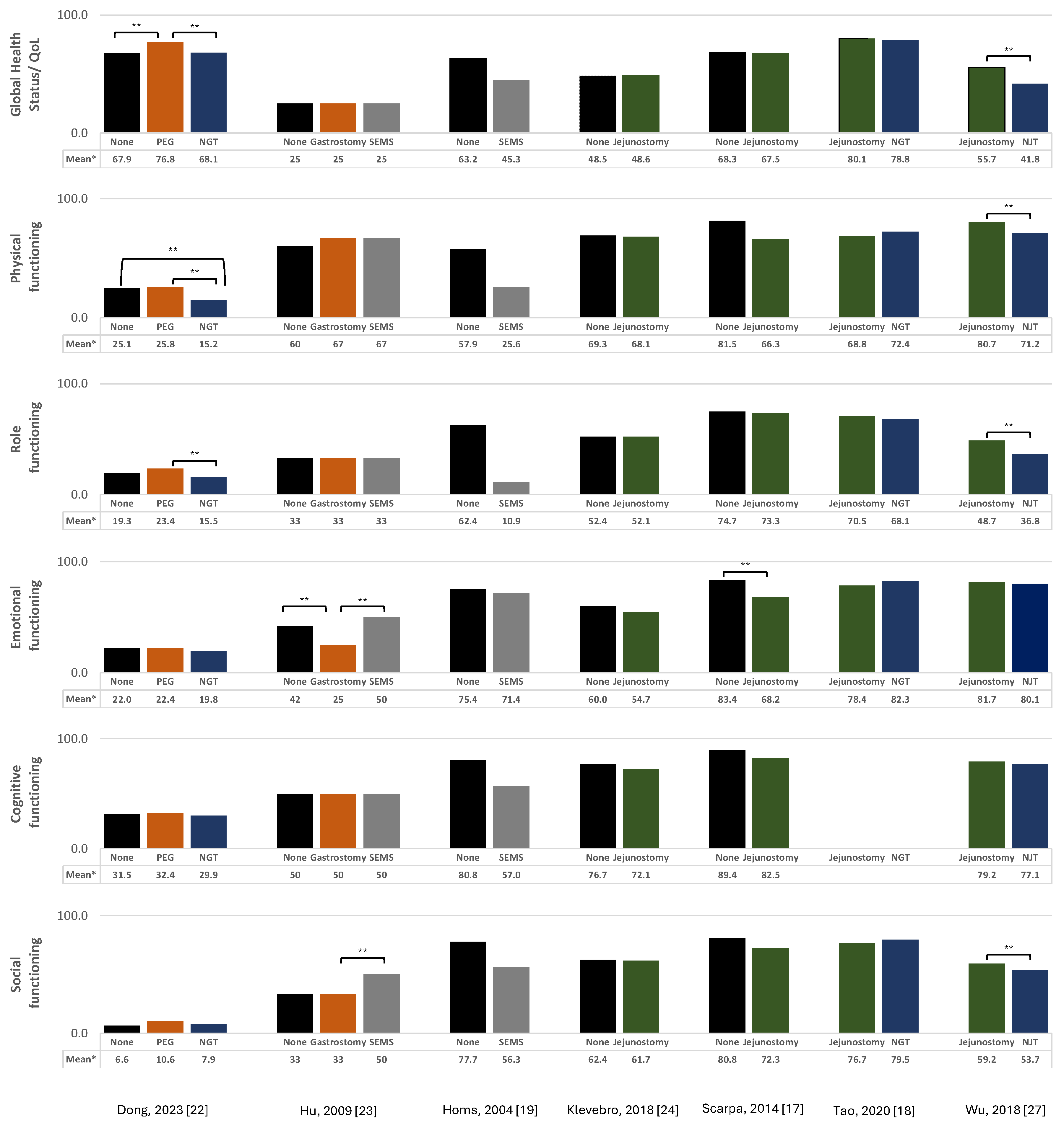
3.1. Overall Quality of Life
3.1.1. Jejunostomy
3.1.2. Nasogastric/Nasojejunal Tube
3.1.3. Gastrostomy
3.1.4. Esophageal Stent
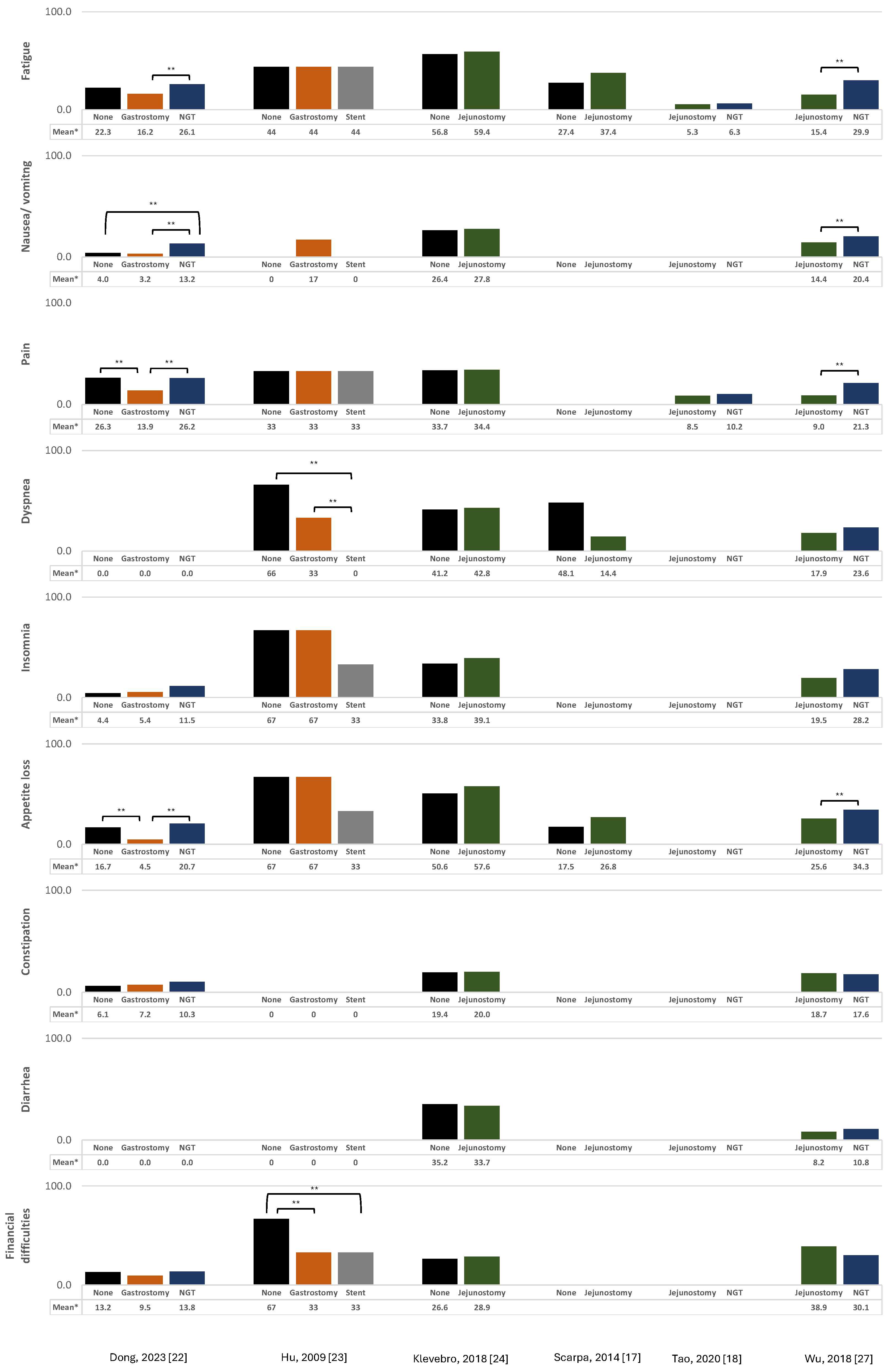
3.2. Pain
3.3. Depression
4. Discussion
4.1. Jejunostomy vs. Alternatives
4.2. Esophageal Stents vs. Alternatives
4.3. Selection Bias
4.4. Limitations of Current Research and Future Approaches
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| 1st Author, Year of Publication (REF) | Study Description | Subjects’ Characteristics | Technique Evaluated | PRO Evaluated (PRO Measurement) | Timing of Assessment and Results Presented | Control of Confounding |
|---|---|---|---|---|---|---|
| Dong, 2023 [22] | Country: China. Study design: Prospective cohort study. Aim: To analyze the differences in the nutritional status, chemoradiotherapy-related adverse events, and QoL of patients with esophageal cancer receiving nutrition through oral intake, PEG, and NGT approaches during concurrent chemoradiotherapy. Study population: Patients with esophageal squamous cell carcinoma (18–75 years) who were scheduled to receive concurrent chemoradiotherapy. Sample size: N = 141; “PEG group”: N = 74; “NGT group”: N = 29; “Control group (oral intake)”: N = 38. | Age (number [%]): <60 years: 36 (48.6%) vs. 11 (37.9%) vs. 13 (34.2%); ≥60 years: 38 (51.4%) vs. 18 (62.1%) vs. 25 (65.8%) in the PEG, NGT, and control groups, respectively. Cancer stage (number [%]): stage II-III: 37 (50.0%) vs. 10 (34.5%) vs. 26 (68.4%); stage IV: 37 (0.0%) vs. 19 (65.5%) vs. 12 (31.6%) in the PEG, NGT, and control groups, respectively. Type of carcinoma: squamous cell carcinoma. | PEG vs. NGT vs. none | QoL (EORTC QLQ-C30) | Mean (SD) in scores before and after concurrent chemoradiotherapy. | -- |
| Wang, 2021 [26] | Country: China. Study design: Retrospective cohort study. Aim: To compare the changes in nutritional status, the safety of the procedures, and the overall survival of patients with esophageal cancer and dysphagia who underwent stent insertion or percutaneous gastrostomy. Study population: Patients with dysphagia who underwent gastrostomy or stent insertion. Sample size: N = 113; “SEMS group”: N = 47; “RIG group”: N = 66. | Age (median [IQR]): 76.0 (9.0) vs. 75.0 (6.0) years in the SEMS and RIG groups, respectively. Cancer stage (number [%]): stage II/III: 22 (31.9%) vs. 20 (30.3%); stage IV: 25 (68.1%) vs. 46 (69.7%) in the SEMS and RIG groups, respectively. Type of carcinoma (number [%]): squamous cell carcinoma: 38 (80.9%) vs. 60 (90.9%); adenocarcinoma: 9 (19.1%) vs. 6 (9.1%) in the SEMS and RIG groups, respectively. | SEMS vs. RIG | Pain (Visual Analog Scale) | Number (%) of patients with local severe pain (score of 7 or greater). | -- |
| Martin, 2020 [25] | Country: United States of America. Study design: Retrospective cohort study. Aim: To assess the efficacy and complications of palliative external-beam radiotherapy compared with esophageal stent placement among a large, multi-institutional cohort of veterans diagnosed with metastatic esophageal cancer. Study population: Veterans with esophageal cancer stage IV, diagnosed between 2000 and 2015, treated with palliative external-beam radiotherapy and/or esophageal stenting. Sample size: N = 766; “Stent group”: N = 31%; “Control group (Radiotherapy)”: N = 41%. | Age: -- Cancer stage: stage IV. Type of carcinoma: -- | Stent 1 vs. none | Pain [Likert scale (0–10)] | Estimate from linear mixed effects model. | Charlson comorbidity index score, income, population density, and tumor location. |
| Tao, 2020 [18] | Country: China. Study design: Randomized controlled trial. Aim: To assess two common enteral nutrition methods after minimally invasive McKeown esophagectomy. Study population: Patients who underwent minimally invasive McKeown esophagectomy. Sample size: N = 120; “Jejunostomy group”: N = 58; “NGT group”: N = 62. | Age (mean [range]): 65 (41–81) vs. 64 (39–82) years in the jejunostomy and NGT groups, respectively. Cancer stage (number): stage I: 12 (20.7) vs. 19 (30.6); stage II: 21 (36.2) vs. 23 (37.1); stage III: 16 (27.6) vs. 16 (25.8); stage IV: 9 (12.6) vs. 4 (6.5) in the jejunostomy and NGT groups, respectively. Type of carcinoma: squamous cell carcinoma. | Surgical jejunostomy vs. NGT | QoL (EORTC QLQ-C30) | Mean (SE) scores preoperatively and one week and one month after surgery. | -- |
| Klevebro, 2018 [24] | Country: Sweden. Study design: Prospective cohort study. Aim: To assess the influence of jejunostomy on postoperative outcomes other than weight development in patients who underwent esophagectomy for esophageal cancer. Study population: Patients operated on for esophageal or gastroesophageal junctional cancer. Sample size: N = 397; “Jejunostomy group”: N = 178; “Control group”: N = 219. | Age (number [%]): <60 years: 41 (23%) vs. 58 (26%); 60–74 years: 102 (57%) vs. 128 (58%); >74 years: 35 (20%) vs. 33 (15%) in the jejunostomy and control groups, respectively. Cancer stage (number [%]): stage 0-I: 39 (22%) vs. 44 (20%); stage II: 51 (29%) vs. 69 (32%); stage III: 72 (40%) vs. 89 (41%); stage IV: 16 (9%) vs. 17 (8%) in the jejunostomy and control groups, respectively. Type of carcinoma (number [%]): squamous cell carcinoma: 53 (30%) vs. 44 (20%); adenocarcinoma: 125 (70%) vs. 175 (80%) in the jejunostomy and control groups, respectively. | Surgical jejunostomy vs. none | QoL (EORTC QLQ-C30 and EORTC QLQ-OES 18) | Adjusted mean scores (95% CI) six months after esophagectomy. | Age, sex, histological tumor type, tumor stage, Charlson comorbidity index, surgical technique, neoadjuvant therapy, baseline body mass index, and weight loss at baseline. |
| Wu, 2018 [27] | Country: China. Study design: Prospective cohort study. Aim: To investigate the effect of 3 months home enteral nutrition on health-related QoL and nutritional status of esophageal cancer patients who were preoperatively malnourished. Study population: Patients diagnosed with esophageal and esophagogastric junction cancer and suitable for potentially curative resection with intrathoracic anastomosis. Sample size: N = 142; “Jejunostomy group”: N = 67; “NJT group”: N = 75. | Age (median [range]): 62 (45–80) vs. 61 (43–80) years in the jejunostomy and NJT groups, respectively. Cancer stage (number [%]): stage 0/I: 13 (19.4%) vs. 10 (13.3%); stage II: 27 (40.3%) vs. 36 (48.0%); stage III: 27 (40.3%) vs. 29 (38.6%) in the jejunostomy and NJT groups, respectively. Type of carcinoma (number [%]): squamous cell carcinoma: 61 (91.0%) vs. 71 (94.7%); adenocarcinoma: 6 (9.0%) vs. 4 (5.3%) in the jejunostomy and NJT groups, respectively. | Surgical jejunostomy vs. NJT | QoL (EORTC QLQ-C30) | Mean (SD) scores before surgery and two weeks and three months after surgery. | -- |
| Pain (Visual Analog Scale) | Median (range) scores within 72 h post-surgery. | -- | ||||
| Yu, 2018 [28] | Country: Taiwan. Study design: Non-randomized controlled trial. Aim: To evaluate esophageal squamous cell carcinoma patients who received neoadjuvant or definite chemoradiation radiotherapy to compare the efficacy, safety, and QoL among patients using different methods to maintain enteral nutrition. Study population: Incident esophageal squamous cell carcinoma patients who received neoadjuvant or definite chemoradiation radiotherapy. Sample size: N = 81; “SEMS group”: N = 7; “Jejunostomy/gastrostomy group”: N = 26; “NGT group”: N = 19; “Control group (oral intake)”: N = 29. | Age (mean [SD]): 57.2 (13.7) vs. 57.8 (7.8) vs. 53.6 (6.7) vs. 57.8 (9.8) years in the SEMS, jejunostomy/gastrostomy, NGT, and control groups, respectively. Cancer stage (number [%]): stage I/II: 0 (0%) vs. 3 (11.5%) vs. 1(5.3%) vs. 3 (10.3); stage III: 7 (100.0%) vs. 19 (73.1%) vs. 16 (84.2) vs. 19 (65.5%); stage IV: 0 (0.0%), 4 (15.4%), 2 (10.5%), 7 (24.1%) in the SEMS, jejunostomy/gastrostomy, NGT, and control groups, respectively. Type of carcinoma: squamous cell carcinoma. | SEMS vs. surgical jejunostomy/gastrostomy vs. NGT vs. none | QoL (EORTC QLQ-C30 and EORTC QLQ-OES18) | Mean difference (between 4 and 5 weeks after completion of CRT and at cancer diagnosis) in the scores. | -- |
| Depression (Patient Health Questionnaire) | Mean difference (between 4 and 5 weeks after completion of CRT and at cancer diagnosis) in the scores. | -- | ||||
| Scarpa, 2014 [17] | Country: Italy. Study design: Retrospective cohort study. Aim: To evaluate the impact of jejunostomy during esophagectomy for cancer on postoperative health-related QoL. Study population: Patients who underwent esophagectomy for cancer. Sample size: N = 109; “Jejunostomy group”: N = 40; “Non-jejunostomy group”: N = 69. | Age (median [IQR]): 64 (58–71) vs. 59 (51–67) in the jejunostomy and non-jejunostomy groups, respectively. Cancer stage (number [%]): stage 0: 7 (17.5%) vs. 18 (26.1%); stage I-II: 12 (30.0%) vs. 31 (44.9%); stage III-IV: 21 (54.5%) vs. 20 (29.9%) in the jejunostomy and non-jejunostomy groups, respectively. Type of carcinoma (number [%]): squamous cell carcinoma: 21 (53.8%) vs. 12 (17.4%); adenocarcinoma: 18 (46.2%) vs. 57 (52.6%) in the jejunostomy and non-jejunostomy groups, respectively. | Surgical jejunostomy vs. none | QoL (EORTC QLQ-C30 and EORTC QLQ-OES 18) | Mean (SD) scores at admission and after surgery and mean (SD) score differences between scores at admission and after surgery. | General linear models adjusted for age, tumor site, and pathologic stage 2. |
| Shenfine, 2009 [29] | Country: United Kingdom. Study design: Randomized controlled trial. Aim: To compare the clinical effectiveness and cost-effectiveness of SEMSs with other palliative therapies. Study population: Patients with dysphagia due to esophageal cancer. Sample size: N = 101; “SEMS group”: N = 53 3; “Control group (non-SEMS group)”: N = 48. | Age (mean [SD]): 74.6 (11.4) vs. 76.9 (9.0) in the SEMS and control groups, respectively. Cancer stage: -- Type of carcinoma: squamous cell carcinoma: 15 (28.3%) vs. 16 (32.0%); adenocarcinoma: 32 (60.4%) vs. 31 (62.0%) in the SEMS and control groups, respectively. | SEMS vs. none | QoL (Spitzer QL Index and Euroqol (EQ)-5D) | Mean (SD) scores at admission and six weeks after. | -- |
| Hu, 2009 [23] | Country: China. Study design: Nan-randomized controlled trial. Aim: To compare the survival time and QoL of patients who have received different treatments for tracheoesophageal/bronchoesophageal fistula. Study population: Patients diagnosed with tracheoesophageal/ bronchoesophageal fistula secondary to esophageal squamous. Sample size: N = 27; “Gastrostomy group”: N = 7; “SEMS group”: N = 13; “Control group (conservative therapy)”: N = 7. | Age (mean [SD]): 57.33 (8.60) vs. 56.83 (7.72) vs. 59.56 (4.77) years in the gastrostomy, SEMS, and control groups, respectively. Cancer stage: -- Type of carcinoma: squamous cell carcinoma. | Surgical gastrostomy vs. SEMS vs. none | QoL (EORTC QLQ-C30 and EORTC QLQ-OES 18) | Median (IQR) scores two weeks after gastrostomy or stent insertion (two weeks after admission for the control group). | -- |
| Homs, 2004 [19] | Country: The Netherlands. Study design: Randomized controlled trial. Aim: To compare metal stent placement and single-dose brachytherapy with respect to generic and disease-specific health-related QoL. Study population: Patients with inoperable cancer of the esophagus or esophagogastric junction due to metastatic disease and/or a poor medical condition with progressive dysphagia. Sample size: N = 209; “SEMS group”: N = 108; “Control group (Single dose brachytherapy)”: N = 101. | Age (mean [SD]): 69 (11) vs. 69 (13) in the SEMS and control groups, respectively. Cancer stage: -- Type of carcinoma (number [%]): squamous cell carcinoma: 29 (27%) vs. 29 (29%); adenocarcinoma: 75 (69%) vs. 69 (68%); other: 4 (4%) vs. 3 (3%) in the SEMS and control groups, respectively. | SEMS vs. none | QoL (EORTC QLQ-C30, EORTC QLQ OES-23, and Euroqol (EQ)-5D) | Mean (95%CI) scores before treatment and 12 months after treatment. | -- |
| Pain (Visual Analog Scale) | Mean (95%CI) scores before treatment and 12 months after treatment. | -- |
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today (version 1.1). Available online: https://gco.iarc.who.int/today (accessed on 4 July 2024).
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Haiducu, C.; Buzea, A.; Mirea, L.E.; Dan, G.A. The prevalence and the impact of sarcopenia in digestive cancers. A systematic review. Rom. J. Intern. Med. 2021, 59, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef] [PubMed]
- Hébuterne, X.; Lemarié, E.; Michallet, M.; de Montreuil, C.B.; Schneider, S.M.; Goldwasser, F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. J. Parenter. Enter. Nutr. 2014, 38, 196–204. [Google Scholar] [CrossRef]
- Nitenberg, G.; Raynard, B. Nutritional support of the cancer patient: Issues and dilemmas. Crit. Rev. Oncol. Hematol. 2000, 34, 137–168. [Google Scholar] [CrossRef]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.G.; et al. ESPEN practical guideline: Clinical nutrition in surgery. Clin. Nutr. 2021, 40, 4745–4761. [Google Scholar] [CrossRef]
- Nunes, G.; Fonseca, J.; Barata, A.T.; Dinis-Ribeiro, M.; Pimentel-Nunes, P. Nutritional Support of Cancer Patients without Oral Feeding: How to Select the Most Effective Technique? GE Port. J. Gastroenterol. 2020, 27, 172–184. [Google Scholar] [CrossRef]
- Mohapatra, S.; Santharaman, A.; Gomez, K.; Pannala, R.; Kachaamy, T. Optimal Management of Dysphagia in Patients with Inoperable Esophageal Cancer: Current Perspectives. Cancer Manag. Res. 2022, 14, 3281–3291. [Google Scholar] [CrossRef]
- Weijs, T.J.; Berkelmans, G.H.; Nieuwenhuijzen, G.A.; Ruurda, J.P.; van Hillegersberg, R.; Soeters, P.B.; Luyer, M.D. Routes for early enteral nutrition after esophagectomy. A systematic review. Clin. Nutr. 2015, 34, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- FDA. Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims; FDA: Rockville, MD, USA, 2009. [Google Scholar]
- Covidence Systematic Review Software; Veritas Health Innovation: Melbourne, Australia, 2024.
- Scarpa, M.; Cavallin, F.; Noaro, G.; Pinto, E.; Alfieri, R.; Cagol, M.; Castoro, C. Impact of jejunostomy during esophagectomy for cancer on health related quality of life. Chin. J. Cancer Res. 2014, 26, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Zhang, Y.; Zhu, S.; Ni, Z.; You, Q.; Sun, X.; Xu, D. A Prospective Randomized Trial Comparing Jejunostomy and Nasogastric Feeding in Minimally Invasive McKeown Esophagectomy. J. Gastrointest. Surg. 2020, 24, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Homs, M.Y.; Essink-Bot, M.L.; Borsboom, G.J.; Steyerberg, E.W.; Siersema, P.D. Quality of life after palliative treatment for oesophageal carcinoma—a prospective comparison between stent placement and single dose brachytherapy. Eur. J. Cancer 2004, 40, 1862–1871. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A. WebPlotDigitizer. Available online: https://automeris.io/WebPlotDigitizer.html (accessed on 27 March 2024).
- Hong, Q.N.; Pluye, P.; Fàbregues, S.; Bartlett, G.; Boardman, F.; Cargo, M.; Dagenais, P.; Gagnon, M.P.; Griffiths, F.; Nicolau, B.; et al. Improving the content validity of the mixed methods appraisal tool: A modified e-Delphi study. J. Clin. Epidemiol. 2019, 111, 49–59.e41. [Google Scholar] [CrossRef]
- Dong, J.; Dai, Z.; Cao, F.; Zhang, W.; Zhang, T.; Chen, X.; Chen, Y.; Zhao, F.; Li, J.; Du, Q.; et al. Effects of PEG in patients with esophageal squamous cell carcinoma during concurrent chemoradiotherapy: A prospective study. Gastrointest. Endosc. 2023, 98, 901–910.e903. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, Y.F.; Chen, L.Q.; Zhu, Z.J.; Liu, L.X.; Wang, Y.; Kou, Y.L. Comparative study of different treatments for malignant tracheoesophageal/bronchoesophageal fistulae. Dis. Esophagus 2009, 22, 526–531. [Google Scholar] [CrossRef]
- Klevebro, F.; Johar, A.; Lagergren, J.; Lagergren, P. Outcomes of nutritional jejunostomy in the curative treatment of esophageal cancer. Dis. Esophagus 2019, 32, doy113. [Google Scholar] [CrossRef]
- Martin, E.J.; Bruggeman, A.R.; Nalawade, V.V.; Sarkar, R.R.; Qiao, E.M.; Rose, B.S.; Murphy, J.D. Palliative Radiotherapy Versus Esophageal Stent Placement in the Management of Patients With Metastatic Esophageal Cancer. J. Natl. Compr. Cancer Netw. 2020, 18, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wen, Q.; Zhang, Y.; Yuan, J.; Gao, C.; Hu, H.; Chen, C.; Zheng, L.; Li, F.; Li, Y.; et al. Percutaneous Gastrostomy Compared with Esophageal Stent Placement for the Treatment of Esophageal Cancer with Dysphagia. J. Vasc. Interv. Radiol. 2021, 32, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wu, M.; Wang, Q.; Zhan, T.; Wang, L.; Pan, S.; Chen, G. Home enteral nutrition after minimally invasive esophagectomy can improve quality of life and reduce the risk of malnutrition. Asia Pac. J. Clin. Nutr. 2018, 27, 129–136. [Google Scholar] [CrossRef]
- Yu, F.J.; Shih, H.Y.; Wu, C.Y.; Chuang, Y.S.; Lee, J.Y.; Li, H.P.; Fang, P.T.; Tsai, D.L.; Chou, S.H.; Wu, I.C. Enteral nutrition and quality of life in patients undergoing chemoradiotherapy for esophageal carcinoma: A comparison of nasogastric tube, esophageal stent, and ostomy tube feeding. Gastrointest. Endosc. 2018, 88, 21–31.e24. [Google Scholar] [CrossRef]
- Shenfine, J.; McNamee, P.; Steen, N.; Bond, J.; Griffin, S.M. A randomized controlled clinical trial of palliative therapies for patients with inoperable esophageal cancer. Am. J. Gastroenterol. 2009, 104, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Blazeby, J.M.; Conroy, T.; Hammerlid, E.; Fayers, P.; Sezer, O.; Koller, M.; Arraras, J.; Bottomley, A.; Vickery, C.W.; Etienne, P.L.; et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur. J. Cancer 2003, 39, 1384–1394. [Google Scholar] [CrossRef]
- Blazeby, J.M.; Alderson, D.; Winstone, K.; Steyn, R.; Hammerlid, E.; Arraras, J.; Farndon, J.R. Development of an EORTC questionnaire module to be used in quality of life assessment for patients with oesophageal cancer. The EORTC Quality of Life Study Group. Eur. J. Cancer 1996, 32, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R. EuroQol: The current state of play. Health Policy 1996, 37, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, W.O.; Dobson, A.J.; Hall, J.; Chesterman, E.; Levi, J.; Shepherd, R.; Battista, R.N.; Catchlove, B.R. Measuring the quality of life of cancer patients: A concise QL-index for use by physicians. J. Chronic Dis. 1981, 34, 585–597. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Lee, Y.; Lu, J.Y.; Malhan, R.; Shargall, Y.; Finley, C.; Hanna, W.; Agzarian, J. Effect of routine jejunostomy tube insertion in esophagectomy: A systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2022, 164, 422–432.e417. [Google Scholar] [CrossRef]
- Watson, M.; Trufan, S.; Benbow, J.H.; Gower, N.L.; Hill, J.; Salo, J.C. Jejunostomy at the time of esophagectomy is associated with improved short-term perioperative outcomes: Analysis of the NSQIP database. J. Gastrointest. Oncol. 2020, 11, 421–430. [Google Scholar] [CrossRef]
- Mei, L.X.; Wang, Y.Y.; Tan, X.; Chen, Y.; Dai, L.; Chen, M.W. Is it necessary to routinely perform feeding jejunostomy at the time of esophagectomy? A systematic review and meta-analysis. Dis. Esophagus 2021, 34, doab017. [Google Scholar] [CrossRef]
- Arnold, M.; Rutherford, M.; Lam, F.; Bray, F.; Ervik, M.; Soerjomataram, I. ICBP SURVMARK-2 online tool: International Cancer Survival Benchmarking. Available online: https://gco.iarc.fr/survival/survmark (accessed on 4 July 2024).
- Naher, S.K.; Mercieca-Bebber, R.; Siu, D.; Grimison, P.; Stockler, M.R. Prognostic value of patient reported outcomes in advanced gastro-oesophageal cancer: A systematic review. Intern. Med. J. 2023, 53, 1946–1955. [Google Scholar] [CrossRef]
- Obermannová, R.; Alsina, M.; Cervantes, A.; Leong, T.; Lordick, F.; Nilsson, M.; van Grieken, N.C.T.; Vogel, A.; Smyth, E.C. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 992–1004. [Google Scholar] [CrossRef]
- Shah, M.A.; Hofstetter, W.L.; Kennedy, E.B. Immunotherapy in Patients With Locally Advanced Esophageal Carcinoma: ASCO Treatment of Locally Advanced Esophageal Carcinoma Guideline Rapid Recommendation Update. J. Clin. Oncol. 2021, 39, 3182–3184. [Google Scholar] [CrossRef]
- Shah, M.A.; Kennedy, E.B.; Catenacci, D.V.; Deighton, D.C.; Goodman, K.A.; Malhotra, N.K.; Willett, C.; Stiles, B.; Sharma, P.; Tang, L.; et al. Treatment of Locally Advanced Esophageal Carcinoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 2677–2694. [Google Scholar] [CrossRef]
- Dixit, J.; Gupta, N.; Kataki, A.; Roy, P.; Mehra, N.; Kumar, L.; Singh, A.; Malhotra, P.; Gupta, D.; Goyal, A.; et al. Health-related quality of life and its determinants among cancer patients: Evidence from 12,148 patients of Indian database. Health Qual. Life Outcomes 2024, 22, 26. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Du, L.; Huang, H.; Wang, L.; Zhu, J.; Li, H.; Bai, Y.; Liao, X.; Mao, A.; et al. Health-related quality of life in patients with esophageal cancer or precancerous lesions assessed by EQ-5D: A multicenter cross-sectional study. Thorac. Cancer 2020, 11, 1076–1089. [Google Scholar] [CrossRef]
- Gupta, V.; Allen-Ayodabo, C.; Davis, L.; Zhao, H.; Hallet, J.; Mahar, A.L.; Ringash, J.; Kidane, B.; Darling, G.; Coburn, N.G. Patient-Reported Symptoms for Esophageal Cancer Patients Undergoing Curative Intent Treatment. Ann. Thorac. Surg. 2020, 109, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Schandl, A.R.; Johar, A.; Mälberg, K.; Lagergren, P. Education level and health-related quality of life after oesophageal cancer surgery: A nationwide cohort study. BMJ Open 2018, 8, e020702. [Google Scholar] [CrossRef] [PubMed]
- Minvielle, E.; di Palma, M.; Mir, O.; Scotté, F. The use of patient-reported outcomes (PROs) in cancer care: A realistic strategy. Ann. Oncol. 2022, 33, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Kluetz, P.G.; Chingos, D.T.; Basch, E.M.; Mitchell, S.A. Patient-Reported Outcomes in Cancer Clinical Trials: Measuring Symptomatic Adverse Events With the National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 67–73. [Google Scholar] [CrossRef]

| Studies Included in the Systematic Review (1st Author, Year of Publication) | PROs Reported and Instrument Used for Their Evaluation | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall Quality of Life | Specific Symptoms | |||||||
| Pain | Depression | |||||||
| EORTC QLQ-C30 [30] | EORTC QLQ-OES18 [31] | EORTC QLQ-OES23 [32] | Euroqol (EQ)-5D [33] | Spitzer QL Index [34] | Visual Analog Scale | Likert Scale | Depression Module of the Patient Health Questionnaire (PHQ-9) [35] | |
| Dong, 2023 [22] | ✓ | |||||||
| Wang, 2021 [26] | ✓ | |||||||
| Martin, 2020 [25] | ✓ | |||||||
| Tao, 2020 [18] | ✓ | |||||||
| Klevebro, 2018 [24] | ✓ | ✓ | ||||||
| Wu, 2018 [27] | ✓ | ✓ | ||||||
| Yu, 2018 [28] | ✓ | ✓ | ✓ | |||||
| Scarpa, 2014 [17] | ✓ | ✓ | ||||||
| Hu, 2009 [23] | ✓ | ✓ | ||||||
| Shenfine, 2009 [29] | a | ✓ | ✓ | |||||
| Homs, 2004 [19] | ✓ | ✓ | ✓ | ✓ | ||||
| Category of Study Designs | Methodological Quality Criteria | Studies Included in the Systematic Review | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tao, 2020 [18] | Shenfine, 2009 [29] | Homs, 2004 [19] | Dong, 2023 [22] | Wang, 2021 [26] | Martin, 2020 [25] | Klevebro, 2018 [24] | Wu, 2018 [27] | Yu, 2018 [28] | Scarpa, 2014 [17] | Hu, 2009 [23] | ||
| Quantitative randomized controlled trials | Is randomization appropriately performed? |  |  |  | -- | -- | -- | -- | -- | -- | -- | -- |
| Are the groups comparable at baseline? |  |  |  | -- | -- | -- | -- | -- | -- | -- | -- | |
| Are there complete outcome data? |  |  |  | -- | -- | -- | -- | -- | -- | -- | -- | |
| Are the outcome assessors blinded to the intervention provided? |  |  |  | -- | -- | -- | -- | -- | -- | -- | -- | |
| Did the participants adhere to the assigned intervention? |  |  |  | -- | -- | -- | -- | -- | -- | -- | -- | |
| Quantitative non-randomized | Are the participants representative of the target population? | -- | -- | -- |  |  |  |  |  |  |  |  |
| Are measurements appropriate regarding both the outcome and intervention (or exposure)? | -- | -- | -- |  |  |  |  |  |  |  |  | |
| Are there complete outcome data? | -- | -- | -- |  |  |  |  |  |  |  |  | |
| Are the confounders accounted for in the design and analysis? | -- | -- | -- |  |  |  |  |  |  |  |  | |
| During the study period, is the intervention administered (or exposure occurred) as intended? | -- | -- | -- |  |  |  |  |  |  |  |  | |
 yes;
yes;  no;
no;  the article does not report appropriate information to answer “Yes” or “No” or unclear information related to the criterion was reported.
the article does not report appropriate information to answer “Yes” or “No” or unclear information related to the criterion was reported.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontes, F.; Fernandes, D.; Almeida, A.; Sá, I.; Dinis-Ribeiro, M. Patient-Reported Outcomes after Surgical, Endoscopic, or Radiological Techniques for Nutritional Support in Esophageal Cancer Patients: A Systematic Review. Curr. Oncol. 2024, 31, 6171-6190. https://doi.org/10.3390/curroncol31100460
Fontes F, Fernandes D, Almeida A, Sá I, Dinis-Ribeiro M. Patient-Reported Outcomes after Surgical, Endoscopic, or Radiological Techniques for Nutritional Support in Esophageal Cancer Patients: A Systematic Review. Current Oncology. 2024; 31(10):6171-6190. https://doi.org/10.3390/curroncol31100460
Chicago/Turabian StyleFontes, Filipa, Davide Fernandes, Ana Almeida, Inês Sá, and Mário Dinis-Ribeiro. 2024. "Patient-Reported Outcomes after Surgical, Endoscopic, or Radiological Techniques for Nutritional Support in Esophageal Cancer Patients: A Systematic Review" Current Oncology 31, no. 10: 6171-6190. https://doi.org/10.3390/curroncol31100460
APA StyleFontes, F., Fernandes, D., Almeida, A., Sá, I., & Dinis-Ribeiro, M. (2024). Patient-Reported Outcomes after Surgical, Endoscopic, or Radiological Techniques for Nutritional Support in Esophageal Cancer Patients: A Systematic Review. Current Oncology, 31(10), 6171-6190. https://doi.org/10.3390/curroncol31100460





